Abstract
Myiasis is an ectoparasitic infection caused by the larvae of true flies (Diptera). We came across a rather rare case of myiasis in an immunocompetent 34-year-old man from French Guiana with advanced wound myiasis masquerading as cavitary myiasis and a history of cholesteatoma surgery in the left ear. The Diptera larvae responsible for the disease were isolated and identified using morphological and molecular approaches as Cochliomyia hominivorax. We underline the importance of this parasitosis as the second case of myiasis caused by C. hominivorax and the first case of wound myiasis in this overseas department of France and its incidence in pre-urban areas of the capital, Cayenne, in South America.
1. Introduction
Myiasis is an ectoparasitic disease of human or animal tissues caused by the development of larvae (maggots) of various fly species within the Diptera order [1]. It generally invades vertebrates but occasionally occurs in humans [2]. Human myiasis is a neglected and under-reported disease [3], mainly due to its negative social implications. The infection is more prevalent in tropical and subtropical regions of the world because the warm and humid climatic conditions provide suitable breeding places for flies [4,5,6]. Furthermore, it is more common in rural regions than in urban areas, especially in developing countries with a lack of basic sanitation, close contact with livestock animals, and inadequate garbage disposal [6,7]. Men are more infected than women, mainly due to outdoor activities and less personal hygiene [8]. It generally affects people of low socioeconomic level, immunocompromised, or those with psychiatric disorders [6,9].
The Diptera order is composed of the Nematocera and Brachycera suborders. The latter comprises several important genera responsible for myiasis in humans in the Old World and New World, including Dermatobia, Calliphoridae, Cochliomyia, Cordylobia, Chrysomyia, Cuterebra, Oestrus, Gasterophilus, Hypoderma, Phaenicia (known as Lucilia), Sarcophaga, and Wohlfahrtia [10,11].
Myiasis may be classified in different ways. Based on the affected body location, it can be classified as (i) cutaneous, (ii) oral, (iii) nasal, (iv) cerebral, (v) ocular, (vi) intestinal, and (vii) urogenital myiasis [12,13]. According to the host–parasite relationship, it can be subdivided into obligatory, facultative, or accidental myiasis [12]. On the other hand, it can be considered furunculoid (penetration of the dipteran larva into healthy skin), migratory, cavity, or wound myiasis [14]. Except for cutaneous myiasis, all other aforementioned myiases are leading causes of hospitalization, amputation, reduced mobility, loss of social inclusion, and poor quality of life [15].
There are a variety of predisposing factors, depending on the type of myiasis. Wound myiasis is commonly linked to socioeconomic factors, while cavity myiasis is associated with the presence of animals, and ultimately furunculoid myiasis is related to exposure to mosquitoes or larval-infected tissues (or flies, mango trees, etc.). However, low socioeconomic status, malnutrition, drug use, alcoholism, low oral hygiene, facial trauma, open wounds, mouth-breathing, mental retardation, psychological disorders, advanced age, poor hygiene conditions, vascular disorders, necrosis, immunocompromised conditions, diabetes mellitus, and living in close contact with domestic animals are conditions that favor human myiasis [16]. The severity of myiasis is associated with infested body location and clinical manifestations (e.g., lesions and tissue inflammation); therefore, it requires early diagnosis and intervention.
The diagnosis of myiasis can be challenging, mostly due to numerous fly species associated with infestation, and multiple clinical presentations and symptom variations based on infected-body location. Sometimes, the symptoms are similar to those of other infectious diseases, such as cellulitis, impetigo, herpes zoster, or leishmaniasis [17,18]. Therefore, misdiagnosis is not uncommon. Accurate and prompt diagnosis is important, not only to relieve the patient’s symptoms but also to prevent the establishment of myiasis-causing flies in areas where it is not endemic. The diagnosis mainly relies on the clinico-entomological criteria. The latter includes the identification of fly larvae in the infested tissues or organs. In the case of cavernous lesions, the extraction of larvae in a single session is difficult, and this delay may aggravate the situation. As long as larvae are present, a foul-smelling bloody discharge is observed [19]. Clues that myiasis may be present include recent travel to tropical and sub-tropical endemic regions, non-healing cutaneous lesions, discharge from a central punctum, movement sensation under the skin, pruritus, or pain [20]. Dermoscopy, imaging, ultrasound scanning, and serology are techniques used to diagnose myiasis [21,22,23,24]. The biopsy of the wound may also be advised for accurate microscopic examination. Blood tests, such as a complete blood count, may show increased white blood cells. IgE may also be elevated as another manifestation of the host–parasite interaction. In recent decades, molecular biology has been used in diagnosing myiasis in various countries [25].
The treatment of myiasis essentially consists of the manual removal of larvae, although the removal of larvae from tissue can be difficult due to the larva’s tapered shape and the many rows of spines and hooks that it uses to grip tissue (anterior hooks that function as an anchor). On the other hand, larvae require contact with air to breathe. The application of suffocating occlusive substances, such as Vaseline, glycerol, paraffin, petrolatum, or bacon, restricts oxygen flow and forces the larvae to move on the surface for respiration, consequently facilitating the removal from myiasis lesions [26]. Medications may also be prescribed in some cases of myiasis. Ivermectin is a semisynthetic macrolide antibiotic that is effective and can be prescribed for the treatment of more severe cases [27]. Surgery is typically performed to remove a severe infection such as migratory myiasis (deep migration into tissue in which extraction may not be possible) [28]. Proper hygienic cleaning methods are complementary preventive measures that should be strictly followed for wound cleaning after the removal of the larva.
Cochliomyia hominivorax is a parasitic fly belonging to the Calliphoridae family and Cochliomyia genus. The latter includes four species of C. macellaria, C. hominivorax, C. aldrichi, and C. minima [29]. Cochliomyia hominivorax is known as screwworm and is the main agent of myiasis in the New World [30,31,32,33]. It was first described by a French naval surgeon, Dr. Charles Coquerel, in 1858 when it was responsible for the death of hundreds of prisoners at the Devil’s Island penal colony in French Guiana [34]. It is an obligate ectoparasite in homeothermic vertebrates, either domestic or wild, occasionally including humans. Its larvae produce myiasis and primarily feed on living warm-blooded animals (such as cattle and other livestock). The larvae can cause ocular [35], oral [36], umbilical [37], subcutaneous [38], and nasal [39] infections. In addition, C. hominivorax is responsible for the most common forms of human myiasis [40] and causes serious lesions in the abdomen, lower limbs, and various parts of the head (ears and eyelids) [41]. This feeding of the larvae on the host causes deep, pocket-like lesions in the skin, which can damage infected tissues. However, C. hominivorax infections in humans are less common and occur in patients with the following risk factors: open wounds, poor hygiene, low educational level, alcoholism, immobility, and physical or mental disability [42]. The female flies lay eggs on a superficial wound, injured mucous membrane, or the natural orifice of a warm-blooded animal or humans (such as nostrils, sinuses, mouth, orbits, and genital orifices). One female can deposit up to 400 eggs at a time, and up to 2800 eggs during a 10–30 day lifespan [43]. Eggs hatch between 12 and 24 h into larvae that burrow into the wound or flesh to feed. Unlike typical maggots that feed on dead flesh, C. hominivorax larvae feed on living tissue that allows the larvae to penetrate the tissues [44]. Larvae are pointed at one end and blunt at the other, with dark brown spines circling the body [45]. They possess small spines on each body segment that resemble a screw’s threads. The third instar larvae of C. hominivorax are creamy-white with a robust cylindrical body from 6 to 17 mm long and 1.6 to 3.5 mm wide [46]. They penetrate the wound and burrow deeper, perpendicular to the skin surface. The larvae then continue to feed on the wound fluids and the tissue. The wound enlarges as the larvae feed, and a foul-smelling, bloody discharge develops. There may be hundreds of larvae within the wound. The wound produces a characteristic odor, often not perceived by humans, which attracts other gravid female flies to lay their eggs in the same wounds and aggravates the infective process [47]. After 4–10 days of feeding, larvae fall out on the ground, burrow into the soil, and then transform into pupa. The pupal stage lasts from a week to three months approximately [47]. The rate of development of the immature stages is influenced by environmental and wound temperatures and becomes slower at lower temperatures with no true diapause. The adult screwworm flies emerge and mate after 3–5 days, beginning the cycle again. Adult flies are less frequent than larvae and can be separated from flies of other genera by the confirmation of a deep blue to blue-green metallic body colour with three dark longitudinal stripes on the thorax.
The risk of the introduction of C. hominivorax may be relatively low, but it can be introduced and spread into non-endemic and eradicated areas via the movement of infested hosts, including humans. According to World Organization for Animal Health (WOAH), C. hominivorax is listed as a notifiable infestation for animals that affects livestock, wildlife, and humans in endemic areas and leads to major socioeconomic consequences [48]. Therefore, C. hominovorax infestation can be health-threatening, since it can penetrate living tissues of the body and does not stay subdermal, contrary to most myiasis-causing fly species. Extensive, chronic, or advanced-stage wounds with frequent exposures are commonly infested by this fly species [42,49]. Massive tissue destruction, the loss of eyes and ears, and erosion of bones and nasal sinuses can occur following the C. hominovorax infestation. Severe cases may be accompanied by fever, chills, pain, bleeding from infested sites, and secondary infections. Blood tests may show elevated levels of neutrophils and eosinophils [50]. It can be fatal in untreated severe infestations [51].
Beyond the sanitary problems associated with this species, these infestations affect veterinary sectors, such as the cattle industry. In animals, myiasis caused by C. hominivorax results in a negative economic impact due to a decrease in animal weight and milk production, and an increase in animal death. Infected animals may present with enlarged, draining, and foul-smelling wounds. They may isolate themselves and show signs of discomfort. Infected animals with no intervention may die from secondary infection or toxicity within 7–14 days. A control cost (e.g., cost of larvicides) of USD 2 m per year is estimated for eliminating animal myiasis [52].
Human myiasis caused by C. hominivorax continues to be reported in endemic countries, especially in Central and South America and some Caribbean islands [53,54]. The northern and southern borders of its range are primarily limited due to cold weather [55]. It has been controlled using sterile insect techniques since the 1950s. Millions of mass-produced sterile flies are released per week during a campaign. Eradication has been achieved in the U.S.A. (by 1982), Mexico (by 1991), and most other Central American countries. A permanent barrier was established at the Panama–Colombian border. In this barrier, sterile flies are continuously released to prevent reinfestation in South America [56]. Economic savings in the eradicated areas, spanning the southern US to Panama and parts of the Caribbean, is estimated to be USD 1.3 billion per year [47,57]. Furthermore, eradication has also been achieved in Curacao, the Netherlands Antilles, the British Virgin Islands, the USA Virgin Islands, and Puerto Rico. Nevertheless, human myiasis has been reported in individuals who have traveled to these countries [58]. Based on computer simulation models, C. hominivorax can potentially colonize most of the tropical and semi-tropical regions of the world [59,60]. International livestock trade and movement, as well as human travel, have resulted in the introduction of C. hominivorax to non-endemic areas and previously eradicated areas [61]. Cochliomyia hominivorax was introduced and established in Libya in 1988, [62] presumably by livestock importation from South America [63]. In a similar way, it entered France through an infected dog returning from Brazil [64]; and in Australia by a woman who visited Brazil and Argentina [65]. Despite the announcement of Mexico as a screwworm-free country in 1991, 69 cases were reported in 1992 and 1993, about 200 miles away from the USA border [66]. Similarly, several screwworm reintroductions have been reported in the USA since their complete eradication in the 1980s [60,67]. On the other hand, the control management strategies of these parasitic flies in South America rely mainly on the application of insecticides, which are harmful to the environment and make them less efficient due to the emergence of resistant populations [68]. Furthermore, it affects non-target organisms.
French Guiana is a French overseas department located on the Northeastern coast of South America in which some myiasis cases, including furunculoid and cavitary myiases, have already been reported [23,69]. Nevertheless, in spite of the developed knowledge of myiasis worldwide, little is known about this parasitosis and its causative agents in this tropical country, French Guiana. Herein, we present the case of an immunocompetent patient with long-standing wound myiasis.
2. Case Presentation
A 34-year-old man was consulted twice two weeks apart at the hospital of Cayenne, the main city of French Guiana, for otorrhagia and otalgia in the left ear. He complained of constant noise and tickling coming from the same ear. He was originally from Paris, France, and had lived for several years on Reunion Island, in the Indian Ocean, before moving to French Guiana less than a year earlier. The patient was an agricultural engineer, who worked for environmental protection. He worked mainly in the peri-urban area of Cayenne but regularly hiked in exceptional biogeographical areas, such as the primary forest. His residence was healthy with full access to amenities, located in a low urbanized area and surrounded directly by secondary forests and swamps. The patient presented no stigma of negligence and was aware of necessary local care, hygiene, and dietary rules. Except for smoking, no notion of diabetes mellitus, chronic alcoholism, or immunosuppression, including AIDS, was recorded. He had a history of multiple surgeries in the left ear, including the installation of transtympanic aerators in 1991, diagnosis and removal of cholesteatoma in 2003, 2005, 2008, and 2017, ossiculoplasty in 2008, and tympanoplasty in 2017. He underwent a surgical operation, the so-called open technique, in which a cavity is dug in the temporal bone that opens into the external auditory canal at the level of its posterior wall, forming a sort of cul-de-sac at the bottom of the external auditory canal. The patient suffered from sequential hypoacusis due to cholesteatoma surgery. In addition, he was subjected to occasional otorrhagia in the left ear since its arrival in French Guiana. In mid-December 2020, a strong analgesic drug was prescribed following the patient’s arrival at the emergency department for a painful otorrhagia, leading to hospitalization the next morning.
Based on clinical examination, an ear canal inflammation, along with myiases protruding from the external auditory canal, a non-marginal post-inferior perforation of the left eardrum, and a thick complete graft were observed. MRI demonstrated scarring lesions of the myiasis filling the middle of the ear space (Figure 1A). A CT scan showed the left middle ear filled with tissues enclosing the ossicular prosthesis (Figure 1B). He then underwent treatment, including fulfilling the ear cavity with topical ivermectin (5 crushed tablets of 3 mg) and Vaseline covered by an occlusive dressing for 48 h. An aspiration performed right after this intervention led to the isolation of several tens of maggots from the mastoid cavity. Patient care was continued on an outpatient basis with borated hydrogen peroxide ear baths twice a day for 7 days. Two weeks later, the patient felt an insect entering the left ear’s external auditory canal, crawling and making noise for 10 min without being able to pull it out. A bloody otorrhagia, otalgia, and scratching appeared 2 days after this event, leading to referring the patient again to the emergency department resulting in receiving the same treatment as two weeks ago. Following the observation of a teeming mass of maggots at the top of the external auditory canal close to the eardrum, they were aspirated the next day. No otorrhagia was observed after two days. The outcome was favorable within 3 months, and no relapse was reported by the patient. It is worth mentioning that the patient consulted for a dengue infection diagnosed by NS1 antigen detection a few days after the appearance of myiasis, without an obvious correlation between both medical events.
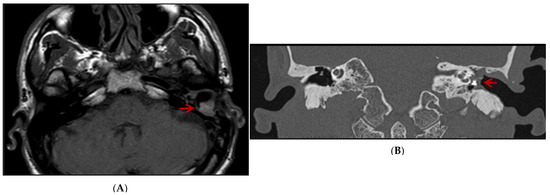
Figure 1.
MRI axial (A) and CT scan coronal (B) images: scar lesion filling the left middle ear of the patient (highlighted by red arrow) compared to the right ear.
In order to confirm the diagnosis and to identify the fly species, the larvae responsible for parasitosis were extracted by the patient himself, preserved in 70% alcohol, and sent to mainland France to the parasitology–mycology department of Avicenna Hospital (Bobigny, France) for an accurate identification. The morphological identification was performed based on the entomological criteria, following the dissection of the larvae and the identification of the cephalopharyngeal skeleton and posterior spiracles under the microscope as Cochliomyia hominivorax [1,70] (Figure 2, Supplementary information S1).
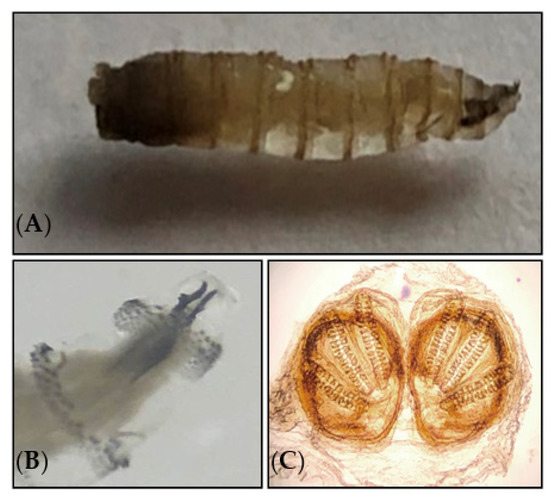
Figure 2.
(A) Third-stage larva of Cochliomyia hominivorax (Calliphoridae). (B) Cephalopharyngeal skeleton. (C) Posterior spiracle of larva examined under a light microscope at 500× magnification.
Morphological identification was further confirmed using a molecular approach. For this purpose, the larvae DNA was extracted using Chelex 10% (Bio-Rad, Hercules, CA, USA) and then subjected to conventional PCR and bidirectional sequencing, targeting 710 bp fragment of the mitochondrial cytochrome oxidase I gene (mtCOI) [71]. BLAST analysis identified the specimen as C. hominivorax according to >99% identity with GenBank sequences. The obtained sequence was deposited in GenBank with the assigned accession number NX532747. An inferred neighbor-joining (NJ) phylogenetic tree of COI sequences belonging to our C. hominivorax specimen (AVC1) and homologous GenBank sequences, constructed using MEGA version 5.0 software, demonstrated a high genetic diversity and significant heterogeneity compared to homonym sequences of other countries, such as from North and South America (Figure 3).
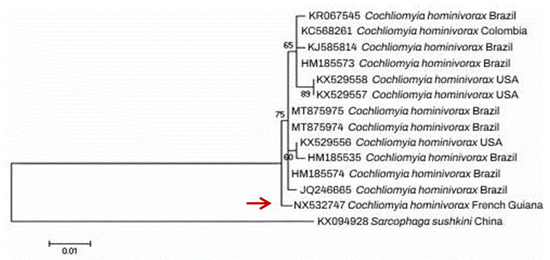
Figure 3.
Neighbor-joining (NJ) tree reconstructed from COI sequence of Cochliomyia hominivorax specimen isolated from our patient (highlighted by red arrow) together with homologous sequences collected from GenBank.
3. Discussion
French Guiana is a coastal department surrounded by Brazil, Suriname, and the Atlantic Ocean, in which more than 90% of its surface is covered by Amazonian rainforest. It possesses a tropical, hot, and humid climate throughout the year, with a relatively dry, slightly warmer season from July to November, and a rainy season from December to June. Due to the abundance of natural resources and rainfall, it is rich in insect species in terms of frequency and biodiversity.
The accurate diagnosis of myiasis infestation relies mainly on the medical background, geographic location, travel history, and clinical examination of an infected patient. However, some complications related to myiasis can occur. These include allergies (due to not completely removing the larvae), secondary pyogenic infection, cellulitis, pneumocephalus, extensive erosion of the tissue or mucous membranes, meningitis, and death (in unattended and untreated cases) [72]. In addition, the presence of larvae in the tissue triggers a local inflammatory response with the migration and proliferation of inflammatory cells, such as neutrophils, mast cells, eosinophils, fibroblasts, and endothelial cells [73]. Furthermore, a complete blood cell count may show high levels of leukocytes and eosinophils [74]. In the case of our patient, an ear canal inflammation along with a painful otorrhagia were the two major complications recorded.
On the other hand, the correct identification of the larvae species is essential for understanding the infestation mechanism and promoting preventive and treatment measures [54,75]. The morphological identification of the larvae relies mainly on the body shape, the mouthparts, posterior spiracles, and the arrangement of cuticular spines [76]. The posterior spiracles are usually visualized by dissection under a stereomicroscope, which allows identifying the fly species responsible for myiasis at the species level. In the present study, the larvae were identified according to the general appearance of the larva (morphological criteria, such as color and size) and examination of the posterior respiratory spiracles as C. hominivorax (Figure 2). In order to confirm morphological identification and to investigate the genetic diversity of fly larva species isolated in this study with counterpart sequences from other endemic countries, a conventional PCR targeting COI fragment was performed that allowed us to further confirm the identity of the larva species. Furthermore, high heterogeneity was observed between the larval specimen and GenBank sequences (Figure 3).
To the best of our knowledge, seven myiasis reports have been documented in French Guiana. It includes three reports of cutaneous myiasis [77,78,79], followed by two reports of furuncular myiasis [80,81], and one report of nosocomial myiasis [82]. Most of the infected patients were males, with inflammatory nodules as the most abundant symptom exhibited. Dermatobia hominis was the most frequently reported fly species responsible for myiasis in this country. A single report of myiasis caused by C. hominivorax was reported (nosocomial myiasis) in a bed-ridden 84 years old woman, who developed right nasal myiasis during his stay at the Cayenne Hospital [82]. In contrast to the previous report, our patient was an immunocompetent young patient with no history of recent travel to other endemic regions where C. hominivorax is prevalent. This report is, therefore, the second case of myiasis caused by C. hominivorax and the first case of wound myiasis in this country. Detailed information on the myiasis cases reported in French Guiana is provided in Table 1. The information gathered in Table 1 is of significant importance not only for the inhabitants but also for the tourists of the Amazonian Forest regarding increasing travel to tropical regions.

Table 1.
Detailed information on the myiasis cases reported in French Guiana.
Various treatments have been proposed for the treatment of myiasis, but most of them are not achievable in outpatient consultation. The management of myiasis usually consists of (i) mechanical removal of the larvae, (ii) surgical debridement of the infested wound tissue, and (iii) irrigation of the wound with antiseptic solutions [83,84]. In addition, topical medications such as ivermectin have been successfully used for the removal of larvae [85,86]. The application of topical ivermectin combined with Vaseline and manual extraction of the maggots was effective in eradicating the ectoparasites with no need for surgery. A favorable evolution and infection cure were observed two weeks post-treatment. The prevention of reinfestation and the spread of larvae are important underlying conditions in the treatment of the disease. This dreadful condition can be prevented by the maintenance of appropriate hygiene, general cleanliness, sanitation, proper wound cleaning, decreasing the number of flies around the wounds, and educating the population susceptible to the disease. In the case of the patient presented in this study, he had no underlying medical condition. However, a large cavity left by several ear surgeries provided a suitable environment for this myiasis colonization.
Wearing protective clothing, using insect repellant, covering wounds, and improving general sanitation and personal hygiene are preventative measures to avoid myiasis [56]. In non-endemic areas, the surveillance of all imported animals is necessary to prevent the entry of screwworms. Despite several cases of negligence or lack of hygienic measures associated with myiasis frequently reported in the literature, this patient had a local debilitating condition (a multi-scarring aspect of his left auditory canal) without any negligence and was aware of the local care of his infested ear. Nevertheless, he was infested during his daily life activities in the vicinity of Cayenne, the capital, and not in the deep forest or rural areas. This case highlights the risk of myiasis infestation outside the known and usual disease zones and the presence of this pathogenic ectoparasite near urban areas.
4. Conclusions
The entomological identification of the ectoparasite and the description of this case are important to raise the knowledge on the Amazonian ectoparasites, which are too often noted and rarely studied. With the increasing number of international travelers to the Amazonian tropical regions, it is important to acquire knowledge on this parasitosis, especially for healthcare workers unfamiliar with myiasis and its etiological agents. Therefore, general practitioners and surgeons in these regions should be better informed about the risk of ectoparasitic colonization in their patients without underlying comorbidities, but with the sequelae of cavities.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics13152575/s1. Supplementary information S1: Third-stage larva of Cochliomyia hominivorax.
Author Contributions
Conceptualization: L.E., A.I. and M.A.; methodology: A.M., W.H., G.Q., R.B., D.B., H.B.R. and L.E.; investigation: M.A., A.M., W.H., J.N., G.Q., R.B., D.B., L.E. and A.I.; data curation: A.M., W.H., G.Q., R.B., D.B., L.E. and M.A.; writing—original draft preparation: M.A. and L.E.; writing—review and editing: M.A., A.M., R.B., L.E. and A.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from our patient involved in this study. Furthermore, written informed consent was obtained from the patient to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zumpt, F. Myiasis in Man and Animals in the Old World; Butterworth: London, UK, 1965. [Google Scholar]
- Robbins, K.; Khachemoune, A. Cutaneous myiasis: A review of the common types of myiasis. Int. J. Dermatol. 2010, 49, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.J.; Wall, R.L.; Stevens, J.R. Traumatic Myiasis: A Neglected Disease in a Changing World. Annu. Rev. Entomol. 2016, 61, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Onyeama, C.O.; Njai, P.C. Cutaneous myiasis (Tumbu fly larvae): A case report. Niger. J. Paediatr. 2005, 32, 26–27. [Google Scholar] [CrossRef]
- McGarry, J.W. Tropical myiases: Neglected and well-travelled. Lancet Infect. Dis. 2014, 14, 672–674. [Google Scholar] [CrossRef]
- Kuria, S.K.; Oyedeji, A.O. Human myiasis cases originating and reported in Africa for the last two decades (1998–2018): A review. Acta Trop. 2020, 210, 105590. [Google Scholar] [CrossRef]
- Antunes, A.A.; Santos Tde, S.; Avelar, R.L.; Martins Neto, E.C.; Macedo Neres, B.; Laureano Filho, J.R. Oral and maxillofacial myiasis: A case series and literature review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, e81–e85. [Google Scholar] [CrossRef]
- Kumar, G.V.; Sowmya, G.; Shivananda, S. Chrysomya bezziana oral myiasis. J. Glob. Infect. Dis. 2011, 3, 393–395. [Google Scholar] [CrossRef]
- Dutto, M.; Pellegrino, M.; Vanin, S. Nosocomial myiasis in a patient with diabetes. J. Hosp. Infect. 2013, 83, 74–76. [Google Scholar] [CrossRef]
- Manickam, A.; Sengupta, S.; Saha, J.; Basu, S.K.; Ranjan Das, J.; Sannigrahi, R. Myiasis of the tracheostomy wound: A case report with review of literature. Otolaryngology 2015, 5, 198. [Google Scholar]
- Villalobos, G.; Vega-Memije, M.E.; Maravilla, P. Myiasis caused by Dermatobia hominis: Countries with increased risk for travelers going to neotropic areas. Int. J. Dermatol. 2016, 55, 1060–1068. [Google Scholar] [CrossRef]
- Goddard, J. Physician’s Guide to Arthropods of Medical Importance: Flies whose Maggots cause Myiasis Inhumans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1996; Volume 2, pp. 169–187. [Google Scholar]
- World Health Organization: History of the Development of the ICD. Available online: https://icd.who.int/browse10/2019/en#/B87 (accessed on 1 January 2021).
- Shearer, D.; Wall, R. Veterinary Entomology: Athropod Ectoparasites of Veterinary Importance; Chapman and Hall: London, UK; New York, NY, USA, 1997; p. 203. [Google Scholar]
- Bowering, C.K. Diabetic foot ulcers. Pathophysiology, assessment, and therapy. Can Fam. Physician 2001, 47, 1007–1016. [Google Scholar] [PubMed]
- Singh, A.; Singh, Z. Incidence of myiasis among humans-a review. Parasitol. Res. 2015, 114, 3183–3199. [Google Scholar] [CrossRef] [PubMed]
- Pallai, L.; Hodge, J.; Fishman, S.J.; Millikan, L.E.; Phelps, R.G. Case report: Myiasis—The botfly boil. Am. J. Med. Sci. 1992, 303, 245–248. [Google Scholar]
- Caissie, R.; Beaulieu, F.; Giroux, M.; Berthod, F.; Landry, P. Cutaneous myiasis: Diagnosis, treatment, and prevention. J. Oral Maxillofac. Surg. 2008, 66, 560–568. [Google Scholar] [CrossRef]
- Sherman, R.A. Wound myiasis in urban and suburban United States. Arch. Intern. Med. 2000, 160, 2004–2014. [Google Scholar] [CrossRef]
- Adisa, C.; Mbanaso, A. Furuncular myiasis of the breast caused by the larvae of the tumbu fly (Cordylobia anthropophaga). BMC Surg. 2004, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Szczurko, C.; Dompmartin, A.; Moreau, A.; Belloy, F.; Remond, B.; Leroy, D. Ultrasonography of furuncular cutaneous myiasis: Detection of Dermatobia hominis larvae and treatment. Int. J. Dermatol. 1994, 33, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Giangaspero, A.; Marangi, M.; Balotta, A.; Venturelli, C.; Szpila, K.; Di Palma, A. Wound myiasis caused by Sarcophaga (Liopygia) Argyrostoma (robineau-desvoidy) (Diptera: Sarcophagidae): Additional evidences of the morphological identification dilemma and molecular investigation. Sci. World J. 2017, 2017, 9064531. [Google Scholar] [CrossRef]
- Noutsis, C.; Millikan, L.E. Myiasis. Dermatol Clin. 1994, 12, 729–736. [Google Scholar] [CrossRef]
- Gontijo, J.R.V.; Bittencourt, F.V. Wound myiasis: The role of entodermoscopy. An. Bras. Dermatol. 2018, 93, 746–748. [Google Scholar] [CrossRef]
- Marangi, M.; Hall, M.J.; Aitken, A.; Ready, P.D.; Giangaspero, A. Origins of Wohlfahrtia magnifica in Italy based on the identification of mitochondrial cytochrome b gene haplotypes. Parasitol. Res. 2016, 115, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, E.; Gaskin, K.; Wojcik, S. Sonographic Detection of Cutaneous Myiasis. Clin. Pract. Cases Emerg. Med. 2019, 3, 438–439. [Google Scholar] [CrossRef] [PubMed]
- Puthran, N.; Hegde, V.; Anupama, B.; Andrew, S. Ivermectin treatment for massive orbital myiasis in an empty socket with concomitant scalp pediculosis. Indian J. Ophthalmol. 2012, 60, 225–227. [Google Scholar] [CrossRef]
- Sunny, B.; Sulthana, L.; James, A.; Sivakumar, T. Maggot Infestation: Various Treatment Modalities. J. Am. Coll. Clin. Wound Spec. 2018, 8, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, T. Keys to the genera and species of blow flies (Diptera: Calliphoridae) of America North of Mexico. Proc. Entomol. Soc. 2006, 108, 689–725. [Google Scholar]
- Neira, P.; Muñoz, N.; Cantero, D. Miasis auricular por Cochliomyia hominivorax (Diptera: Calliphoridae) (Coquerel, 1858) [Auricular myiasis cause by Cochliomyia hominivorax (Diptera: Calliphoridae) (Coquerel, 1858)]. Rev. Med. Chil. 2002, 130, 907–909. [Google Scholar] [CrossRef]
- Menghi, C.I.; Gatta, C.L.; Oliva, A. Otomiasis por Cochliomyia hominivorax en dos niños del conurbano bonaerense, Argentina [Otomyiasis by Cochliomyia hominivorax in two children from the outskirts of Buenos Aires, Argentina]. Rev. Argent. Microbiol. 2010, 42, 176–178. [Google Scholar]
- Lindsay, R.; Stancil, J.; Ray, J.M. Myiasis of facial wounds by Cochliomyia hominivorax sustained in a natural disaster in Haiti. Otolaryngol. Head Neck Surg. 2010, 143, 595–596. [Google Scholar] [CrossRef]
- Costa-Júnior, L.M.; Chaves, D.P.; Brito, D.R.B.; Santos, V.A.F.D.; Costa-Júnior, H.N.; Barros, A.T.M. A review on the occurrence of Cochliomyia hominivorax (Diptera: Calliphoridae) in Brazil. Rev. Bras. Parasitol. Vet. 2019, 28, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Reichard, R.E.; Vargas-Teran, M.; Abu Sowa, M. Myiasis: The battle continues against screwworm infestation. Public Health Pract. 1992, 13, 130–137. [Google Scholar]
- Khurana, S.; Biswal, M.; Bhatti, H.S.; Pandav, S.S.; Gupta, A.; Chatterjee, S.S.; Lyngdoh, W.V.; Malla, N. Ophthalmomyiasis: Three cases from North India. Indian J. Med. Microbiol. 2010, 28, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Lima Júnior, S.M.; Asprino, L.; Prado, A.P.; Moreira, R.W.; de Moraes, M. Oral myiasis caused by Cochliomyia hominivorax treated nonsurgically with nitrofurazone: Report of 2 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, e70–e73. [Google Scholar] [CrossRef] [PubMed]
- Duro, E.A.; Mariluis, J.C.; Mulieri, P.R. Umbilical myiasis in a human newborn. J. Perinatol. 2007, 27, 250–251. [Google Scholar] [CrossRef]
- Trombetta, L.; Oliva, A.; Galache, V.; Bava, J.; Troncoso, A. Cutaneous myiasis due to Cochliomyia hominivorax in a drug user. J. Infect. Dev. Ctries 2009, 3, 873–876. [Google Scholar] [CrossRef]
- Tai, R.; Marsh, M.A.; Rao, R.; Kurniali, P.C.; DiNino, E.; Meharg, J.V. Nasal Myiasis Caused by Cochliomyia Hominivorax in the United States: A Case Report. Am. J Infect Dis. 2011, 7, 107–109. [Google Scholar]
- Acha, P.N.; Szyfres, B. Zoonosis y Enfermedades Transmisibles Comunes al Hombre y a los Animale; Volumen III. Parasitosis; Organización Panamericana de la Salud: Washington, DC, USA, 2003; 230p. [Google Scholar]
- Visciarelli, E.; Costamagna, S.; Lucchi, L.; Basabe, N. Miasis Humana en Bahía Blanca, Argentina: Periodo 2000/2005. Neotropical. Entomol. 2007, 36, 605–611. [Google Scholar] [CrossRef]
- Batista-da-Silva, J.A.; Moya-Borja, G.E.; Queiroz, M.M. Factors of susceptibility of human myiasis caused by the New World screw-worm, Cochliomyia hominivorax in São Gonçalo, Rio de Janeiro, Brazil. J. Insect. Sci. 2011, 11, 14. [Google Scholar] [CrossRef]
- Wittenberg, R.; Cock, M.J.W. (Eds.) Invasive Alien Species: A Toolkit of Best Prevention and Management Practices; Cabi Publishing: Wallingford, UK, 2001. [Google Scholar]
- Concha, C.; Palavesam, A.; Guerrero, F.D.; Sagel, A.; Li, F.; Osborne, J.A.; Hernandez, Y.; Pardo, T.; Quintero, G.; Vasquez, M.; et al. A transgenic male-only strain of the New World screwworm for an improved control program using the sterile insect technique. BMC Biol. 2016, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Stadler, F. A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics; Open Book Publishers: Cambridge, UK, 2022; pp. 121–142. [Google Scholar]
- Laake, E.W.; Cushing, E.C.; Parish, H.E. Biology of the Primary Screwworm Fly, Cochliomyia americana, and a Comparison of its Stages with those of C. macellaria. United States Dep. Agricul. Tech. Bull. 1936, 500, 24. [Google Scholar]
- Vargas-Terán, M.; Spradbery, J.P.; Hofmann, H.C.; Tweddle, N.E. Impact of screwworm eradication programmes using the sterile insect. Technique. In Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Altuna, M.; Hickner, P.V.; Castro, G.; Mirazo, S.; Pérez de León, A.A.; Arp, A.P. New World screwworm (Cochliomyia hominivorax) myiasis in feral swine of Uruguay: One Health and transboundary disease implications. Parasit. Vectors 2021, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Villamil-Gómez, W.E.; Cardona-Ospina, J.A.; Prado-Ojeda, J.S.; Hernández-Prado, H.; Figueroa, M.; Causil-Morales, P.N.; Pérez-Reyes, K.; Palechor-Ocampo, L.A.; Rodríguez-Morales, A.J. Pin-Site Myiasis Caused by Screwworm Fly in Nonhealed Wound, Colombia. Emerg Infect Dis. 2019, 25, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.S. Tropical skin diseases in British military personnel. J. R. Army. Med. Corps. 2013, 159, 224–228. [Google Scholar] [CrossRef]
- Fatal scalp myiasis: Autopsy finding of Cochliomyia hominivorax (diptera: Calliphoridae) in the brain cavity. Can. Soc. Forensic Sci. J. 2007, 40, 183–186.
- Coronado, A.; Kowalski, A. Current status of the New World screwworm Cochliomyia hominivorax in Venezuela. Med. Vet. Entomol. 2009, 23, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Duque, F.L.; Ardila, C.M. Oral myiasis caused by the screwworm Cochliomyia hominivorax treated with subcutaneous ivermectin and creolin: Report of six cases after trauma. Dent. Traumatol. 2011, 27, 404–407. [Google Scholar] [CrossRef]
- Francesconi, F.; Lupi, O. Myiasis. Clin. Microbiol. Rev. 2012, 25, 79–105. [Google Scholar] [CrossRef]
- Carvalho, R.A.; Azeredo-Espin, A.M.L.; Torres, T.T. Deep Sequencing of New World Screw-Worm Transcripts to Discover Genes Involved in Insecticide Resistance. BMC Genom. 2010, 11, 695. [Google Scholar] [CrossRef]
- Mastrangelo, T.; Fresia, P.; Lyra, M.L.; Rodrigues, R.A.; Azeredo-Espin, A.M.L. Genetic diversity and population structure of the New World screwworm fly from the Amazon region of Brazil. Acta Trop. 2014, 138, S26–S33. [Google Scholar] [CrossRef] [PubMed]
- Arp, A.P.; Quintero, G.; Sagel, A.; Batista, R.G.; Phillips, P.L.; Hickner, P.V. The microbiome of wild and mass-reared new world screwworm, Cochliomyia hominivorax. Sci Rep. 2022, 12, 1042. [Google Scholar] [CrossRef] [PubMed]
- Mehr, Z.; Powers, N.R.; Konkol, K.A. Myiasis in a wounded soldier returning from Panama. J. Med. Entomol. 1991, 28, 553–554. [Google Scholar] [CrossRef]
- Sutherst, R.W.; Spradbery, J.P.; Maywald, G.F. The potential geographical distribution of the Old World screw-worm fly, Chrysomya bezziana. Med. Vet. Entomol. 1989, 3, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Mayer, D.G.; Atzeni, M.G.; Butler, D.G. Adaptation of CLIMEX for spatial screwworm fly population dynamics. Math. Comput. Simul. 1992, 33, 439–444. [Google Scholar] [CrossRef]
- Spradbery, J.P. Screw-worm fly: A tale of two species. Agricul. Zoolog. Rev. 1994, 6, 1–62. [Google Scholar]
- E1-Azazy, O.M.E. Wound myiasis caused by Cochliomyia hominivorax in Libya. Vet. Rec. 1989, 124, 103. [Google Scholar] [CrossRef]
- Beesley, W.N. The New World screw-worm fly in north Africa. Ann. Trop. Med. Parasitol. 1991, 85, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Chermette, R. A case of canine otitis due to screwworm, Cochliomyia hominivorax, in France. Vet. Record. 1989, 124, 641. [Google Scholar] [CrossRef] [PubMed]
- Searson, J.; Sanders, L.; Davis, G.; Tweddle, N.; Thornber, P. Screwworm Fly Myiasis in an Overseas Traveller—A Case Report. Commun. Dis. Intell. 1992, 16, 239–240. [Google Scholar]
- Galvin, T.J.; Wyss, J.H. Screwworm eradication program in Central America. Ann. N. Y. Acad. Sci. 1996, 791, 233–240. [Google Scholar] [CrossRef]
- Garris, G. On the front of defense: Veterinarian averts screwworm outbreak. J. Am. Vet. Med. Assoc. 1998, 212, 159. [Google Scholar]
- Tandonnet, S.; Cardoso, G.A.; Mariano-Martins, P.; Monfardini, R.D.; Cunha, V.A.S.; de Carvalho, R.A.; Torres, T.T. Molecular basis of resistance to organophosphate insecticides in the New World screw-worm fly. Parasit. Vectors. 2020, 13, 562. [Google Scholar] [CrossRef]
- Pradinaud, R.; Rivierez, E. Myiase furonculeuse de la paupière supérieure en Guyane Française [Furuncular myiasis of the upper eyelid in French Guiana]. Bull. Soc. Fr. Dermatol. Syphiligr. 1968, 75, 808–810. [Google Scholar] [PubMed]
- Spradbery, J.P. A Manual for the Diagnosis of the Screwworm Fly, Fisheries and Forestry; Department of Agriculture: Canberra, Australia, 2002; p. 2. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Szakacs, T.A.; MacPherson, P.; Sinclair, B.J.; Gill, B.D.; McCarthy, A.E. Nosocomial myiasis in a Canadian intensive care unit. CMAJ. 2007, 177, 719–720. [Google Scholar] [CrossRef][Green Version]
- Carvalho, R.W.; Santos, T.S.; Antunes, A.A.; Laureano Filho, J.R.; Anjos, E.D.; Catunda, R.B. Oral and maxillofacial myiasis associated with epidermoid carcinoma: A case report. J. Oral Sci. 2008, 50, 103–105. [Google Scholar] [CrossRef]
- Shenouda, M.; Enten, G.; Nguyen, T.; Mangar, D.; Camporesi, E. Human Botfly: A Case Report and Overview of Differential Diagnosis. J. Investig. Med. High Impact Case Rep. 2018, 6, 2324709618801692. [Google Scholar] [CrossRef]
- Ruiz-Zapata, J.D.; Figueroa-Gutiérrez, L.M.; Mesa-Franco, J.A.; Moreno-Gutierrez, P.A. Umbilical Myiasis by Cochliomyia hominivorax in an Infant in Colombia. Front. Med. (Lausanne) 2020, 6, 292. [Google Scholar] [CrossRef]
- Mathison, B.A.; Pritt, B.S. Laboratory identification of arthropod ectoparasites. Clin. Microbiol. Rev. 2014, 27, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Brumpt, L.C.; Poulet, J. Observation à paris d’un cas de myiase sous-cutanée à Dermatobia contractée en Guyane. Bull. Soc. Pathol. Exot. Fil. 1965, 58, 88–92. [Google Scholar]
- Schreiber, H.A.; Renkl, A.C.; Lapinski, W.; Scharffetter-Kochanek, K.; Weiss, J.M. Myiasis after study trip to French Guiana. J. Dtsch. Dermatol. Ges. 2010, 8, 357–359. [Google Scholar] [CrossRef]
- Graveriau, C.; Peyron, F. Cutaneous myiasis. Travel Med. Infect. Dis. 2017, 16, 70–71. [Google Scholar] [CrossRef]
- Blaizot, R.; Vanhecke, C.; Le Gall, P.; Duvignaud, A.; Receveur, M.C.; Malvy, D. Furuncular myiasis for the Western dermatologist: Treatment in outpatient consultation. Int. J. Dermatol. 2018, 57, 227–230. [Google Scholar] [CrossRef]
- Couppié, P.; Roussel, M.; Rabarison, P.; Sockeel, M.J.; Sainte-Marie, D.; Marty, C.; Carme, B. Nosocomial nasal myiasis owing to Cochliomyia hominivorax: A case in French Guiana. Int. J. Dermatol. 2005, 44, 302–303. [Google Scholar] [CrossRef]
- Denion, E.; Dalens, P.H.; Couppié, P.; Aznar, C.; Sainte-Marie, D.; Carme, B.; Petitbon, J.; Pradinaud, R.; Gérard, M. External ophthalmomyiasis caused by Dermatobia hominis. A retrospective study of nine cases and a review of the literature. Acta Ophthalmol. Scand. 2004, 82, 576–584. [Google Scholar]
- Sherman, R.A. Maggot therapy for foot and leg wounds. Int. J. Low Extrem. Wounds 2002, 1, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Pierre-filho, P.D.T.P.; Minguini, N.; Pierre, L.M.; Pierre, A.M. Use of ivermectin in the treatment of orbital myiasis caused by Cochliomyia hominivorax. Scand. J. Infect. Dis. 2004, 36, 503–505. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.; Moncada, L.; Molano, A.; Valderrama, S.; Gualtero, S.; Franco-Paredes, C. Role of ivermectin in the treatment of severe orbital myiasis due to Cochliomyia hominivorax. Clin. Infect. Dis. 2006, 43, e57–e59. [Google Scholar] [CrossRef]
- Tay, S.Y.; Ramasamy, B.R.; Watson, D.A.; Montoya, M. Treatment of nasal myiasis with ivermectin irrigation. BMJ Case Rep. 2018, 2018, bcr2017224142. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).