Digital Tomosynthesis: Review of Current Literature and Its Impact on Diagnostic Bronchoscopy
Abstract
:1. Introduction
2. Electromagnetic Navigational Bronchoscopy (ENB)
3. Radial EBUS
4. Robotic Bronchoscopy
5. Clinical Data Using Robotic Bronchoscopy
6. Variables Impacting Diagnostic Yield
- Pre-procedural incentive spirometry can help recruit lung volume and prevent atelectasis [20].
- The use of 100% oxygen during pre-oxygenation can induce absorption atelectasis, so the lowest tolerable FiO2 should be used [21].
- Lengthy intubation times may increase the risk of atelectasis. General anesthesia using total intravenous anesthesia (TIVA) with propofol and muscle paralysis is optimal [22].
- Application of PEEP throughout induction can also prevent atelectasis [22].
- Higher PEEP with the lowest tolerable FiO2 as guided by oxygen saturation should be maintained. PEEP of up to 10–12 cm H2O may be beneficial for upper lobe biopsies, and higher PEEP may be needed for lower lobe biopsies [22].
- Recruitment maneuvers immediately after intubation can reverse any intubation atelectasis. This is especially important if intubation was difficult or prolonged [21].
7. Cone Beam CT
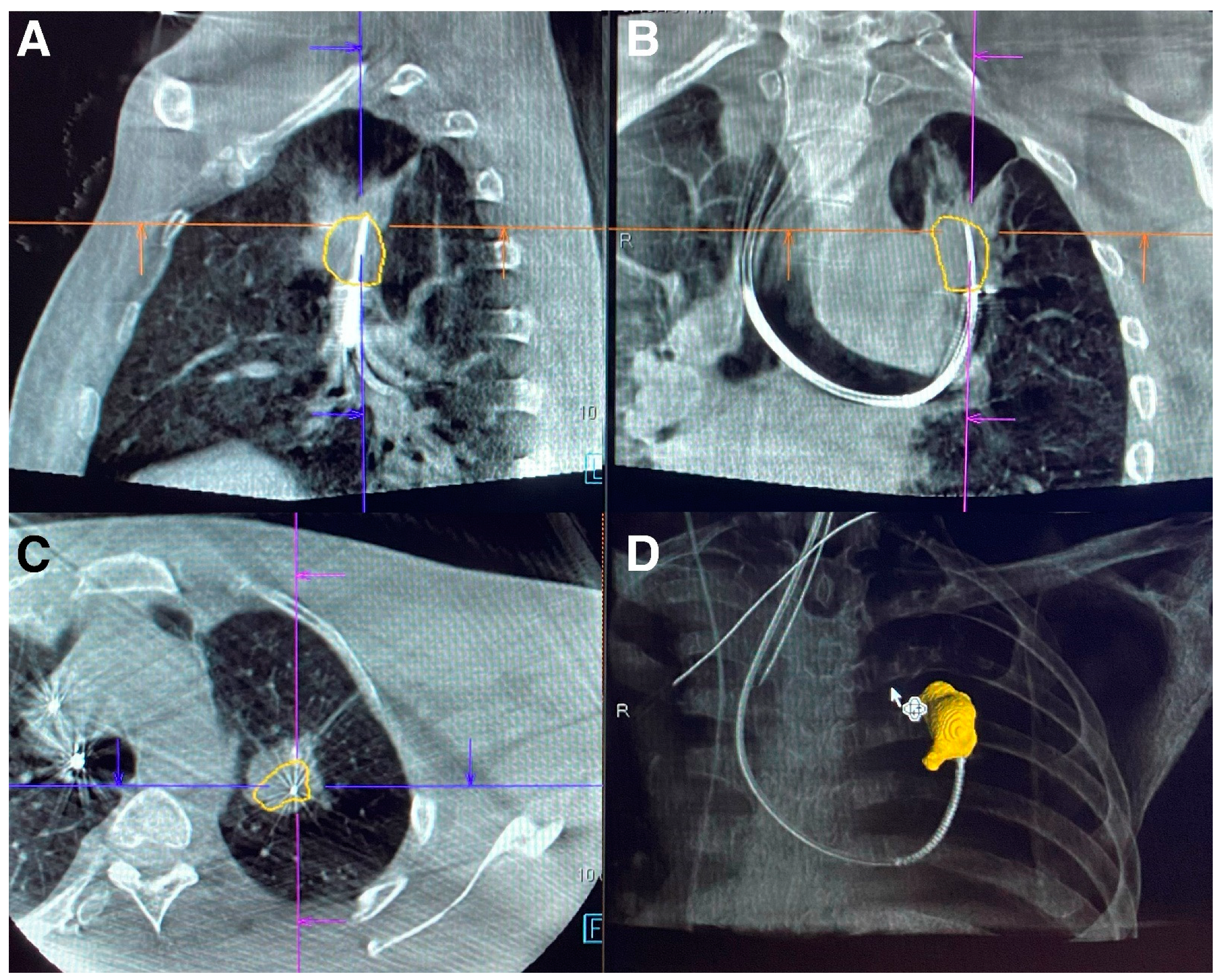
8. Digital Tomosynthesis-Based Imaging Devices
9. IllumisiteTM
10. LungVisionTM
11. Robotic Bronchoscopy with Digital Tomosynthesis and Electromagnetic Navigation
12. Mobile Cone Beam
13. Clinical Data for IllumisiteTM
14. Clinical Data for LungVisionTM
15. Clinical Data for CIOS Spin
16. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tuddenham, W.J. Glossary of terms for thoracic radiology: Recommendations of the Nomenclature Committee of the Fleischner Society. Am. J. Roentgenol. 1984, 143, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Gould, M.K.; Tang, T.; Liu, I.-L.A.; Lee, J.; Zheng, C.; Danforth, K.N.; Kosco, A.E.; Di Fiore, J.L.; Suh, D.E. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am. J. Respir. Crit. Care Med. 2015, 192, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 13 March 2023).
- Flores, R.; Patel, P.; Alpert, N.; Pyenson, B.; Taioli, E. Association of Stage Shift and Population Mortality Among Patients with Non–Small Cell Lung Cancer. JAMA Netw. Open 2021, 4, e2137508. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Ghobrial, M.; Mazzone, P.J. Models to Estimate the Probability of Malignancy in Patients with Pulmonary Nodules. Ann. Am. Thorac. Soc. 2018, 15, 1117–1126. [Google Scholar] [CrossRef]
- Mazzone, P.J.; Lam, L. Evaluating the Patient with a Pulmonary Nodule: A Review. JAMA 2022, 327, 264–273. [Google Scholar] [CrossRef]
- DiBardino, D.M.; Yarmus, L.B.; Semaan, R.W. Transthoracic needle biopsy of the lung. J. Thorac. Dis. 2015, 7, S304–S316. [Google Scholar] [PubMed]
- Baaklini, W.A.; Reinoso, M.A.; Gorin, A.B.; Sharafkaneh, A.; Manian, P. Diagnostic Yield of Fiberoptic Bronchoscopy in Evaluating Solitary Pulmonary Nodules. Chest 2000, 117, 1049–1054. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, Y.; Greif, J.; Becker, H.D.; Ernst, A.; Mehta, A. Real-time electromagnetic navigation bronchoscopy to peripheral lung lesions using overlaid CT images: The first human study. Chest 2006, 129, 988–994. [Google Scholar] [CrossRef]
- Folch, E.E.; Pritchett, M.A.; Nead, M.A.; Bowling, M.R.; Murgu, S.D.; Krimsky, W.S.; Murillo, B.A.; LeMense, G.P.; Minnich, D.J.; Bansal, S.; et al. Electromagnetic Navigation Bronchoscopy for Peripheral Pulmonary Lesions: One-Year Results of the Prospective, Multicenter NAVIGATE Study. J. Thorac. Oncol. 2019, 14, 445–458. [Google Scholar] [CrossRef] [Green Version]
- Kurimoto, N.; Murayama, M.; Yoshioka, S.; Nishisaka, T.; Inai, K.; Dohi, K. Assessment of Usefulness of Endobronchial Ultrasonography in Determination of Depth of Tracheobronchial Tumor Invasion. Chest 1999, 115, 1500–1506. [Google Scholar] [CrossRef]
- Ali, M.S.; Trick, W.; Mba, B.I.; Mohananey, D.; Sethi, J.; Musani, A.I. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: A systematic review and meta-analysis. Respirology 2017, 22, 443–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.; Chenna, P.; Loiselle, A.; Massoni, J.; Mayse, M.; Misselhorn, D. Radial Probe Endobronchial Ultrasound for Peripheral Pulmonary Lesions. A 5-Year Institutional Experience. Ann. Am. Thorac. Soc. 2014, 11, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Diddams, M.J.; Lee, H.J. Robotic Bronchoscopy: Review of Three Systems. Life 2023, 13, 354. [Google Scholar] [CrossRef]
- Chaddha, U.; Kovacs, S.P.; Manley, C.; Hogarth, D.K.; Cumbo-Nacheli, G.; Bhavani, S.V.; Kumar, R.; Shende, M.; Egan, J.P., 3rd; Murgu, S. Robot-assisted bronchoscopy for pulmonary lesion diagnosis: Results from the initial multicenter experience. BMC Pulm. Med. 2019, 19, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalchiem-Dekel, O.; Connolly, J.G.; Lin, I.-H.; Husta, B.C.; Adusumilli, P.S.; Beattie, J.A.; Buonocore, D.J.; Dycoco, J.; Fuentes, P.; Jones, D.R.; et al. Shape-Sensing Robotic-Assisted Bronchoscopy in the Diagnosis of Pulmonary Parenchymal Lesions. Chest 2022, 161, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Lee-Mateus, A.Y.; Reisenauer, J.; Garcia-Saucedo, J.C.; Abia-Trujillo, D.; Buckarma, E.H.; Edell, E.S.; Grage, R.A.; Bowman, A.W.; Labarca, G.; Johnson, M.M.; et al. Robotic-assisted bronchoscopy versus CT-guided transthoracic biopsy for diagnosis of pulmonary nodules. Respirology 2023, 28, 66–73. [Google Scholar] [CrossRef]
- Pritchett, M.A.; Bhadra, K.; Calcutt, M.; Folch, E. Virtual or reality: Divergence between preprocedural computed tomography scans and lung anatomy during guided bronchoscopy. J. Thorac. Dis. 2020, 12, 1595–1611, Erratum in J. Thorac. Dis. 2020, 12, 4593–4595. [Google Scholar] [CrossRef]
- Pritchett, M.A.; Lau, K.; Skibo, S.; Phillips, K.A.; Bhadra, K. Anesthesia considerations to reduce motion and atelectasis during advanced guided bronchoscopy. BMC Pulm. Med. 2021, 21, 240. [Google Scholar] [CrossRef]
- Eltorai, A.E.M.; Baird, G.L.; Eltorai, A.S.; Pangborn, J.; Antoci, V.; Cullen, H.A.; Paquette, K.; Connors, K.; Barbaria, J.; Smeals, K.J.; et al. Perspectives on Incentive Spirometry Utility and Patient Protocols. Respir. Care 2018, 63, 519–531. [Google Scholar] [CrossRef] [Green Version]
- Nimmagadda, U.; Salem, M.R.; Crystal, G.J. Preoxygenation: Physiologic basis, benefits, and potential risks. Anesth. Analg. 2017, 124, 507–511. [Google Scholar] [CrossRef]
- Galway, U.; Zura, A.; Khanna, S.; Wang, M.; Turan, A.; Ruetzler, K. Anesthetic considerations for bronchoscopic procedures: A narrative review based on the Cleveland Clinic experience. J. Thorac. Dis. 2019, 11, 3156–3170. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, L.; Weidlich, G.A. Cone Beam CT vs. Fan Beam CT: A Comparison of Image Quality and Dose Delivered Between Two Differing CT Imaging Modalities. Cureus 2016, 8, e778. [Google Scholar] [CrossRef] [Green Version]
- Park, S.C.; Kim, C.J.; Han, C.H.; Lee, S.M. Factors associated with the diagnostic yield of computed tomography-guided transbronchial lung biopsy. Thorac. Cancer 2017, 8, 153–158. [Google Scholar] [CrossRef]
- Piro, R.; Fontana, M.; Casalini, E.; Taddei, S.; Bertolini, M.; Iori, M.; Facciolongo, N. Cone beam CT augmented fluoroscopy allows safe and efficient diagnosis of a difficult lung nodule. BMC Pulm. Med. 2021, 21, 327. [Google Scholar] [CrossRef]
- Schultz, F.W.; Zoetelief, J. Dose conversion coefficients for interventional procedures. Radiat. Prot. Dosim. 2005, 117, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Li, G. Patient radiation dose and protection from cone-beam computed tomography. Imaging Sci. Dent. 2013, 43, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Vrieze, T.J.; Bruesewitz, M.R.; Kofler, J.M.; DeLone, D.R.; Pallanch, J.F.; Lindell, E.P.; McCollough, C.H. Dose and Image Quality Evaluation of a Dedicated Cone-Beam CT System for High-Contrast Neurologic Applications. Am. J. Roentgenol. 2010, 194, W193–W201. [Google Scholar] [CrossRef]
- Hohenforst-Schmidt, W.; Banckwitz, R.; Zarogoulidis, P.; Vogl, T.; Darwiche, K.; Goldberg, E.; Huang, H.; Simoff, M.; Li, Q.; Browning, R.; et al. Radiation Exposure of Patients by Cone Beam CT during Endobronchial Navigation—A Phantom Study. J. Cancer 2014, 5, 192–202. [Google Scholar] [CrossRef] [Green Version]
- Ali, E.A.; Takizawa, H.; Kawakita, N.; Sawada, T.; Tsuboi, M.; Toba, H.; Takashima, M.; Matsumoto, D.; Yoshida, M.; Kawakami, Y.; et al. Transbronchial Biopsy Using an Ultrathin Bronchoscope Guided by Cone-Beam Computed Tomography and Virtual Bronchoscopic Navigation in the Diagnosis of Pulmonary Nodules. Respiration 2019, 98, 321–328. [Google Scholar] [CrossRef]
- Casal, R.F.; Sarkiss, M.; Jones, A.K.; Stewart, J.; Tam, A.; Grosu, H.B.; Ost, D.E.; Jimenez, C.A.; Eapen, G.A. Cone beam computed tomography-guided thin/ultrathin bronchoscopy for diagnosis of peripheral lung nodules: A prospective pilot study. J. Thorac. Dis. 2018, 10, 6950–6959. [Google Scholar] [CrossRef]
- Kheir, F.; Thakore, S.R.; Becerra, J.P.U.; Tahboub, M.; Kamat, R.; Abdelghani, R.; Fernandez-Bussy, S.; Kaphle, U.R.; Majid, A. Cone-Beam Computed Tomography-Guided Electromagnetic Navigation for Peripheral Lung Nodules. Respiration 2021, 100, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, M.A.; Schampaert, S.; de Groot, J.A.; Schirmer, C.C.; van der Bom, I. Cone-Beam CT With Augmented Fluoroscopy Combined with Electromagnetic Navigation Bronchoscopy for Biopsy of Pulmonary Nodules. J. Bronc.-Interv. Pulmonol. 2018, 25, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Benn, B.S.; Romero, A.O.; Lum, M.; Krishna, G. Robotic-Assisted Navigation Bronchoscopy as a Paradigm Shift in Peripheral Lung Access. Lung 2021, 199, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Styrvoky, K.; Schwalk, A.; Pham, D.; Chiu, H.T.; Rudkovskaia, A.; Madsen, K.; Carrio, S.; Kurian, E.M.; Casas, L.D.L.; Abu-Hijleh, M. Shape-Sensing Robotic-Assisted Bronchoscopy with Concurrent use of Radial Endobronchial Ultrasound and Cone Beam Computed Tomography in the Evaluation of Pulmonary Lesions. Lung 2022, 200, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Machida, H.; Yuhara, T.; Tamura, M.; Ishikawa, T.; Tate, E.; Ueno, E.; Nye, K.; Sabol, J.M. Whole-Body Clinical Applications of Digital Tomosynthesis. Radiographics 2016, 36, 735–750. [Google Scholar] [CrossRef] [Green Version]
- Ziedses des Plantes, G. Eine neue methode zur differenzierung in der roentgenographie planigraphie. Acta Radiol. 1932, 13, 182–192. [Google Scholar] [CrossRef]
- Roy, D.N.G.; Kruger, R.A.; Yih, B.; Del Rio, P. Selective plane removal in limited angle tomographic imaging. Med. Phys. 1985, 12, 65–70. [Google Scholar]
- Warp, R.J.; Godfrey, D.J.; Dobbins, I.I.I.J.T. Applications of Matrix Inversion Tomosynthesis. Proc. SPIE 2000, 3977, 376–383. [Google Scholar]
- Dobbins, J.T., 3rd; Godfrey, D.J. Digital X-ray tomosynthesis: Current state of the art and clinical potential. Phys. Med. Biol. 2003, 48, R65–R106. [Google Scholar] [CrossRef]
- Sabol, J.M. A Monte Carlo estimation of effective dose in chest tomosynthesis. Med. Phys. 2009, 36, 5480–5487. [Google Scholar] [CrossRef]
- Maravilla, K.R.; Murry, R.C., Jr.; Horner, S. Digital tomosynthesis: Technique for electronic reconstructive tomography. Am. J. Roentgenol. 1983, 141, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Alabousi, M.; Zha, N.; Salameh, J.-P.; Samoilov, L.; Sharifabadi, A.D.; Pozdnyakov, A.; Sadeghirad, B.; Freitas, V.; McInnes, M.D.F.; Alabousi, A. Digital breast tomosynthesis for breast cancer detection: A diagnostic test accuracy systematic review and meta-analysis. Eur. Radiol. 2020, 30, 2058–2071. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, K.; Rickman, O.B.; Mahajan, A.K.; Hogarth, D.K. “Tool-in-lesion” Accuracy of Galaxy System—A Robotic Electromagnetic Navigation BroncHoscopy with Integrated Tool-in-lesion-Tomosynthesis Technology: The MATCH Study. J. Bronchol. Interv. Pulmonol 2023. ahead of print. [Google Scholar] [CrossRef]
- Sheth, N.M.; Zbijewski, W.; Jacobson, M.W.; Abiola, G.; Kleinszig, G.; Vogt, S.; Soellradl, S.; Bialkowski, J.; Anderson, W.S.; Weiss, C.R.; et al. Mobile C-Arm with a CMOS detector: Technical assessment of fluoroscopy and Cone-Beam CT imaging performance. Med. Phys. 2018, 45, 5420–5436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aboudara, M.; Roller, L.; Rickman, O.; Lentz, R.J.; Pannu, J.; Chen, H.; Maldonado, F. Improved diagnostic yield for lung nodules with digital tomosynthesis-corrected navigational bronchoscopy: Initial experience with a novel adjunct. Respirology 2020, 25, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.K.; Blaj, M.; Stahl, J.; Speicher, J.; Anciano, C.; Hudson, S.; Kragel, E.A.; Bowling, M.R. Evaluation of Electromagnetic Navigational Bronchoscopy Using Tomosynthesis-Assisted Visualization, Intraprocedural Positional Correction and Continuous Guidance for Evaluation of Peripheral Pulmonary Nodules. J. Bronc.-Interv. Pulmonol. 2023, 30, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Bowling, M.R.; Kohan, M.W.; Walker, P.; Efird, J.; Ben Or, S. The Effect of General Anesthesia Versus Intravenous Sedation on Diagnostic Yield and Success in Electromagnetic Navigation Bronchoscopy. J. Bronc.-Interv. Pulmonol. 2015, 22, 5–13. [Google Scholar] [CrossRef]
- Low, S.W.; Lentz, R.J.; Chen, H.; Katsis, J.; Aboudara, M.C.; Whatley, S.; Paez, R.; Rickman, O.B.; Maldonado, F. Shape-Sensing Robotic-Assisted Bronchoscopy vs Digital Tomosynthesis-Corrected Electromagnetic Navigation Bronchoscopy: A Comparative Cohort Study of Diagnostic Performance. Chest 2023, 163, 977–984. [Google Scholar] [CrossRef]
- Pertzov, B.; Gershman, E.; Izhakian, S.; Heching, M.; Amor, S.M.; Rosengarten, D.; Kramer, M.R. The LungVision navigational platform for peripheral lung nodule biopsy and the added value of cryobiopsy. Thorac. Cancer 2021, 12, 2007–2012. [Google Scholar] [CrossRef]
- Cicenia, J.; Bhadra, K.; Sethi, S.; Nader, D.A.; Whitten, P.; Hogarth, D.K. Augmented Fluoroscopy: A New and Novel Navigation Platform for Peripheral Bronchoscopy. J. Bronc.-Interv. Pulmonol. 2021, 28, 116–123. [Google Scholar] [CrossRef]
- Hedstrom, G.; Wagh, A.A. Combining Real-time 3-D imaging and augmented fluoroscopy with robotic bronchoscopy for the diagnosis of peripheral lung lesions. Chest 2022, 162, A2082. [Google Scholar] [CrossRef]
- Kalchiem-Dekel, O.; Fuentes, P.; Bott, M.J.; Beattie, J.A.; Lee, R.P.; Chawla, M.; Husta, B.C. Multiplanar 3D fluoroscopy redefines tool-lesion relationship during robotic-assisted bronchoscopy. Respirology 2021, 26, 120–123. [Google Scholar] [CrossRef]
- Reisenauer, J.; Duke, J.D.; Kern, R.; Fernandez-Bussy, S.; Edell, E. Combining Shape-Sensing Robotic Bronchoscopy with Mobile Three-Dimensional Imaging to Verify Tool-in-Lesion and Overcome Divergence: A Pilot Study. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 177–185. [Google Scholar] [CrossRef]
- Sadoughi, A.; Virdi, S. Mobile 3D Intraprocedural Fluoroscopy in Combination with Ultrathin Bronchoscopy for Biopsy of Peripheral Lung Nodules. J. Bronc-Interv. Pulmonol. 2021, 28, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, M.; Bashour, S.I.; Khan, A.; Chintalapani, G.; Kleinszig, G.; Casal, R.F. Mobile Cone-Beam CT-Assisted Bronchoscopy for Peripheral Lung Lesions. Diagnostics 2023, 13, 827. [Google Scholar] [CrossRef] [PubMed]


| Attributes | MonarchTM RB Platform | IonTM RB System |
|---|---|---|
| Bronchoscope | Articulating bronchoscope within an articulating sheath | Single ultrathin bronchoscope with integrated shape-sensing technology |
| Diameter | 6.0 mm (sheath), 4.4 mm (scope) | 3.5 mm |
| Working channel | 2.1 mm | 2 mm |
| Vision probe | Integrated camera | Removable vision probe |
| Articulation range | Up to 130 degrees (sheath), up to 180 degrees (scope) | Up to 180 degrees |
| Control mechanism | Video-game-style controller with two thumb-sticks | Ball mouse and scroll wheel |
| Navigation system | Electromagnetic navigation with sensors on chest | Shape-sensing technology |
| Monitor display | Vision, navigation, REBUS, and CT overlay | Navigation, fluoroscopy, virtual overlay, and either vision or REBUS |
| Cost | Above mid six-figure USD | Above mid six-figure USD |
| Relative Cost † | Provides 3D Reconstruction Images * | Incorporates into Conventional C-Arm Based Fluoroscope * | Corrects Navigation Pathway Based on Realtime Nodule Positioning * | Provides Multi-Plane Images * | Device |
|---|---|---|---|---|---|
| Low six-figure, USD | No | Yes | Yes—Illumisite Platform only | No | IllumisiteTM |
| Mid to high five-figure, USD | Yes | Yes | Yes—LungVision Platform Only | Yes | LungVisionTM |
| Mid six-figure, USD | Yes | No—Standalone C-Arm with CMOS sensor to provide CBCT images | Yes—Ion Robotic Bronchoscopy Platform Only | Yes | CIOS Spin |
| Platform | * Diagnostic Yield % | Study Type | Complications |
|---|---|---|---|
| IllumisiteTM | 79% | Retrospective comparative study between ENB with digital tomosynthesis (n = 67) vs. standard ENB (n = 100) [46] | Pneumothorax 1.5% |
| IllumisiteTM | 87% | Retrospective, single-center review study [47] | Pneumothorax 2.5% |
| IllumisiteTM | 80% | Retrospective comparative study; ENB combined with digital tomosynthesis (n = 133) vs. shape sensing robotic bronchoscopy (n = 170) [49] | Pneumothorax 1.8% |
| LungVisionTM | 77.8% (73–82%) | Retrospective, single-center study (n = 63) [50] | Pneumothorax 1.6% |
| LungVisionTM | 75% | Prospective, multi-center study (n = 55) [51] | None reported |
| LungVisionTM | 84% | Retrospective, single-center study (n = 45) [52] | Pneumothorax 8% |
| CIOS Spin | Not reported (Tool in Lesion 90%) | Feasibility case series (n = 10) [53] | None reported |
| CIOS Spin | 93% | Prospective, single-center study (n = 30) [54] | None reported |
| CIOS Spin | 100% | Case series (n = 4) [55] | None reported |
| CIOS Spin | 78% | Retrospective, single-center study (n = 51) [56] | Pneumothorax 3.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, A.; Sarkar, A.; Husnain, S.M.N.; Adkinson, B.C.; Sadoughi, A.; Sarkar, A. Digital Tomosynthesis: Review of Current Literature and Its Impact on Diagnostic Bronchoscopy. Diagnostics 2023, 13, 2580. https://doi.org/10.3390/diagnostics13152580
Jain A, Sarkar A, Husnain SMN, Adkinson BC, Sadoughi A, Sarkar A. Digital Tomosynthesis: Review of Current Literature and Its Impact on Diagnostic Bronchoscopy. Diagnostics. 2023; 13(15):2580. https://doi.org/10.3390/diagnostics13152580
Chicago/Turabian StyleJain, Anant, Adrish Sarkar, Shaikh Muhammad Noor Husnain, Brian Cody Adkinson, Ali Sadoughi, and Abhishek Sarkar. 2023. "Digital Tomosynthesis: Review of Current Literature and Its Impact on Diagnostic Bronchoscopy" Diagnostics 13, no. 15: 2580. https://doi.org/10.3390/diagnostics13152580





