Establishing a Novel Diagnostic Framework Using Handheld Point-of-Care Focused-Echocardiography (HoPE) for Acute Left-Sided Cardiac Valve Emergencies: A Bayesian Approach for Emergency Physicians in Resource-Limited Settings
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Synthesis of Results
2.3. Clinical Cases
2.4. Synthesis of a Diagnostic Framework
3. Results
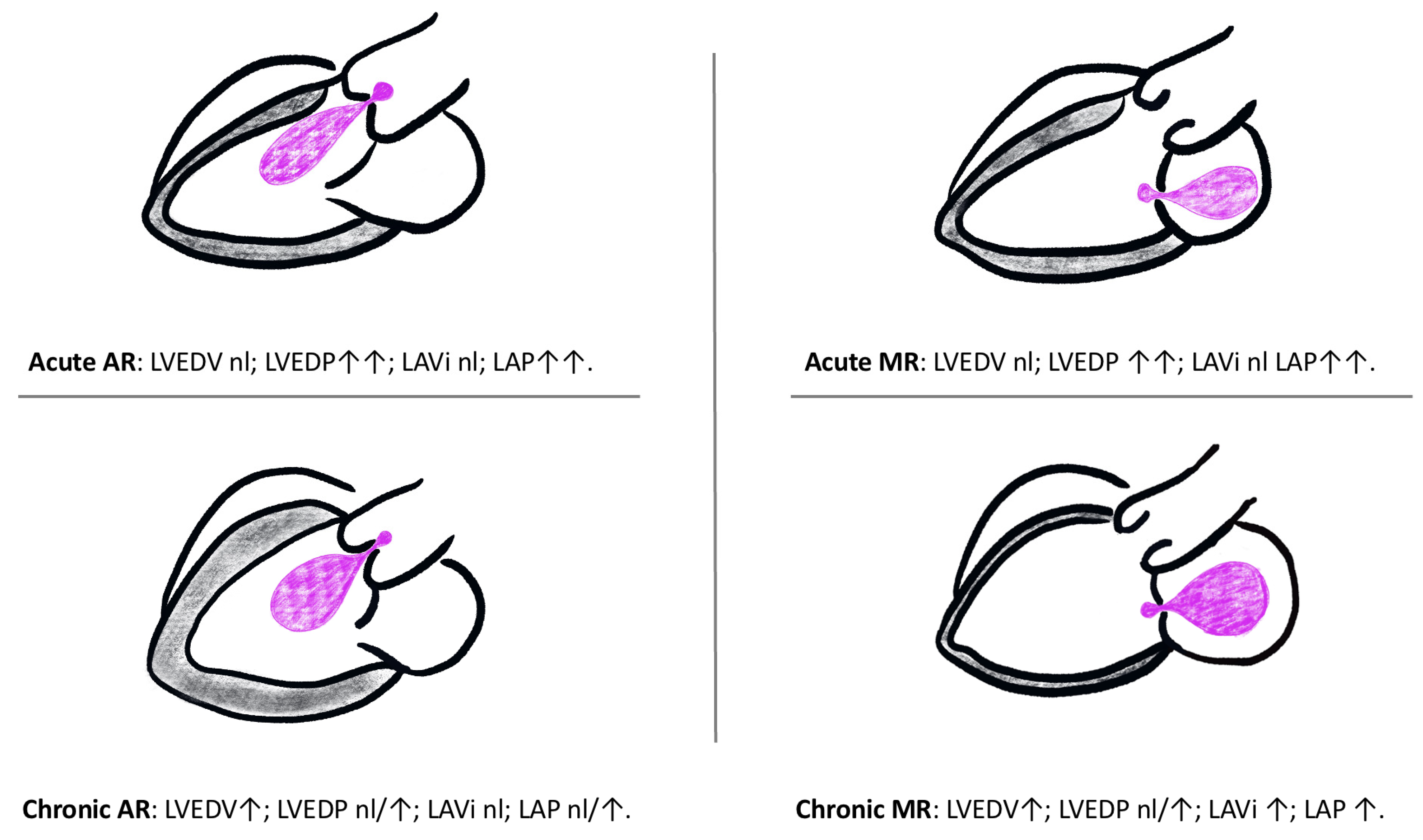
3.1. Left-Sided Cardiac Haemodynamics
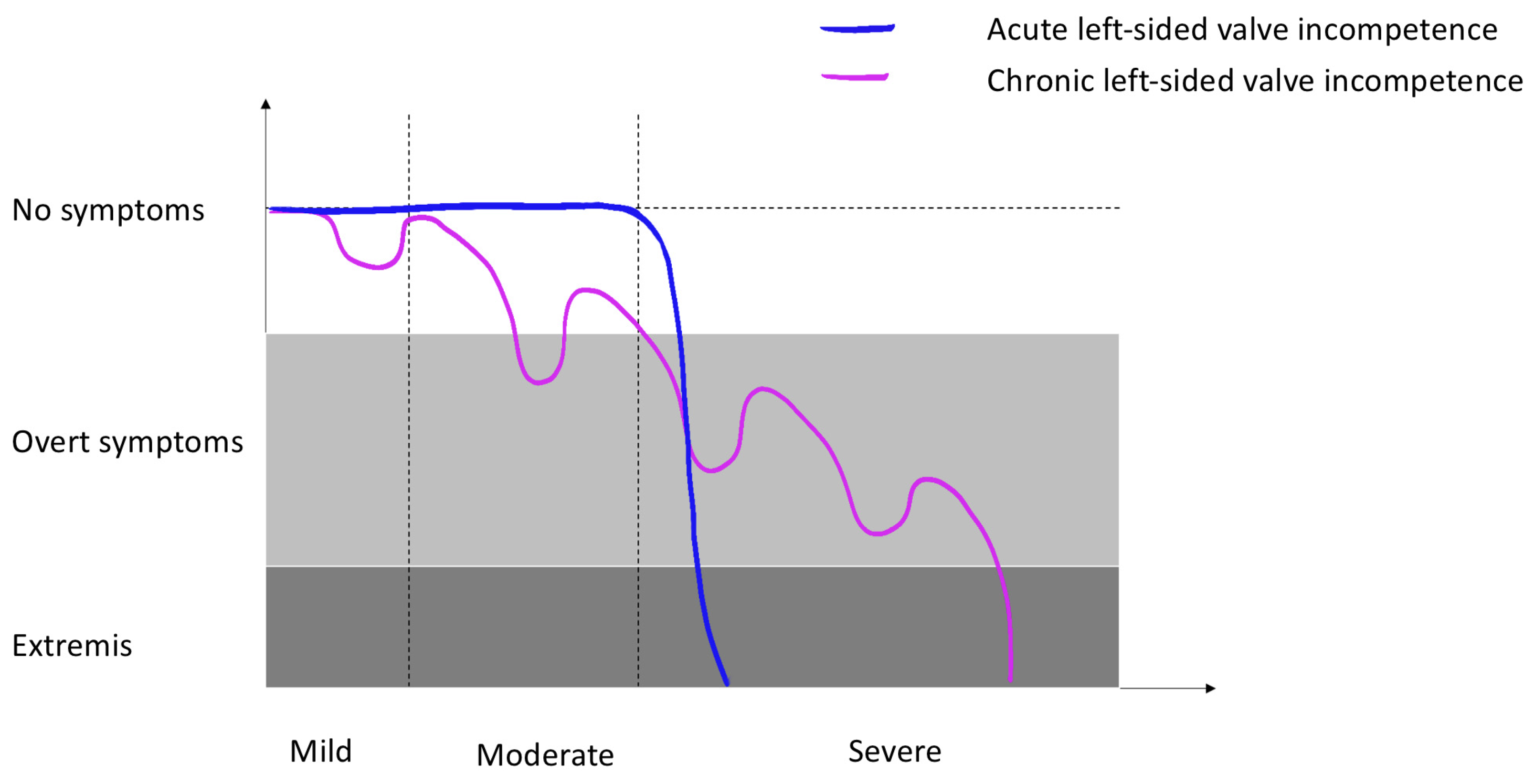
3.2. Chronic Left-Sided Valve Incompetence
3.3. Acute Severe Left-Sided Valve Incompetence
3.4. Echocardiography
3.5. Handheld Point-of-Care Focused-Echocardiography (HoPE)
- A.
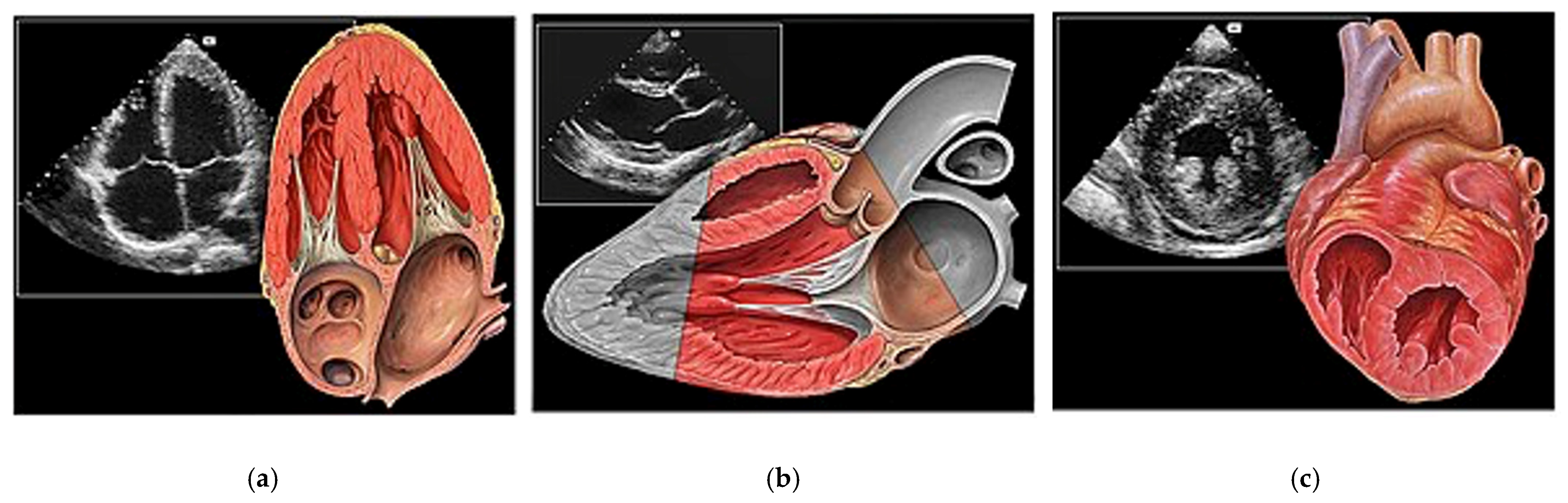
- Basic Cardiac Views (Figure 3)The standard echocardiographic views required to assess cardiac valvular structure and function include the parasternal long-axis (PLAX), parasternal short-axis (PSAX), apical four-chamber (A4C) and subcostal (SC) views. These views facilitate the visualisation of the aortic, mitral, tricuspid, and pulmonic valves, thus enabling the identification of valvular abnormalities [38].
- B.
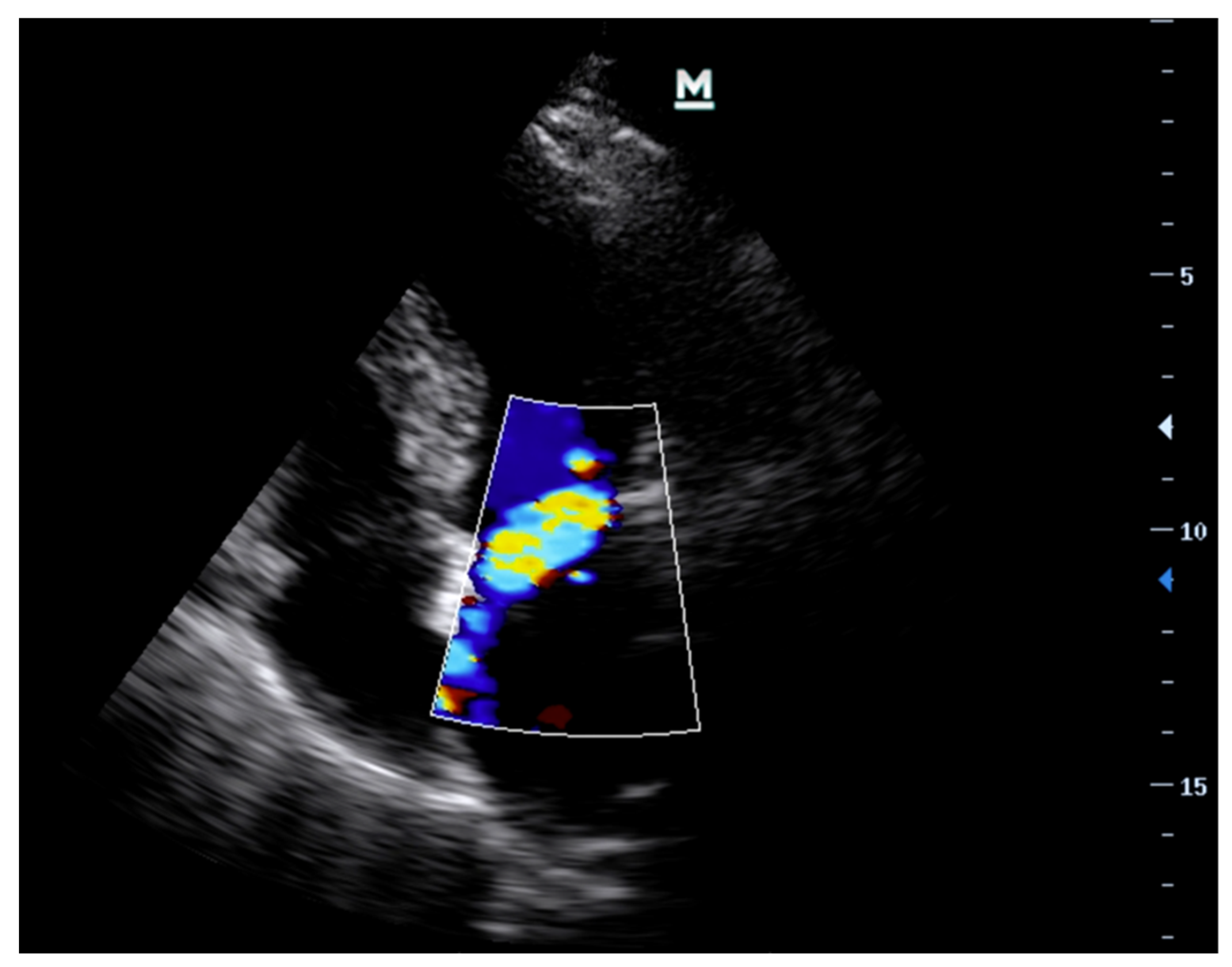
- Imaging ModesVarious ultrasound modalities are used to assess the structure and function of heart valves. Two-dimensional (2D) imaging is generally used for structural assessment, colour flow doppler (CFD) is used to evaluate blood flow across the valves, and continuous-wave (CWD) and pulsed-wave doppler (PWD) techniques are used to measure flow velocities and gradients across valves. Integrating these imaging modalities can offer a comprehensive assessment of valvular function and aid in identifying specific pathologies (Table 1) [27,38].
- C.
- Clinical Integration
| Echocardiographic Technique | Acute Mitral Regurgitation | Acute Aortic Regurgitation |
|---|---|---|
| 2D B-Mode | ||
| Valve Morphology | Flail, prolapsing or perforated leaflet. Ruptured chordae tendineae. Ruptured head of papillary muscle. Vegetations. | Root dilatation and dissection flaps. Vegetations. Torn valve cusps (usually due to trauma). |
| Left Atrium | Variable. Could be of normal size if pure acute severe MR. Usually enlarged with any degree of chronic MR. | Variable. Normal-size if pure acute severe AR. Usually enlarged if any degree of chronicity |
| Left Ventricle | Normal-size if acute severe MR. Dilated if acute-on-chronic severe MR. | Normal-size if acute severe AR. Dilated if acute-on-chronic severe AR. |
| M-Mode | Premature diastolic closure of the mitral valve. | |
| Colour Flow Doppler (CFD) | Colour flow is not a good marker for assessment of severity of MR or AR. However, it may assist with determining aetiology—usually eccentric jets if prolapse/flail in the direction that is away from the prolapsing/flail leaflet i.e., anterior mitral valve leaflet prolapse leads to a posteriorly directed jet. | Central jet, typically directed towards the posterior wall of the left ventricle. |
| Continuous Wave Doppler (CWD) | Increase in forward flow velocity with “V-wave” cut off. Equal colour density between forward flow and regurgitant doppler traces. Systolic flow reversal in 3 out of 4 pulmonary veins. | Increase in forward flow velocity. Pressure half time <200 ms. Doppler trace of AR ends before the end of diastolic due to early equilisation of aortic and LV pressures. Equal colour density between forward flow and regurgitant doppler traces. |
| Pulse Wave Doppler (PWD) | Systolic reversal of flow in pulmonary veins. | Diastolic flow reversal in the descending thoracic aorta. |
3.6. POCUS Protocols
3.7. HoPE for Left-Sided Cardiac Valve Evaluation
- Left ventricular failure (8%),
- Dysrhythmia (2%),
- Cardiac tamponade (1%).
- They reported no instances of acute valvular dysfunction.
3.8. Medical Diagnostics
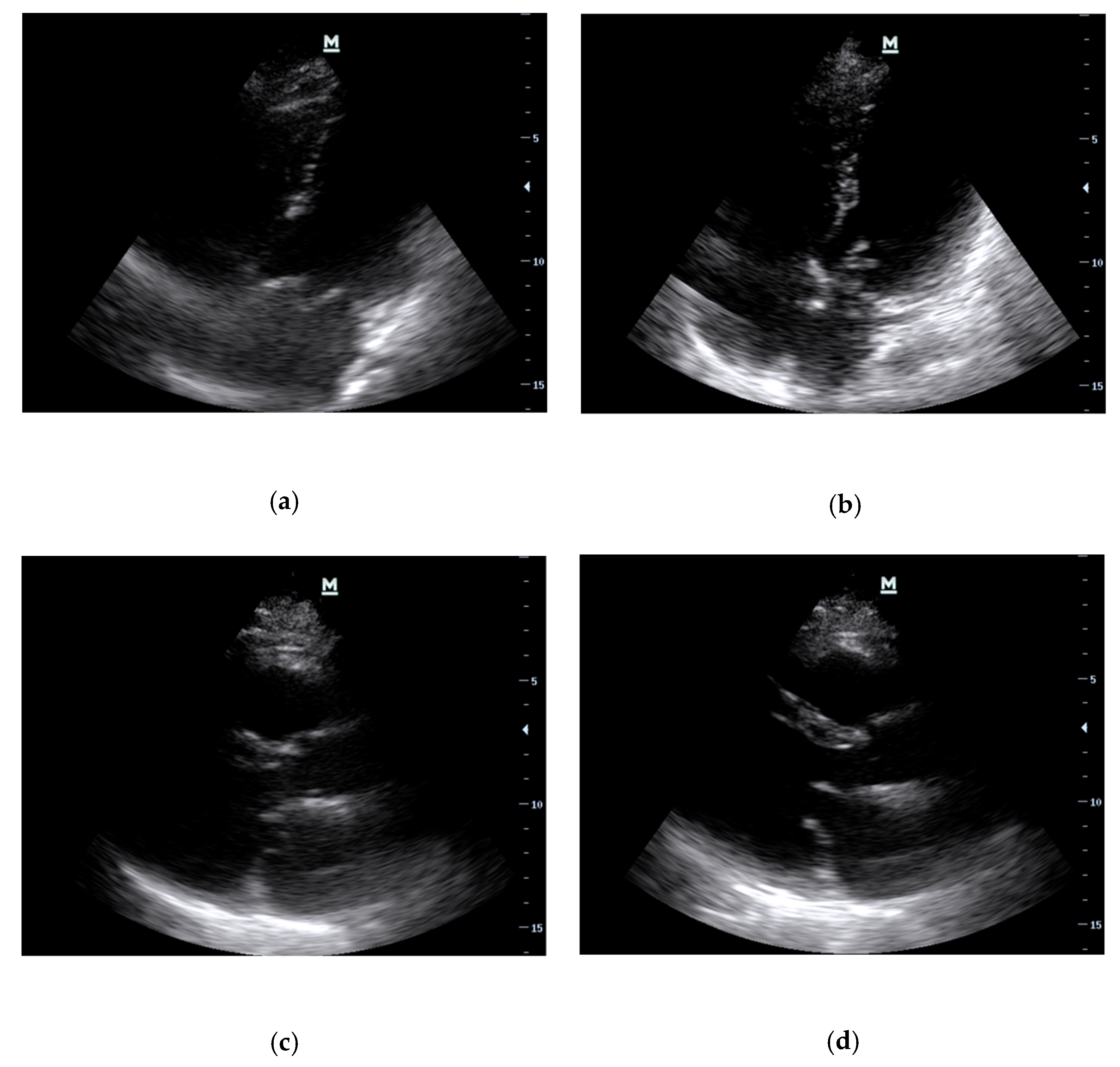
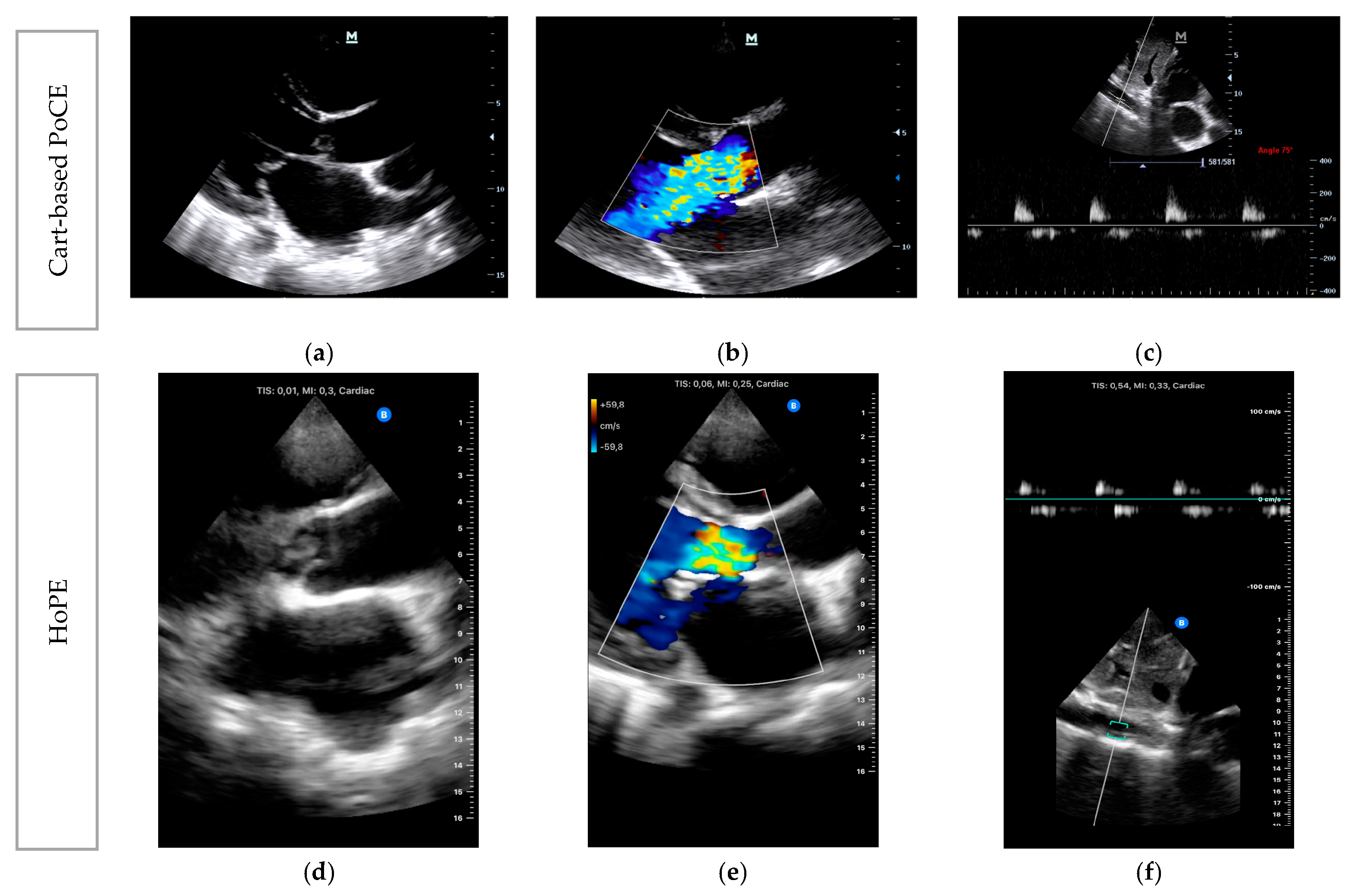
- Case 1: Acute Mitral Regurgitation due to Flail Leaflet
- Case 2: Acute Severe Aortic Regurgitation due to Infective Endocarditis
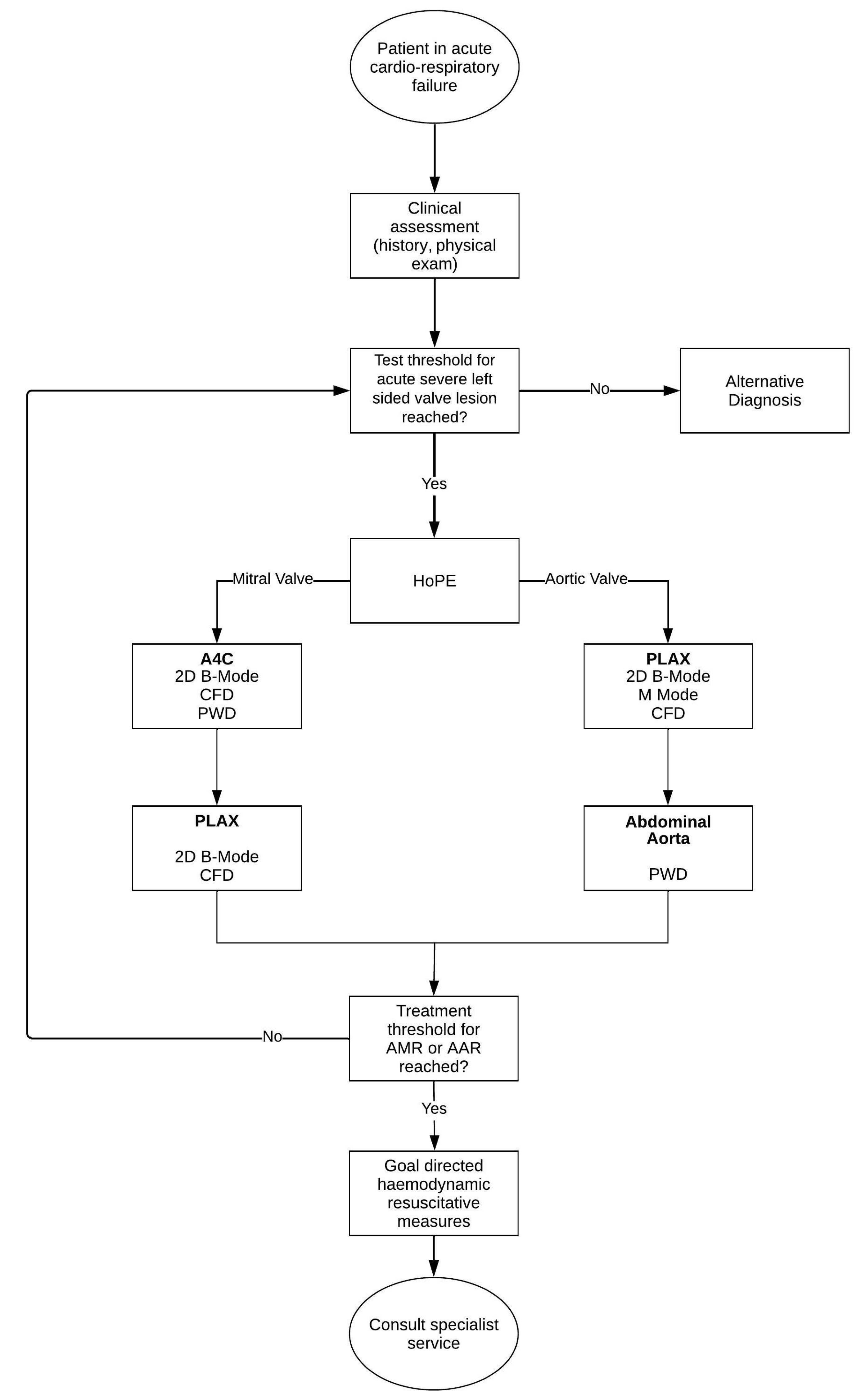
3.9. Synthesis of a Novel, Iterative, Diagnostic Framework for Acute Severe Left-Side Valve Emergencies
- Is there severe valve dysfunction?
- Does the identified valve dysfunction explain the current clinical condition of the patient?
- How does this finding contribute to the patient’s management plan?
- If the treatment threshold for either AMR or AAR is reached, the physician (or treating team) simultaneously contacts a specialty service while instating context specific haemodynamic resuscitative measure—the discussion of which are beyond the scope of this review.
- If the treatment threshold is not reached, the iterative process prompts the physician to re-evaluate the probability of left-side valve disease; if it continues to breach the test threshold, the physician continues to test. Alternatively, the physician has to consider other diagnoses.
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stout, K.K.; Verrier, E.D. Acute Valvular Regurgitation. Circulation 2009, 119, 3232–3241. [Google Scholar] [CrossRef] [PubMed]
- Akodad, M.; Schurtz, G.; Adda, J.; Leclercq, F.; Roubille, F. Management of valvulopathies with acute severe heart failure and cardiogenic shock. Arch. Cardiovasc. Dis. 2019, 112, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Sigal, A.; Costa, S. Managing Acute Cardiac Valvular Emergencies in the Emergency Department. Emerg. Med. Pract. 2022, 24, 1–24. [Google Scholar]
- McClung, J.A. Native and Prosthetic Valve Emergencies. Cardiol. Rev. 2016, 24, 14–18. [Google Scholar] [CrossRef]
- Bacci, M.; Patel, K.; Cabrera, G.; Kalivoda, E.J. Bedside Echocardiography Diagnosis of Tricuspid Valve Infective Endocarditis in the Emergency Department. Cureus 2022, 14, e29541. [Google Scholar] [CrossRef] [PubMed]
- Bruce, C.J.; Connolly, H.M. Right-Sided Valve Disease Deserves a Little More Respect. Circulation 2009, 119, 2726–2734. [Google Scholar] [CrossRef]
- Watanabe, N. Acute mitral regurgitation. Heart 2019, 105, 671–677. [Google Scholar] [CrossRef]
- Voit, J.; Otto, C.M.; Burke, C.R. Acute native aortic regurgitation: Clinical presentation, diagnosis and management. Heart 2022, 108, 1651–1660. [Google Scholar] [CrossRef]
- Alam, L.; Lasam, G.; Fishberg, R.; Powell, D. Acute Severe Mitral Regurgitation Secondary to Ischemic Papillary Muscle Rupture: A Case Report. Cureus 2021, 13, e13996. [Google Scholar] [CrossRef]
- Aluru, J.S.; Barsouk, A.; Saginala, K.; Rawla, P.; Barsouk, A. Valvular Heart Disease Epidemiology. Med. Sci. 2022, 10, 32. [Google Scholar] [CrossRef]
- Nkomo, V.T. Epidemiology of valvular heart diseases in Africa. SA Heart 2017, 6, 12–18. [Google Scholar] [CrossRef][Green Version]
- Coffey, S.; Roberts-Thomson, R.; Brown, A.; Carapetis, J.; Chen, M.; Enriquez-Sarano, M.; Zühlke, L.; Prendergast, B.D. Global epidemiology of valvular heart disease. Nat. Rev. Cardiol. 2021, 18, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Elder, A.; Japp, A.; Verghese, A. How valuable is physical examination of the cardiovascular system? BMJ 2016, 354, i3309. [Google Scholar] [CrossRef] [PubMed]
- Draper, J.; Chambers, J. Detecting heart valve disease: Can we do better? Br. J. Gen. Pract. 2016, 66, 156–157. [Google Scholar] [CrossRef][Green Version]
- Bernard, S.; Deferm, S.; Bertrand, P.B. Acute valvular emergencies. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Bian, Y.; Wang, G.; Liu, W.; Gao, L.; Pan, Y.; Cao, S.; Yuan, Q.; Wei, S.; Xu, F.; et al. Trends in point-of-care ultrasound protocols in the emergency department and intensive care unit: A review. Emerg. Crit. Care Med. 2022, 3, 64–69. [Google Scholar] [CrossRef]
- Atkinson, P.R.; Milne, J.; Diegelmann, L.; Lamprecht, H.; Stander, M.; Lussier, D.; Pham, C.; Henneberry, R.; Fraser, J.M.; Howlett, M.K.; et al. Does Point-of-Care Ultrasonography Improve Clinical Outcomes in Emergency Department Patients with Undifferentiated Hypotension? An International Randomized Controlled Trial from the SHoC-ED Investigators. Ann. Emerg. Med. 2018, 72, 478–489. [Google Scholar] [CrossRef]
- Becker, D.M.; Tafoya, C.A.; Becker, S.L.; Kruger, G.H.; Tafoya, M.J.; Becker, T.K. The use of portable ultrasound devices in low- and middle-income countries: A systematic review of the literature. Trop. Med. Int. Health 2016, 21, 294–311. [Google Scholar] [CrossRef]
- Beaton, A.; Aliku, T.; Okello, E.; Lubega, S.; McCarter, R.; Lwabi, P.; Sable, C. The Utility of Handheld Echocardiography for Early Diagnosis of Rheumatic Heart Disease. J. Am. Soc. Echocardiogr. 2014, 27, 42–49. [Google Scholar] [CrossRef]
- Barjaktarevic, I.; Kenny, J.S.; Berlin, D.; Cannesson, M. The Evolution of Ultrasound in Critical Care: From Procedural Guidance to Hemodynamic Monitor. J. Ultrasound Med. 2020, 40, 401–405. [Google Scholar] [CrossRef]
- Kimambo, D.; Kennedy, S.; Kifai, E.; Kailembo, N.; Eichberg, C.; Markosky, S.; Shah, I.; Powers, E.; Zwerner, P.; Dorman, S.E.; et al. Feasibility of point-of-care cardiac ultrasound performed by clinicians at health centers in Tanzania. BMC Cardiovasc. Disord. 2021, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; Edvardsen, T.; Delgado, V.; Dulgheru, R.; Pepi, M.; Cosyns, B.; Dweck, M.R.; Garbi, M.; et al. Recommendations for the imaging assessment of prosthetic heart valves: A report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2016, 17, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.S.; Barber, M.S.; Kienle, G.S.; Aronson, J.K.; von Schoen-Angerer, T.; Tugwell, P.; Kiene, H.; Helfand, M.; Altman, D.G.; Sox, H.; et al. CARE guidelines for case reports: Explanation and elaboration document. J. Clin. Epidemiol. 2017, 89, 218–235. [Google Scholar] [CrossRef]
- Chioncel, O.; Adamo, M.; Nikolaou, M.; Parissis, J.; Mebazaa, A.; Yilmaz, M.B.; Hassager, C.; Moura, B.; Bauersachs, J.; Harjola, V.; et al. Acute Heart Failure and Valvular Heart Disease: A Scientific Statement of the Heart Failure Association (HFA), the Association for Acute Cardi-Vascular Care (ACVC) and the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC. Eur. J. Heart Fail. 2023. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E.; Moscovitz, H.L.; Amram, S.S.; Lasser, R.P.; Sapin, S.O.; Himmelstein, A.; Ravitch, M.M.; Gordon, A.J. The Hemodynamics of the Left Side of the Heart as Studied by Simultaneous Left Atrial, Left Ventricular, and Aortic Pressures; Particular Reference to Mitral Stenosis. Circulation 1955, 12, 69–81. [Google Scholar] [CrossRef]
- Badeer, H.S. Hemodynamics for Medical Students. Adv. Physiol. Educ. 2001, 25, 44–52. [Google Scholar] [CrossRef]
- Spencer, K.T.; Kimura, B.J.; Korcarz, C.E.; Pellikka, P.A.; Rahko, P.S.; Siegel, R.J. Focused Cardiac Ultrasound: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2013, 26, 567–581. [Google Scholar] [CrossRef]
- Weekes, A.J.; Quirke, D.P. Emergency Echocardiography. Emerg. Med. Clin. N. Am. 2011, 29, 759–787. [Google Scholar] [CrossRef]
- Popescu, B.A.; Andrade, M.J.; Badano, L.P.; Fox, K.F.; Flachskampf, F.A.; Lancellotti, P.; Varga, A.; Sicari, R.; Evangelista, A.; Nihoyannopoulos, P.; et al. European Association of Echocardiography recommendations for training, competence, and quality improvement in echocardiography. Eur. J. Echocardiogr. 2009, 10, 893–905. [Google Scholar] [CrossRef]
- Fraser, A.G.; Monaghan, M.J.; van der Steen, A.F.W.; Sutherland, G.R. A concise history of echocardiography: Timeline, pioneers, and landmark publications. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1130–1143. [Google Scholar] [CrossRef]
- Maleki, M.; Esmaeilzadeh, M. The Evolutionary Development of Echocardiography. Iran. J. Med. Sci. 2012, 37, 222–232. [Google Scholar] [PubMed]
- Neskovic, A.N.; Edvardsen, T.; Galderisi, M.; Garbi, M.; Gullace, G.; Jurcut, R.; Dalen, H.; Hagendorff, A.; Lancellotti, P.; The European Association of Cardiovascular Imaging Document Reviewers; et al. Focus cardiac ultrasound: The European Association of Cardiovascular Imaging viewpoint. Eur. Heart J.-Cardiovasc. Imaging 2014, 15, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Farsi, D.; Hajsadeghi, S.; Hajighanbari, M.J.; Mofidi, M.; Hafezimoghadam, P.; Rezai, M.; Mahshidfar, B.; Abiri, S.; Abbasi, S. Focused cardiac ultrasound (FOCUS) by emergency medicine residents in patients with suspected cardiovascular diseases. J. Ultrasound 2017, 20, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Chamsi-Pasha, M.A.; Sengupta, P.P.; Zoghbi, W.A. Handheld Echocardiography. Circulation 2017, 136, 2178–2188. [Google Scholar] [CrossRef]
- Dewar, Z.E.; Wu, J.; Hughes, H.; Adnani, A.; Do, G.C.; Ovedovitz, L.; Rittenberger, J.C. A comparison of handheld ultrasound versus traditional ultrasound for acquisition of RUSH views in healthy volunteers. J. Am. Coll. Emerg. Physicians Open 2020, 1, 1320–1325. [Google Scholar] [CrossRef]
- Savino, K.; Ambrosio, G. Handheld Ultrasound and Focused Cardiovascular Echography: Use and Information. Medicina 2019, 55, 423. [Google Scholar] [CrossRef]
- Neskovic, A.N.; Skinner, H.; Price, S.; Via, G.; De Hert, S.; Stankovic, I.; Galderisi, M.; Donal, E.; Muraru, D.; Sloth, E.; et al. Focus cardiac ultrasound core curriculum and core syllabus of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 475–481. [Google Scholar] [CrossRef]
- Yamada, H.; Ito, H.; Fujiwara, M. Cardiac and vascular point-of-care ultrasound: Current situation, problems, and future prospects. J. Med. Ultrason. 2022, 49, 601–608. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Ternus, B.; Eleid, M.; Rihal, C. Structural Heart Disease Emergencies. J. Intensive Care Med. 2020, 36, 975–988. [Google Scholar] [CrossRef]
- Patel, A.; Tomar, N.S.; Bharani, A. Utility of physical examination and comparison to echocardiography for cardiac diagnosis. Indian Heart J. 2017, 69, 141–145. [Google Scholar] [CrossRef]
- Arntfield, R.T.; Millington, S.J. Point of Care Cardiac Ultrasound Applications in the Emergency Department and Intensive Care Unit—A Review. Curr. Cardiol. Rev. 2012, 8, 98–108. [Google Scholar] [CrossRef]
- Fink, W.; Kamenski, G.; Konitzer, M. Diagnostic protocols—A consultation tool still to be discovered. J. Eval. Clin. Pract. 2017, 24, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Labovitz, A.J.; Noble, V.E.; Bierig, M.; Goldstein, S.A.; Jones, R.; Kort, S.; Porter, T.R.; Spencer, K.T.; Tayal, V.S.; Wei, K. Focused Cardiac Ultrasound in the Emergent Setting: A Consensus Statement of the American Society of Echocardiography and American College of Emergency Physicians. J. Am. Soc. Echocardiogr. 2010, 23, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Millington, S.J.; Arntfield, R.T. Advanced Point-of-Care Cardiac Ultrasound Examination: Doppler Applications, Valvular Assessment, and Advanced Right Heart Examination. Glob. Heart 2013, 8, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Romine, D.; Flannigan, M.; Reynolds, J.C. Unexpected Causes and Complications of ST-Segment Myocardial Infarction That Highlight the Importance and Limitations of Point-of-Care Ultrasound in the Emergency Department. Cureus 2023, 15, e35754. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Ravindranath, S. Is it septic or cardiogenic shock? Papillary muscle rupture masquerading as sepsis. Australas. J. Ultrasound Med. 2019, 22, 51–55. [Google Scholar] [CrossRef]
- Bustam, A.; Azhar, M.N.; Veriah, R.S.; Arumugam, K.; Loch, A. Performance of emergency physicians in point-of-care echocardiography following limited training. Emerg. Med. J. 2013, 31, 369–373. [Google Scholar] [CrossRef]
- Frederiksen, C.A.; Juhl-Olsen, P.; Andersen, N.H.; Sloth, E. Assessment of cardiac pathology by point-of-care ultrasonography performed by a novice examiner is comparable to the gold standard. Scand. J. Trauma Resusc. Emerg. Med. 2013, 21, 87. [Google Scholar] [CrossRef]
- Marbach, J.A.; Almufleh, A.; Di Santo, P.; Jung, R.; Simard, T.; McInnes, M.; Salameh, J.-P.; McGrath, T.A.; Millington, S.J.; Diemer, G.; et al. Comparative Accuracy of Focused Cardiac Ultrasonography and Clinical Examination for Left Ventricular Dysfunction and Valvular Heart Disease: A Systematic Review and Meta-Analysis. Ann. Intern. Med. 2019, 171, 264. [Google Scholar] [CrossRef]
- Milne, J.; Atkinson, P.; Lewis, D.; Fraser, J.; Diegelmann, L.; Olszynski, P.; Stander, M.; Lamprecht, H. Sonography in Hypotension and Cardiac Arrest (SHoC): Rates of Abnormal Findings in Undifferentiated Hypotension and During Cardiac Arrest as a Basis for Consensus on a Hierarchical Point of Care Ultrasound Protocol. Cureus 2016, 8, e564. [Google Scholar] [CrossRef] [PubMed]
- Puga, J.L.; Krzywinski, M.; Altman, N. Bayes’ theorem. Nat. Methods 2015, 12, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Bours, M.J. Bayes’ rule in diagnosis. J. Clin. Epidemiol. 2021, 131, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Norman, G.; Barraclough, K.; Dolovich, L.; Price, D. Iterative diagnosis. BMJ 2009, 339, b3490. [Google Scholar] [CrossRef] [PubMed]
- Tsalatsanis, A.; Hozo, I.; Kumar, A.; Djulbegovic, B. Dual Processing Model for Medical Decision-Making: An Extension to Diagnostic Testing. PLoS ONE 2015, 10, e0134800. [Google Scholar] [CrossRef] [PubMed]
- Kitabchi, A.E.; Umpierrez, G.E.; Miles, J.M.; Fisher, J.N. Hyperglycemic Crises in Adult Patients with Diabetes. Diabetes Care 2009, 32, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Chardoli, M. Valvular Emergencies Part 1: Diagnosis and Management of Severe Mitral Regurgitation Made Easy! 2023. Available online: https://recapem.com/valvular-emergencies-part-1-diagnosis-and-management-of-severe-mitral-regurgitation-made-easy-2/ (accessed on 28 March 2023).
- Santangelo, G.; Bursi, F.; Faggiano, A.; Moscardelli, S.; Simeoli, P.S.; Guazzi, M.; Lorusso, R.; Carugo, S.; Faggiano, P. The Global Burden of Valvular Heart Disease: From Clinical Epidemiology to Management. J. Clin. Med. 2023, 12, 2178. [Google Scholar] [CrossRef]
- Messika-Zeitoun, D.; Burwash, I.G.; Thierry, M. Challenges in the diagnosis and management of valve disease: The case for the specialist valve clinic. Echo Res. Pract. 2019, 6, T1–T6. [Google Scholar] [CrossRef]
- Arden, C.; Chambers, J.B.; Sandoe, J.; Ray, S.; Prendergast, B.; Taggart, D.; Westaby, S.; Grothier, L.; Wilson, J.; Campbell, B.; et al. Can we improve the detection of heart valve disease? Heart 2013, 100, 271–273. [Google Scholar] [CrossRef]
- Yan, B.P.; Fok, J.C.-Y.; Wong, T.H.-Y.; Tse, G.; Lee, A.; Yang, X.-S.; Sun, J.-P. Junior medical student performed focused cardiac ultrasound after brief training to detect significant valvular heart disease. IJC Heart Vasc. 2018, 19, 41–45. [Google Scholar] [CrossRef]
- Jenkins, S.; Shiha, M.G.; Yones, E.; Wardley, J.; Ryding, A.; Sawh, C.; Flather, M.; Morris, P.; Swift, A.J.; Vassiliou, V.S.; et al. Cardiovascular examination using hand-held cardiac ultrasound. J. Echocardiogr. 2021, 20, 1–9. [Google Scholar] [CrossRef]
- Hothi, S.S.; Sprigings, D.; Chambers, J. Point-of-care cardiac ultrasound in acute medicine–the quick scan. Clin. Med. 2014, 14, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Sachpekidis, V.; Papadopoulou, S.-L.; Kantartzi, V.; Styliadis, I.; Nihoyannopoulos, P. A Novel Handheld Echocardiography Device with Continuous-Wave Doppler Capability: Implications for the Evaluation of Aortic Stenosis Severity. J. Am. Soc. Echocardiogr. 2022, 35, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Medow, M.A.; Lucey, C.R. A qualitative approach to Bayes’ theorem. BMJ Evid.-Based Med. 2011, 16, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Luciani, D.; Cavuto, S.; Antiga, L.; Miniati, M.; Monti, S.; Pistolesi, M.; Bertolini, G. Bayes pulmonary embolism assisted diagnosis: A new expert system for clinical use. Emerg. Med. J. 2007, 24, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.N.; Saxhaug, L.M. Handheld ultrasound in training–The future is getting smaller! J. Intensive Care Soc. 2020, 22, 220–229. [Google Scholar] [CrossRef]
- Vaishnav, M.; Sedgwick, J. Point-of-care echocardiography-A road to future or a step backwards. Australas. J. Ultrasound Med. 2019, 22, 26–31. [Google Scholar] [CrossRef]
- Sliwa, K.; Wilkinson, D.; Hansen, C.; Ntyintyane, L.; Tibazarwa, K.; Becker, A.; Stewart, S. Spectrum of heart disease and risk factors in a black urban population in South Africa (the Heart of Soweto Study): A cohort study. Lancet 2008, 371, 915–922. [Google Scholar] [CrossRef]
- Ganas, U.; Malan, J.J.; Bruijns, S.R. A descriptive study of the use of cardiac point of care ultrasound (PoCUS) in public emergency centres in Cape Town. Afr. J. Emerg. Med. 2020, 10, 239–242. [Google Scholar] [CrossRef]
- Khanyi, H.B.; Naicker, B. The use of point-of-care ultrasound in a regional emergency department in KwaZulu-Natal, South Africa. South Afr. Fam. Pract. 2021, 63, 6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekambaram, K.; Hassan, K. Establishing a Novel Diagnostic Framework Using Handheld Point-of-Care Focused-Echocardiography (HoPE) for Acute Left-Sided Cardiac Valve Emergencies: A Bayesian Approach for Emergency Physicians in Resource-Limited Settings. Diagnostics 2023, 13, 2581. https://doi.org/10.3390/diagnostics13152581
Ekambaram K, Hassan K. Establishing a Novel Diagnostic Framework Using Handheld Point-of-Care Focused-Echocardiography (HoPE) for Acute Left-Sided Cardiac Valve Emergencies: A Bayesian Approach for Emergency Physicians in Resource-Limited Settings. Diagnostics. 2023; 13(15):2581. https://doi.org/10.3390/diagnostics13152581
Chicago/Turabian StyleEkambaram, Kamlin, and Karim Hassan. 2023. "Establishing a Novel Diagnostic Framework Using Handheld Point-of-Care Focused-Echocardiography (HoPE) for Acute Left-Sided Cardiac Valve Emergencies: A Bayesian Approach for Emergency Physicians in Resource-Limited Settings" Diagnostics 13, no. 15: 2581. https://doi.org/10.3390/diagnostics13152581
APA StyleEkambaram, K., & Hassan, K. (2023). Establishing a Novel Diagnostic Framework Using Handheld Point-of-Care Focused-Echocardiography (HoPE) for Acute Left-Sided Cardiac Valve Emergencies: A Bayesian Approach for Emergency Physicians in Resource-Limited Settings. Diagnostics, 13(15), 2581. https://doi.org/10.3390/diagnostics13152581






