Video-Based versus On-Site Neonatal Pain Assessment in Neonatal Intensive Care Units: The Impact of Video-Based Neonatal Pain Assessment in Real-World Scenario on Pain Diagnosis and Its Artificial Intelligence Application
Abstract
1. Introduction
2. Methods
2.1. Setting and Participants
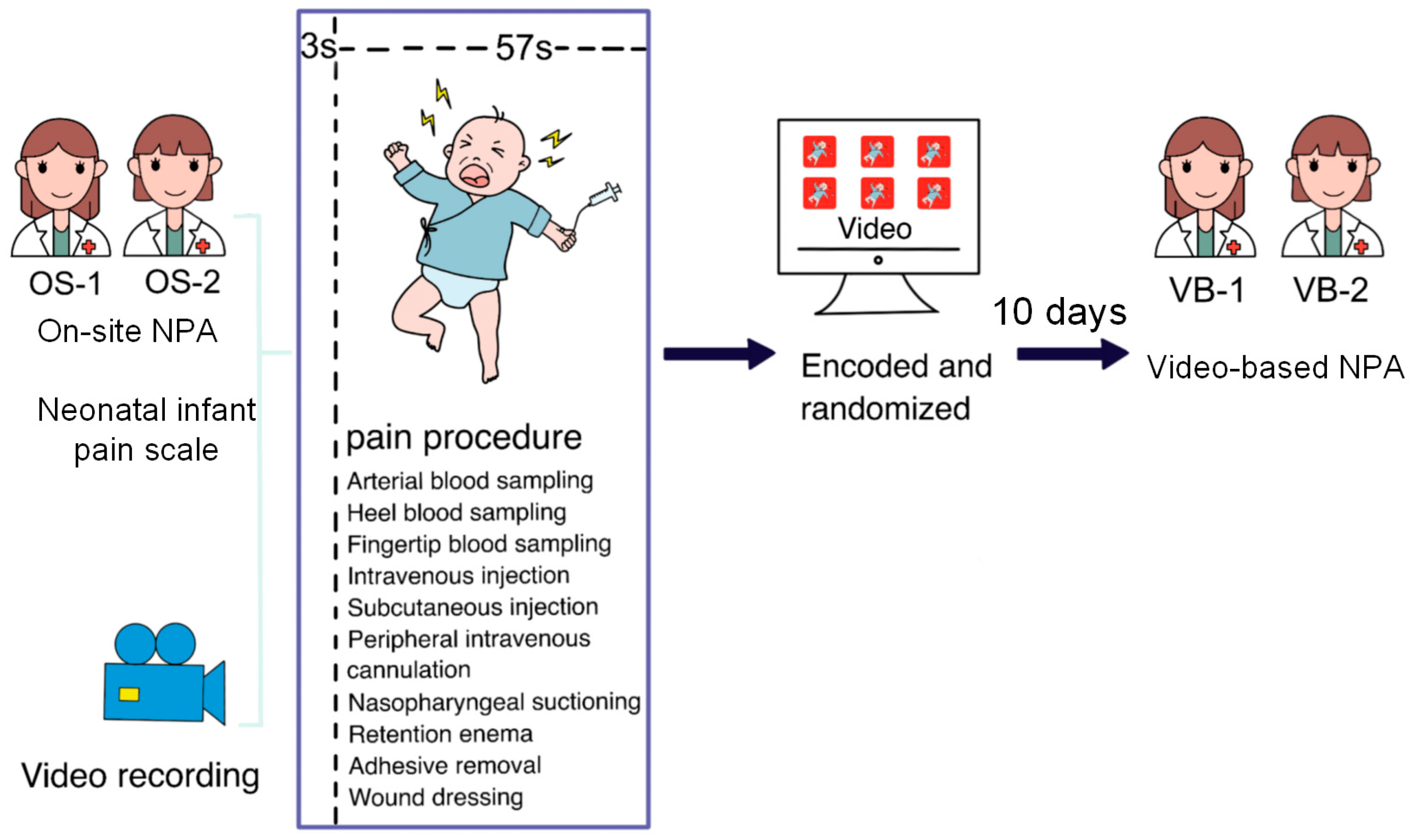
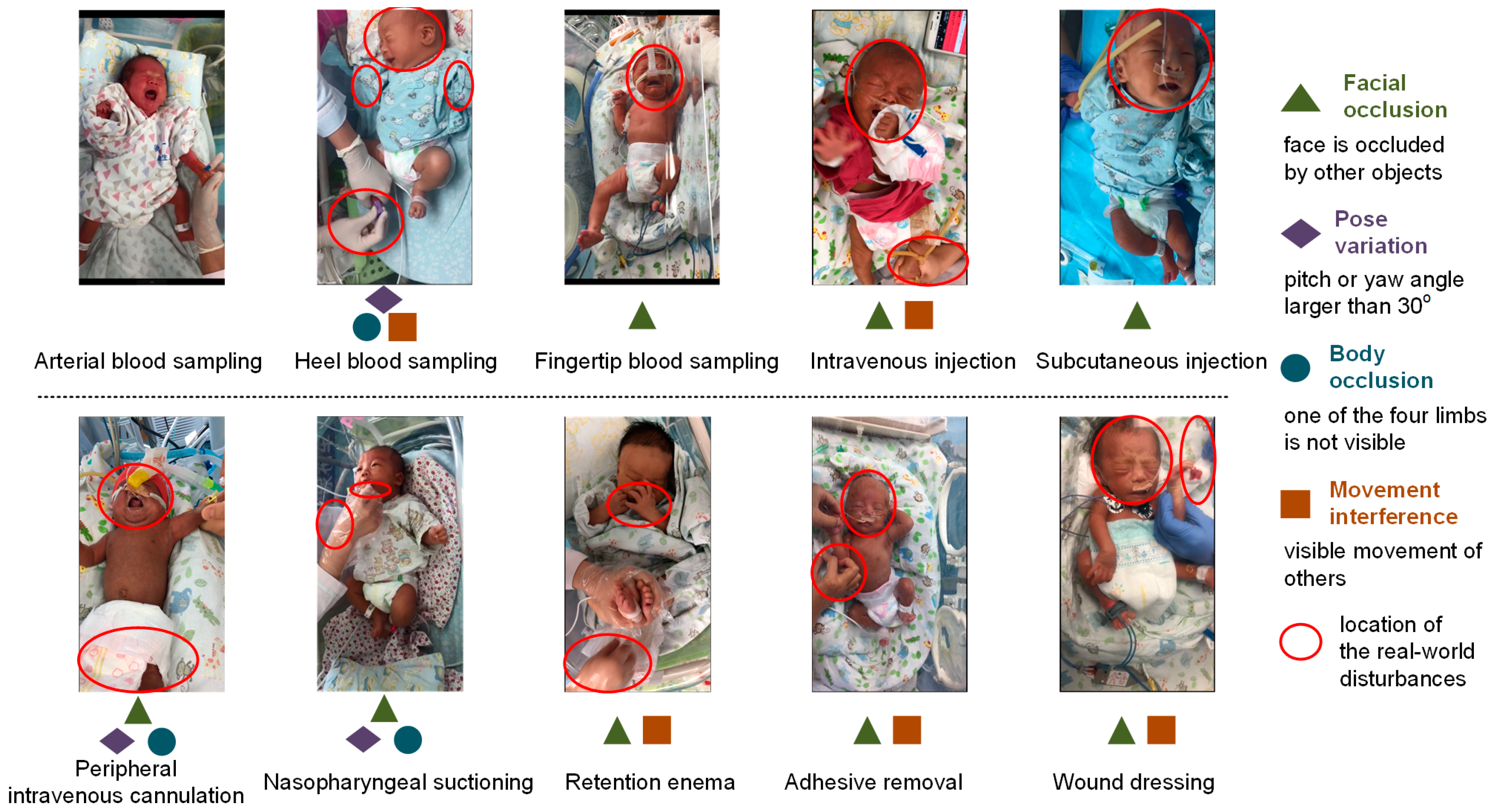
2.2. Video Recording in Real-World Scenario
2.3. Pain Assessment
2.4. OS-NPA and VB-NPA
2.5. Data Analysis
3. Results
3.1. Study Population
3.2. Intra-rater Reliability and Inter-Rater Reliability
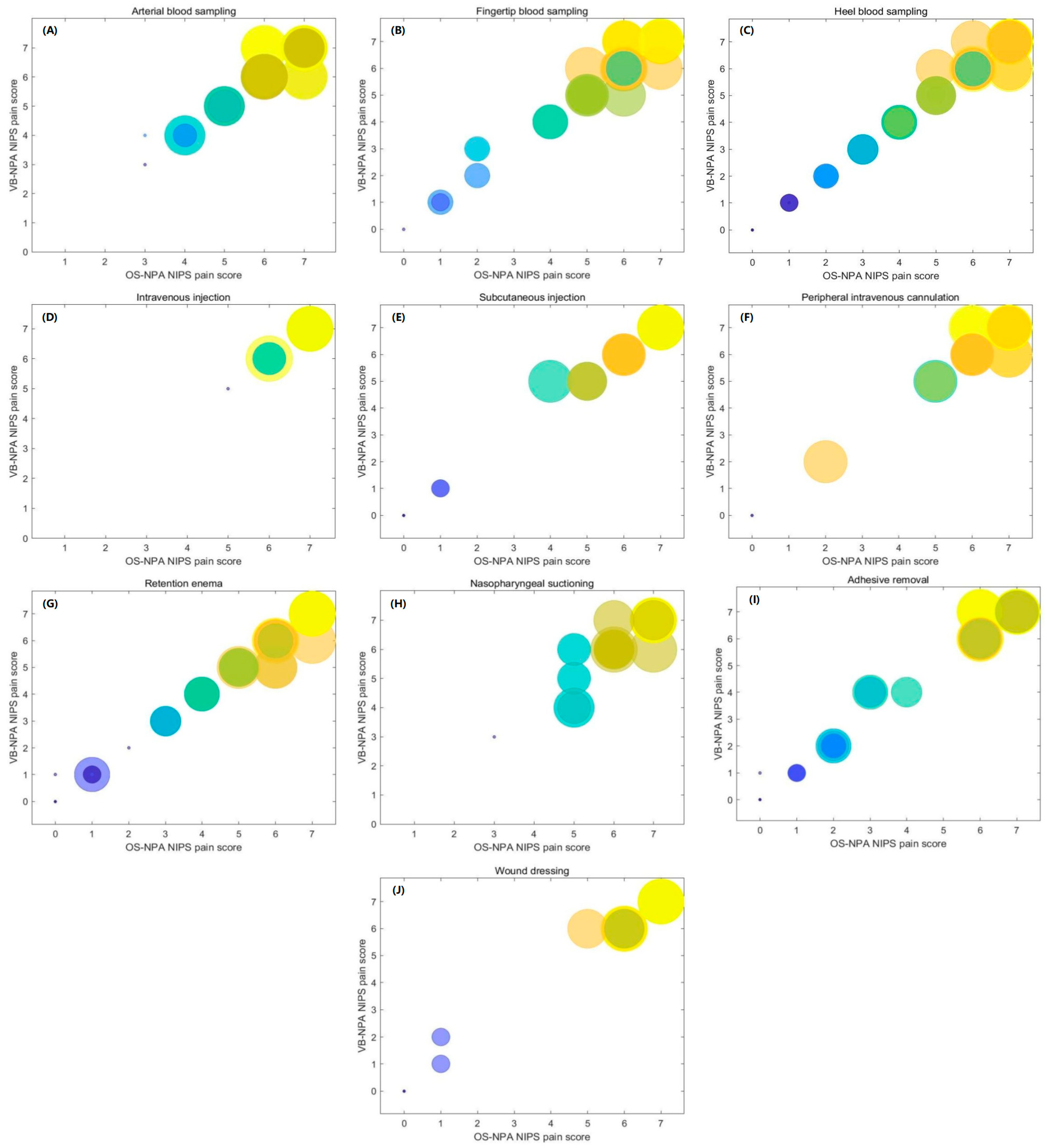
3.3. Comparison of the NIPS Pain Grades between OS-NPA and VB-NPA
3.4. Impact of Different Label Sources on AI Methods
4. Discussion
4.1. High Evaluation Consistency of the VB-NPA
4.2. VB-NPA for NIPS Pain Grades
4.3. VB-NPA for AI-NPA
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carbajal, R.; Rousset, A.; Danan, C.; Coquery, S.; Nolent, P.; Ducrocq, S.; Saizou, C.; Lapillonne, A.; Granier, M.; Durand, P.; et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA 2008, 300, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Courtois, E.; Droutman, S.; Magny, J.F.; Merchaoui, Z.; Durrmeyer, X.; Roussel, C.; Biran, V.; Eleni, S.; Vottier, G.; Renolleau, S.; et al. Epidemiology and neonatal pain management of heelsticks in intensive care units: EPIPPAIN 2, a prospective observational study. Int. J. Nurs. Stud. 2016, 59, 79–88. [Google Scholar] [CrossRef]
- Cruz, M.D.; Fernandes, A.M.; Oliveira, C.R. Epidemiology of painful procedures performed in neonates: A systematic review of observational studies. Eur. J. Pain 2016, 20, 489–498. [Google Scholar] [CrossRef]
- Meesters, N.J.; Simons, S.H.P.; van Rosmalen, J.; Holsti, L.; Reiss, I.K.M.; van Dijk, M. Acute pain assessment in prematurely born infants below 29 weeks: A long way to go. Clin. J. Pain 2019, 35, 975–982. [Google Scholar] [CrossRef]
- Anand, K.J.S. Clinical importance of pain and stress in preterm neonates. Neonatology 1998, 73, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Slater, L.; Asmerom, Y.; Boskovic, D.S.; Bahjri, K.; Plank, M.S.; Angeles, K.R.; Phillips, R.; Deming, D.; Ashwal, S.; Hougland, K.; et al. Procedural pain and oxidative stress in premature neonates. J. Pain 2012, 13, 590–597. [Google Scholar] [CrossRef]
- Walker, S.M. Long-term effects of neonatal pain. Semin. Fetal Neonatal Med. 2019, 24, 101005. [Google Scholar] [CrossRef]
- Perry, M.; Tan, Z.; Chen, J.; Weidig, T.; Xu, W.; Cong, X.S. Neonatal pain: Perceptions and current practice. Crit. Care Nurs. Clin. 2018, 30, 549–561. [Google Scholar] [CrossRef]
- Sposito, N.P.B.; Rossato, L.M.; Bueno, M.; Kimura, A.F.; Costa, T.; Guedes, D.M.B. Assessment and management of pain in newborns hospitalized in a neonatal intensive care unit: A cross-sectional study. Rev. Lat.-Am. Enferm. 2017, 25, e2931. [Google Scholar] [CrossRef]
- Franck, L.S.; Bruce, E. Putting pain assessment into practice: Why is it so painful? Pain Res. Manag. 2009, 14, 13–20. [Google Scholar] [CrossRef]
- Zamzmi, G.; Kasturi, R.; Goldgof, D.; Zhi, R.; Ashmeade, T.; Sun, Y. A review of automated pain assessment in infants: Features, classification tasks, and databases. IEEE Rev. Biomed. Eng. 2017, 11, 77–96. [Google Scholar] [CrossRef]
- Merboth, M.K.; Barnason, S. Managing pain: The fifth vital sign. Nurs. Clin. N. Am. 2000, 35, 375–383. [Google Scholar] [CrossRef]
- Grunauer, M.; Mikesell, C.; Bustamante, G.; Cobo, G.; Sánchez, S.; Román, A.M.; Icaza-Freire, A.P.; Gavilanes, A.W.D.; Wang, N.E.; PICU-MIC Research Group. Pain assessment and management in pediatric intensive care units around the world, an international, multicenter study. Front. Pediatr. 2021, 9, 746489. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.; Alcock, D.; McGrath, P.; Kay, J.; MacMurray, S.B.; Dulberg, C. The development of a tool to assess neonatal pain. Neonatal Netw. 1993, 12, 59–66. [Google Scholar] [CrossRef] [PubMed]
- da Motta, G.d.C.P.; Schardosim, J.M.; da Cunha, M.L.C. Neonatal infant pain scale: Cross-cultural adaptation and validation in Brazil. J. Pain Symptom Manag. 2015, 50, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Napiórkowska-Orkisz, M.; Gutysz-Wojnicka, A.; Tanajewska, M.; Sadowska-Krawczenko, I. Evaluation of methods to minimize pain in newborns during capillary blood sampling for screening: A randomized clinical trial. Int. J. Environ. Res. Public Health 2022, 19, 870. [Google Scholar] [CrossRef]
- Suraseranivongse, S.; Kaosaard, R.; Intakong, P.; Pornsiriprasert, S.; Karnchana, Y.; Kaopinpruck, J.; Sangjeen, K. A comparison of postoperative pain scales in neonates. Br. J. Anaesth. 2006, 97, 540–544. [Google Scholar] [CrossRef]
- Yao, W.Y.; Petrini, M.; Deng, W.L.; Wu, H.L.; Tu, H.X. Effects of glucose administering approaches on reducing neonatal pain during heel lance procedures. Chin. J. Nurs. 2011, 46, 637–639. (In Chinese) [Google Scholar]
- Zamzmi, G.; Pai, C.-Y.; Goldgof, D.; Kasturi, R.; Ashmeade, T.; Sun, Y. A comprehensive and context-sensitive neonatal pain assessment using computer vision. IEEE Trans. Affect. Comput. 2019, 13, 28–45. [Google Scholar] [CrossRef]
- Min, L.; Sun, Y.; de With, P.H.N. Video-based infant monitoring using a CNN-LSTM scheme. Proc. SPIE 2021, 11597, 1159717. [Google Scholar]
- Salekin, M.S.; Zamzmi, G.; Goldgof, D.; Kasturi, R.; Ho, T.; Sun, Y. Multimodal spatio-temporal deep learning approach for neonatal postoperative pain assessment. Comput. Biol. Med. 2021, 129, 104150. [Google Scholar] [CrossRef] [PubMed]
- Dagnaes-Hansen, J.; Mahmood, O.; Bube, S.; Bjerrum, F.; Subhi, Y.; Rohrsted, M.; Konge, L. Direct observation vs. video-based assessment in flexible cystoscopy. J. Surg. Educ. 2018, 75, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Salekin, M.S.; Mouton, P.R.; Zamzmi, G.; Patel, R.; Goldgof, D.; Kneusel, M.; Elkins, S.L.; Murray, E.; Coughlin, M.E.; Maguire, D.; et al. Future roles of artificial intelligence in early pain management of newborns. Paediatr. Neonatal Pain 2021, 3, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhu, H.; Mei, L.; Luo, F.; Chen, X.; Zhao, Y.; Chen, S.; Pan, Y. Artificial intelligence based pain assessment technology in clinical application of real-world neonatal blood sampling. Diagnostics 2022, 12, 1831. [Google Scholar] [CrossRef]
- Scaffidi, M.A.; Grover, S.C.; Carnahan, H.; Yu, J.J.; Yong, E.; Nguyen, G.C.; Ling, S.C.; Khanna, N.; Walsh, C.M. A prospective comparison of live and video-based assessments of colonoscopy performance. Gastrointest. Endosc. 2018, 87, 766–775. [Google Scholar] [CrossRef]
- Sun, Y.; Kommers, D.; Wang, W.; Joshi, R.; Shan, C.; Tan, T.; Aarts, R.M.; van Pul, C.; Andriessen, P.; de With, P.H.N. Automatic and continuous discomfort detection for premature infants in a NICU using video-based motion analysis. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 5995–5999. [Google Scholar]
- Hoti, K.; Chivers, P.T.; Hughes, J.D. Assessing procedural pain in infants: A feasibility study evaluating a point-of-care mobile solution based on automated facial analysis. Lancet Digit. Health 2021, 3, e623–e634. [Google Scholar] [CrossRef]
- Branco, A.; Fekete, S.M.W.; Rugolo, L.M.S.S.; Rehder, M.I. The newborn pain cry: Descriptive acoustic spectrographic analysis. Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 539–546. [Google Scholar] [CrossRef]
- Almeida, M.; Zhuang, Y.; Ding, W.; Crouter, S.E.; Chen, P. Mitigating class-boundary label uncertainty to reduce both model bias and variance. ACM Trans. Knowl. Discov. Data 2021, 15, 1–18. [Google Scholar] [CrossRef]
| Variables | Total Amount (Proportion, %) |
|---|---|
| Gender | |
| Female | 252 (42.14) |
| Male | 346 (57.86) |
| Delivery mode | |
| Spontaneous delivery | 270 (45.15) |
| Cesarean section | 328 (54.85) |
| Pain procedure | |
| Arterial blood sampling | 81 (13.55) |
| Heel blood sampling | 61 (10.20) |
| Fingertip blood sampling | 85 (14.21) |
| Intravenous injection | 12 (2.01) |
| Subcutaneous injection | 46 (7.69) |
| Peripheral intravenous cannulation | 76 (12.7) |
| Nasopharyngeal suctioning | 65 (10.87) |
| Retention enema | 73 (12.21) |
| Adhesive removal | 68 (11.37) |
| Wound dressing | 31 (5.18) |
| Label Source 1 | Label Source 2 | ICC | 95% CI | p-Value |
|---|---|---|---|---|
| OS-1 | VB-1 | 0.976 (single) 0.988 (averages) | 0.972–0.980 0.986–0.990 | <0.001 <0.001 |
| OS-2 | VB-2 | 0.983 (single) 0.992 (averages) | 0.980–0.986 0.990–0.993 | <0.001 <0.001 |
| OS-1 | VB-2 | 0.976 (single) 0.988 (averages) | 0.972–0.979 0.986–0.990 | <0.001 <0.001 |
| OS-1 | OS-2 | 0.986 (single) 0.993 (averages) | 0.984–0.986 0.992–0.994 | <0.001 <0.001 |
| OS-2 | VB-1 | 0.976 (single) 0.988 (averages) | 0.972–0.979 0.986–0.990 | <0.001 <0.001 |
| VB-1 | VB-2 | 0.983 (single) 0.992 (averages) | 0.980–0.986 0.990–0.993 | <0.001 <0.001 |
| VB-NPA | Kappa Value | p-Value | |||||
|---|---|---|---|---|---|---|---|
| No Pain | Mild | Moderate | Severe | ||||
| OS-NPA | No pain | 97 | 1 | 0 | 0 | 0.926 | <0.001 |
| Mild | 2 | 8 | 4 | 1 | |||
| Moderate | 0 | 1 | 32 | 3 | |||
| Severe | 0 | 0 | 6 | 443 | |||
| Label Source | Test | OS-1 | OS-2 | ||||
|---|---|---|---|---|---|---|---|
| Training | Method | [19] | [20] | [21] | [19] | [20] | [21] |
| OS-1 | average | 0.759 | 0.783 | 0.828 | 0.752 | 0.779 | 0.826 |
| std | 0.044 | 0.040 | 0.036 | 0.034 | 0.031 | 0.028 | |
| OS-2 | average | 0.756 | 0.769 | 0.824 | 0.776 | 0.786 | 0.826 |
| std | 0.036 | 0.039 | 0.035 | 0.028 | 0.024 | 0.035 | |
| VB-1 | average | 0.746 | 0.769 | 0.819 | 0.732 | 0.754 | 0.803 |
| std | 0.038 | 0.038 | 0.028 | 0.022 | 0.023 | 0.027 | |
| VB-2 | average | 0.737 | 0.747 | 0.801 | 0.749 | 0.761 | 0.808 |
| std | 0.037 | 0.042 | 0.023 | 0.019 | 0.031 | 0.021 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhu, H.; Mei, L.; Shu, Q.; Cheng, X.; Luo, F.; Zhao, Y.; Chen, S.; Pan, Y. Video-Based versus On-Site Neonatal Pain Assessment in Neonatal Intensive Care Units: The Impact of Video-Based Neonatal Pain Assessment in Real-World Scenario on Pain Diagnosis and Its Artificial Intelligence Application. Diagnostics 2023, 13, 2661. https://doi.org/10.3390/diagnostics13162661
Chen X, Zhu H, Mei L, Shu Q, Cheng X, Luo F, Zhao Y, Chen S, Pan Y. Video-Based versus On-Site Neonatal Pain Assessment in Neonatal Intensive Care Units: The Impact of Video-Based Neonatal Pain Assessment in Real-World Scenario on Pain Diagnosis and Its Artificial Intelligence Application. Diagnostics. 2023; 13(16):2661. https://doi.org/10.3390/diagnostics13162661
Chicago/Turabian StyleChen, Xiaofei, Huaiyu Zhu, Linli Mei, Qi Shu, Xiaoying Cheng, Feixiang Luo, Yisheng Zhao, Shuohui Chen, and Yun Pan. 2023. "Video-Based versus On-Site Neonatal Pain Assessment in Neonatal Intensive Care Units: The Impact of Video-Based Neonatal Pain Assessment in Real-World Scenario on Pain Diagnosis and Its Artificial Intelligence Application" Diagnostics 13, no. 16: 2661. https://doi.org/10.3390/diagnostics13162661
APA StyleChen, X., Zhu, H., Mei, L., Shu, Q., Cheng, X., Luo, F., Zhao, Y., Chen, S., & Pan, Y. (2023). Video-Based versus On-Site Neonatal Pain Assessment in Neonatal Intensive Care Units: The Impact of Video-Based Neonatal Pain Assessment in Real-World Scenario on Pain Diagnosis and Its Artificial Intelligence Application. Diagnostics, 13(16), 2661. https://doi.org/10.3390/diagnostics13162661








