Cardiac Magnetic Resonance Imaging (CMRI) Applications in Patients with Chest Pain in the Emergency Department: A Narrative Review
Abstract
:1. Introduction
2. Application of CMRI in the Diagnosis of Different Types of Chest Pains
3. Utilizing Machine Learning for the Diagnosis of Chest Pain through CMRI
4. Research Statistics
5. Implementation Results
6. Discussion
- Selection bias: The article does not provide details about the criteria used to select the studies included in the research review. It is important to consider that studies with positive results may be more likely to be published, while studies with negative or inconclusive results may be overlooked. This selection bias can lead to an overestimation of the effectiveness of CMRI in diagnosing chest discomfort.
- Interpretation bias: CMRI interpretation requires expertise and subjective judgment. The article does not mention whether the researchers or reviewers were blinded to the clinical information. If they were not blinded, knowledge of the patient’s clinical status or symptoms could introduce bias into the interpretation of CMRI findings.
- Interobserver variability: Different observers’ interpretations of CMRI may differ. The study offers no inquiry into whether the analysis employed several reviewers or whether steps were taken to evaluate and reduce interobserver variability. The trustworthiness of the study’s findings may be impacted by different reviewers’ inconsistent interpretations of CMRI data.
- Lack of gold standard comparison: Although the article cites the use of CMRI as a substitute for traditional diagnostic methods, it supplies no information regarding the reference standard or gold standard that was used to determine the accuracy of CMRI. It is difficult to adequately assess the genuine diagnostic performance of CMRI without a direct comparison to a recognized gold standard.
- Generalizability: The study populations’ characteristics or the environments in which the investigations included in the research review were carried out are not described in the article, which limits its potential to generalize. The findings’ applicability to other patient demographics or healthcare environments must be taken into account. Depending on the patient’s demographics, comorbidities, and access to knowledge and resources, CMRI may or may not be useful for detecting chest discomfort.
- Potential conflicts of interest: Conflicts of interest that might have existed between the researchers or the funding sources are not addressed in the publication. Financial ties to businesses that make CMRI equipment or drugs related to it can skew the results of studies. Any conflicts of interest must be disclosed in order to maintain transparency and reduce potential bias.
- In addition to the previously mentioned limitations, it is important to address the practical applicability of cardiac MRI in daily clinical practice:
- Feasibility in emergency settings: The paper does not thoroughly discuss the feasibility of performing cardiac MRI in emergency departments or emergency rooms (ED/ER). Given the time-consuming nature of cardiac MRI, it may not be practical to perform this imaging modality in acute situations where timely interventions are crucial.
- Resource utilization: Cardiac MRI requires specialized equipment, trained personnel, and dedicated facilities. Assessing the availability and allocation of these resources, as well as their cost-effectiveness, is crucial in understanding the practicality and sustainability of widespread cardiac MRI implementation.
- Patient selection criteria: Not all patients with chest discomfort or suspected cardiac conditions may be suitable candidates for cardiac MRI due to factors such as contraindications, patient stability, or the urgency of intervention. Understanding the limitations of and specific indications for cardiac MRI in the emergency setting is essential for its optimal utilization and decision-making.
- Limited evidence in the acute setting: The article predominantly focuses on studies conducted in the chronic/subacute setting, where patients were likely admitted to the ward. The lack of effective data on the clinical application of MRI in the acute setting raises concerns about the generalizability of the findings to emergency situations. The article should acknowledge the limitations of the available evidence and highlight the need for further research specifically targeting the acute setting. The generic messages derived from predominantly chronic/subacute studies may not be directly applicable or reproducible in acute clinical scenarios.
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowak, R.M.; Jacobsen, G.; Christenson, R.H.; Moyer, M.; Hudson, M.; McCord, J. Differentiating type 1 and 2 acute myocardial infarctions using the N-terminal pro B-type natriuretic peptide/cardiac troponin T ratio. Am. J. Emerg. Med. 2018, 36, 1849–1854. [Google Scholar] [CrossRef] [PubMed]
- Vasile, V.C.; Jaffe, A.S. High-Sensitivity Cardiac Troponin for the Diagnosis of Patients with Acute Coronary Syndromes. Curr. Cardiol. Rep. 2017, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- DeFilippis, A.P.; Chapman, A.R.; Mills, N.L.; de Lemos, J.A.; Arbab-Zadeh, A.; Newby, L.K.; Morrow, D.A. Assessment and Treatment of Patients With Type 2 Myocardial Infarction and Acute Nonischemic Myocardial Injury. Circulation 2019, 140, 1661–1678. [Google Scholar] [CrossRef]
- Miller, D.D.; Brown, E.W. Artificial Intelligence in Medical Practice: The Question to the Answer? Am. J. Med. 2018, 131, 129–133. [Google Scholar] [CrossRef]
- Cavalier, J.S.; Klem, I. Using Cardiac Magnetic Resonance Imaging to Evaluate Patients with Chest Pain in the Emergency Department. J. Cardiovasc. Imaging 2021, 29, 91. [Google Scholar] [CrossRef]
- Nickander, J.; Themudo, R.; Thalén, S.; Sigfridsson, A.; Xue, H.; Kellman, P.; Ugander, M. The relative contributions of myocardial perfusion, blood volume and extracellular volume to native T1 and native T2 at rest and during adenosine stress in normal physiology. J. Cardiovasc. Magn. Reson. 2019, 21, 73. [Google Scholar] [CrossRef]
- Sirajuddin, A.; Mirmomen, S.M.; Kligerman, S.J.; Groves, D.W.; Burke, A.P.; Kureshi, F.; White, C.S.; Arai, A.E. Ischemic Heart Disease: Noninvasive Imaging Techniques and Findings. RadioGraphics 2021, 41, E990–E1021. [Google Scholar] [CrossRef]
- De Carvalho, F.S.; Mukai, K.; Clayton, J.; Ordovas, K. Cardiac MRI: A preferred method for assessing myocardial ischemia and infarct burden. Appl. Radiol. 2017, 46, 21–29. [Google Scholar] [CrossRef]
- Burrage, M.K.; Shanmuganathan, M.; Masi, A.; Hann, E.; Zhang, Q.; Popescu, I.A.; Soundarajan, R.; Pelado, J.L.; Chow, K.; Neubauer, S. Cardiovascular magnetic resonance stress and rest T1-mapping using regadenoson for detection of ischemic heart disease compared to healthy controls. Int. J. Cardiol. 2021, 333, 239–245. [Google Scholar] [CrossRef]
- Bajaj, N.S.; Singh, S.; Farag, A.; EL-Hajj, S.; Heo, J.; Iskandrian, A.E.; Hage, F.G. The prognostic value of non-perfusion variables obtained during vasodilator stress myocardial perfusion imaging. J. Nucl. Cardiol. 2016, 23, 390–413. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Piechnik, S.K. CMR parametric mapping as a tool for myocardial tissue characterization. Korean Circ. J. 2020, 50, 658–676. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Jang, I.-K.; Beltrame, J.F.; Sicari, R.; Meucci, M.C.; Bode, M.; Gaibazzi, N.; Niccoli, G.; Bucciarelli-Ducci, C.; Crea, F. The evolving role of cardiac imaging in patients with myocardial infarction and non-obstructive coronary arteries. Prog. Cardiovasc. Dis. 2021, 68, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Mark, P.B.; Johnston, N.; Groenning, B.A.; Foster, J.E.; Blyth, K.G.; Martin, T.N.; Steedman, T.; Dargie, H.J.; Jardine, A.G. Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int. 2006, 69, 1839–1845. [Google Scholar] [CrossRef]
- Satoh, H. Distribution of late gadolinium enhancement in various types of cardiomyopathies: Significance in differential diagnosis, clinical features and prognosis. World J. Cardiol. 2014, 6, 585. [Google Scholar] [CrossRef]
- He, C.; Zhu, C.; Han, B.; Hu, H.; Wang, S.; Zhai, C.; Hu, H. Association between anxiety and clinical outcomes in Chinese patients with myocardial infarction in the absence of obstructive coronary artery disease. Clin. Cardiol. 2020, 43, 659–665. [Google Scholar] [CrossRef]
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.P.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur. Heart J. 2016, 38, ehw149. [Google Scholar] [CrossRef]
- Ciliberti, G.; Coiro, S.; Tritto, I.; Benedetti, M.; Guerra, F.; Del Pinto, M.; Finocchiaro, G.; Cavallini, C.; Capucci, A.; Kaski, J.C.; et al. Predictors of poor clinical outcomes in patients with acute myocardial infarction and non-obstructed coronary arteries (MINOCA). Int. J. Cardiol. 2018, 267, 41–45. [Google Scholar] [CrossRef]
- Ziegler, C.E.; Painter, D.M.; Borawski, J.B.; Kim, R.J.; Kim, H.W.; Limkakeng, A.T. Unexpected Cardiac MRI Findings in Patients Presenting to the Emergency Department for Possible Acute Coronary Syndrome. Crit. Pathw. Cardiol. A J. Evid.-Based Med. 2018, 17, 167–171. [Google Scholar] [CrossRef]
- Fagiry, M.A.; Abdelaziz, I.; Davidson, R.; Mahmoud, M.Z. The recent advances, drawbacks, and the future directions of CMRI in the diagnosis of IHD. Sci. Rep. 2021, 11, 14958. [Google Scholar] [CrossRef]
- Wong, N.D. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat. Rev. Cardiol. 2014, 11, 276–289. [Google Scholar] [CrossRef]
- Jalnapurkar, S.; Zarrini, P.; Mehta, P.K.; Thomson, L.E.J.; Agarwal, M.; Samuels, B.A.; Shufelt, C.L.; Eastwood, J.-A.; Berman, D.; Merz, N.B.; et al. Role of Stress Cardiac Magnetic Resonance Imaging in Women With Suspected Ischemia But No Obstructive Coronary Artery Disease. J. Radiol. Nurs. 2017, 36, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Simkowski, J.M.; Wehbe, R.M.; Goergen, J.; Anderson, A.S.; Ghafourian, K.; Okwuosa, I.; Vorovich, E.E.; Ahmad, F.S.; Tibrewala, A.; Pham, D.T.; et al. Unsupervised Machine Learning of LGE Patterns on Cardiac MRI Identifies Patients at Risk for Right Ventricular Failure After LVAD. J. Card. Fail. 2020, 26, S146. [Google Scholar] [CrossRef]
- Alsunbuli, A. The use of cardiac magnetic resonance imaging (CMRI) for adult congenital heart disease patients: Qualitative comparative review. Clin. Med. 2020, 20, s6–s7. [Google Scholar] [CrossRef] [PubMed]
- Grober, Y.; Grober, H.; Wintermark, M.; Jane, J.A.; Oldfield, E.H. Comparison of MRI techniques for detecting microadenomas in Cushing’s disease. J. Neurosurg. 2018, 128, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Moonen, A.; Lal, S.; Ingles, J.; Yeates, L.; Semsarian, C.; Puranik, R. Prevalence of Anderson-Fabry disease in a cohort with unexplained late gadolinium enhancement on cardiac MRI. Int. J. Cardiol. 2020, 304, 122–124. [Google Scholar] [CrossRef]
- Groepenhoff, F.; Eikendal, A.L.M.; Bots, S.H.; van Ommen, A.-M.; Overmars, L.M.; Kapteijn, D.; Pasterkamp, G.; Reiber, J.H.C.; Hautemann, D.; Menken, R.; et al. Cardiovascular imaging of women and men visiting the outpatient clinic with chest pain or discomfort: Design and rationale of the ARGUS Study. BMJ Open 2020, 10, e040712. [Google Scholar] [CrossRef]
- Ranka, S.; Reddy, M.; Noheria, A. Artificial intelligence in cardiovascular medicine. Curr. Opin. Cardiol. 2021, 36, 26–35. [Google Scholar] [CrossRef]
- Itchhaporia, D. Artificial intelligence in cardiology. Trends Cardiovasc. Med. 2022, 32, 34–41. [Google Scholar] [CrossRef]
- Chan, B. The rise of artificial intelligence and the crisis of moral passivity. AI Soc. 2020, 35, 991–993. [Google Scholar] [CrossRef]
- Bernard, O.; Lalande, A.; Zotti, C.; Cervenansky, F.; Yang, X.; Heng, P.-A.; Cetin, I.; Lekadir, K.; Camara, O.; Gonzalez Ballester, M.A.; et al. Deep Learning Techniques for Automatic MRI Cardiac Multi-Structures Segmentation and Diagnosis: Is the Problem Solved? IEEE Trans. Med. Imaging 2018, 37, 2514–2525. [Google Scholar] [CrossRef]
- Sander, J.; de Vos, B.D.; Išgum, I. Automatic segmentation with detection of local segmentation failures in cardiac MRI. Sci. Rep. 2020, 10, 21769. [Google Scholar] [CrossRef] [PubMed]
- Oktay, O.; Ferrante, E.; Kamnitsas, K.; Heinrich, M.; Bai, W.; Caballero, J.; Cook, S.A.; de Marvao, A.; Dawes, T.; O’Regan, D.P.; et al. Anatomically Constrained Neural Networks (ACNNs): Application to Cardiac Image Enhancement and Segmentation. IEEE Trans. Med. Imaging 2018, 37, 384–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, J.; Bello, G.; Schlemper, J.; Bai, W.; Dawes, T.J.W.; Biffi, C.; de Marvao, A.; Doumoud, G.; O’Regan, D.P.; Rueckert, D. Automatic 3D Bi-Ventricular Segmentation of Cardiac Images by a Shape-Refined Multi-Task Deep Learning Approach. IEEE Trans. Med. Imaging 2019, 38, 2151–2164. [Google Scholar] [CrossRef]
- Painchaud, N.; Skandarani, Y.; Judge, T.; Bernard, O.; Lalande, A.; Jodoin, P.-M. Cardiac Segmentation With Strong Anatomical Guarantees. IEEE Trans. Med. Imaging 2020, 39, 3703–3713. [Google Scholar] [CrossRef]
- Park, J.Y.; Noh, Y.; Choi, B.G.; Rha, S.W. CRT-100.48 Prediction of Acute Myocardial Infarction Using a Machine Learning-Based Approach From Data at Admission. JACC Cardiovasc. Interv. 2020, 13, S13. [Google Scholar] [CrossRef]
- Weidlich, V.; Weidlich, G.A. Artificial Intelligence in Medicine and Radiation Oncology. Cureus 2018, 10, e2475. [Google Scholar] [CrossRef] [Green Version]
- Pang, S.; Yu, Z.; Orgun, M.A. A novel end-to-end classifier using domain transferred deep convolutional neural networks for biomedical images. Comput. Methods Programs Biomed. 2017, 140, 283–293. [Google Scholar] [CrossRef]
- Tsubaki, M.; Tomii, K.; Sese, J. Compound–protein interaction prediction with end-to-end learning of neural networks for graphs and sequences. Bioinformatics 2019, 35, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Bai, C.; Zhao, X.-X.; Yuan, W.-F. Artificial Intelligence and Myocardial Contrast Enhancement Pattern. Curr. Cardiol. Rep. 2020, 22, 77. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.; Aggarwal, T.; Ward, C.; Bathla, G.; Jacob, M.; Gerke, A.; Hoffman, E.A.; Nagpal, P. Radiomics Detection of Pulmonary Hypertension via Texture-Based Assessments of Cardiac MRI: A Machine-Learning Model Comparison—Cardiac MRI Radiomics in Pulmonary Hypertension. J. Clin. Med. 2021, 10, 1921. [Google Scholar] [CrossRef]
- Apfaltrer, G.; Lavra, F.; De Cecco, C.N.; Varga-Szemes, A.; van Assen, M.; Mastrodicasa, D.; Scarabello, M.; Eid, M.H.; Griffith, L.P.; Nance, J.W.; et al. Predictive Value of Cardiac CTA, Cardiac MRI, and Transthoracic Echocardiography for Cardioembolic Stroke Recurrence. Am. J. Roentgenol. 2021, 217, 336–346. [Google Scholar] [CrossRef]
- Iwata, K.; Ogasawara, K. Assessment of non-invasive diagnostic imaging modalities efficiency for detecting myocardial ischemia in patients suspected of having stable angina. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Zhao, J.; Zhang, Y. A Myocardial Segmentation Method Based on Adversarial Learning. Biomed Res. Int. 2021, 2021, 6618918. [Google Scholar] [CrossRef] [PubMed]
- Uthoff, J.; Alabed, S.; Swift, A.J.; Lu, H. Geodesically smoothed tensor features for pulmonary hypertension prognosis using the heart and surrounding tissues. In Proceedings of the Medical Image Computing and Computer Assisted Intervention–MICCAI 2020: 23rd International Conference, Lima, Peru, 4–8 October 2020; Proceedings, Part II 23. Springer: Berlin/Heidelberg, Germany, 2020; pp. 253–262. [Google Scholar]
- Alis, D.; Guler, A.; Yergin, M.; Asmakutlu, O. Assessment of ventricular tachyarrhythmia in patients with hypertrophic cardiomyopathy with machine learning-based texture analysis of late gadolinium enhancement cardiac MRI. Diagn. Interv. Imaging 2020, 101, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Sparapani, R.; Dabbouseh, N.M.; Gutterman, D.; Zhang, J.; Chen, H.; Bluemke, D.A.; Lima, J.A.C.; Burke, G.L.; Soliman, E.Z. Detection of Left Ventricular Hypertrophy Using Bayesian Additive Regression Trees: The MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Heart Assoc. 2019, 8, e009959. [Google Scholar] [CrossRef]
- Hombach, V.; Merkle, N.; Kestler, H.A.; Torzewski, J.; Kochs, M.; Marx, N.; Nusser, T.; Burgstahler, C.; Rasche, V.; Bernhardt, P.; et al. Characterization of patients with acute chest pain using cardiac magnetic resonance imaging. Clin. Res. Cardiol. Suppl. 2010, 5, 63–69. [Google Scholar] [CrossRef]
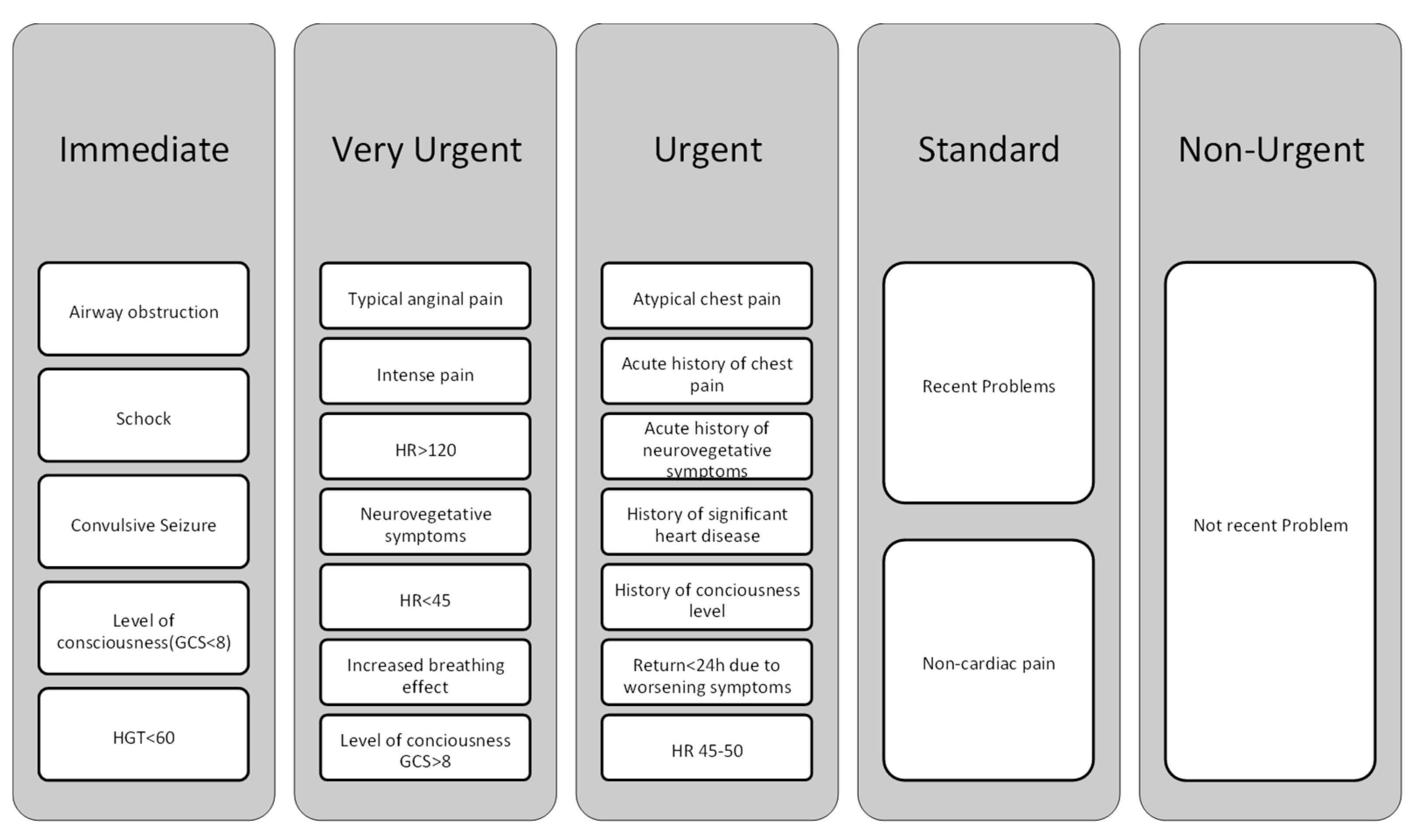

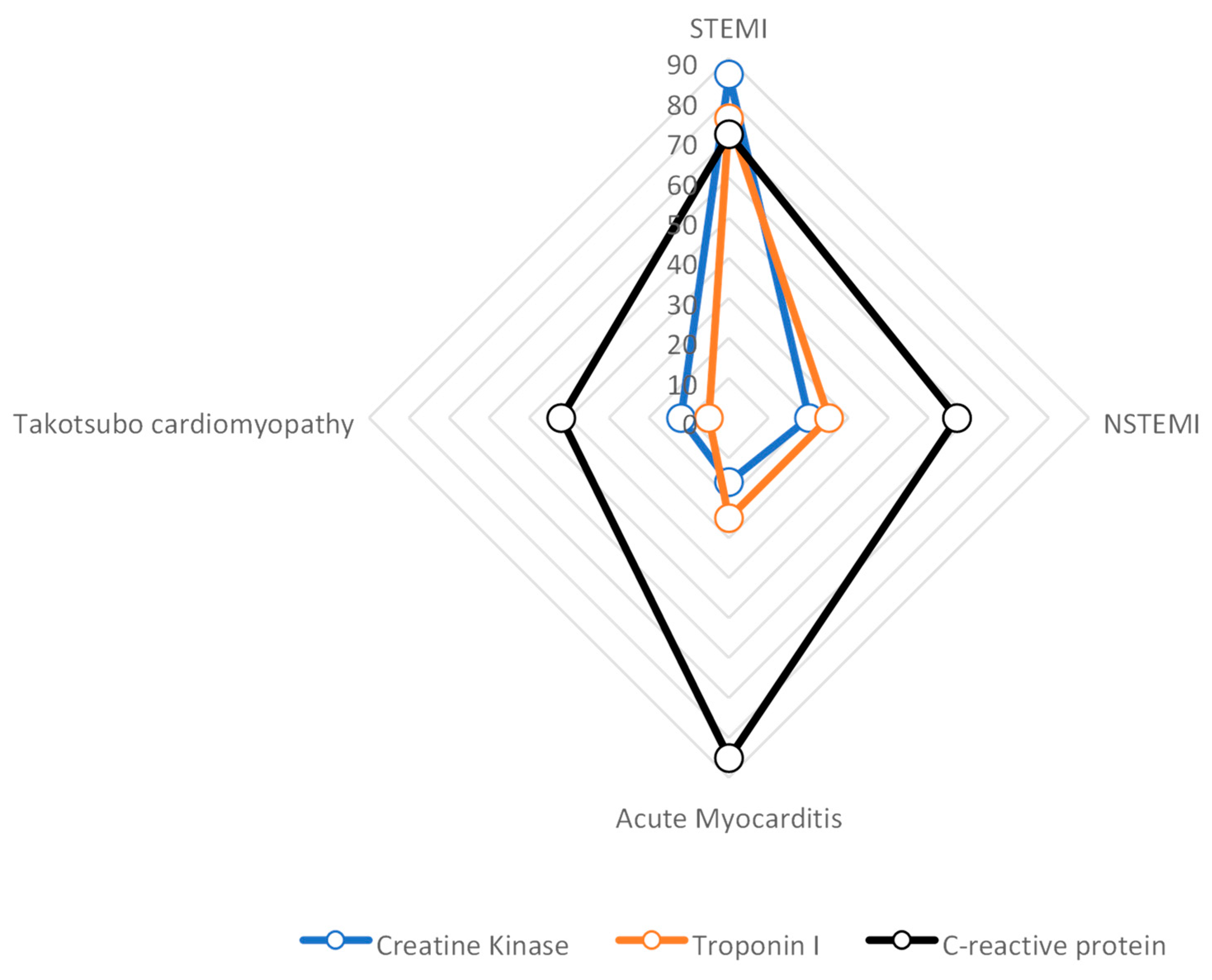
| Author/Year | Disease | Design of the Study | Number of Patients | Region | Aim | Results |
|---|---|---|---|---|---|---|
| Fagiry et al. [19], 2021 | Ischemic heart disease | The study was conducted at the Radiology and Medical Imaging Department, King Fahad Medical City (KFMC), Riyadh, Saudi Arabia. | This study was designed as a prospective cohort study | The study enrolled 100 patients with ischemic heart disease (IHD) | Explore CMRI’s usefulness in IHD diagnosis | CMRI showed sensitivity, specificity, and accuracy in diagnosing IHD compared to angiography |
| Simkowski et al. [22], 2020 | RVF | The study utilized a retrospective observational design | The study included a total of 31 patients who underwent pre-operative CMRI prior to receiving a LVAD | Not mentioned | Apply ML technique to detect LGE patterns in RV dysfunction post LVAD implantation | No significant association found between RVF and LGE in RV myocardium or insertion sites |
| Alsunbuli [23], 2020 | Congenital heart disease | The study utilized a qualitative approach to compare different imaging modalities for grown-up congenital heart diseases (GUCH); it involved a systematic search for evidence from resources such as PUBMED, EMBASE, ScienceDirect, CINAHL, NICE, ESC, ACC/AHA; the studies identified were analyzed for inclusion in the body of evidence | Not mentioned (the study focused on comparing different imaging modalities rather than involving a specific patient cohort) | Not mentioned | Compare imaging modalities and their limitations | CMRI is valuable for diagnosis and follow-up but has limitations with certain conditions and cost |
| Moonen et al. [25], 2020 | Anderson– Fabry disease | A cohort study including 16+ patients with left ventricular hypertrophy, idiopathic dysfunction, and arrhythmia; patients with unexplained late gadolinium enhancement were tested for Fabry disease using genetic testing or dried blood spot test | The study included a total of 79 patients with unexplained LGE on CMRI | Not mentioned | Investigate frequency of Anderson–Fabry disease in people with LGE on CMRI | Patchy mid-wall augmentation observed in inferoseptum region in people with LGE on CMRI |
| Groepenhoff et al. [26], 2020 | Coronary vascular disease | This study estimated coronary vascular disease prevalence in 45+ individuals with chest pain or discomfort, using electronic health record data and expert panel consensus classification | Not mentioned | Not mentioned | Evaluate macrovascular and microvascular CAD in patients with chest pain | Decision-support tool development underway to aid in assessing coronary artery disease based on data [27,28] |
| Grober et al. [24], 2018 | Cushing’s disease | The study evaluated pituitary MRI in Cushing’s disease patients using three techniques: DMRI, CMRI, and SGE, with anonymized annotations and independent reading by three clinicians | The study included 57 patients who had undergone surgery for Cushing’s disease; the patients consisted of 10 males and 47 females, with an age range of 13 to 69 years | Not mentioned | A blinded MRI of the pituitary gland in Cushing’s disease patients was performed | SGE showed better sensitivity for pituitary microadenoma identification in Cushing’s disease patients |
| Jalnapurkar et al. [21], 2017 | Ischemic heart disease | A retrospective analysis of female patients with ischemia and no CAD who underwent stress cardiac magnetic resonance imaging from 2006 to 2007 | The study analyzed 113 consecutive female patients who met the inclusion criteria | Not mentioned | Investigate stress CMRI in women with probable ischemia but no obstructive CAD | Subendocardial perfusion anomalies observed, indicating possible coronary microvascular dysfunction |
| Author/Year | Disease | Design of the Study | Number of Patients | Region | Aim | ML Method |
|---|---|---|---|---|---|---|
| Priya et al. [40], 2021 | Pulmonary hypertension | A pilot radiomics study analyzed 72 CMRI images from 42 patients with pulmonary hypertension and 30 healthy controls, evaluating texture features’ diagnostic performance in predicting PH using various classifier models | The study analyzed CMRIs from a total of 72 patients, including 42 patients with PH and 30 healthy controls | Not mentioned | Classification | Using ensemble models (ridge, elastic net, LASSO, random forest, GBRM, AdaBoost) for non-invasive imaging-based recognition |
| Apfaltrer et al. [41], 2021 | Cardioembolic stroke recurrence | A retrospective analysis of 151 patients with suspected cardioembolic stroke evaluated sensitivity, specificity, predictive value, and diagnostic accuracy using AUC | The study included a total of 151 patients with suspected cardioembolic stroke | Not mentioned | Prediction | Shapiro–Wilk test, chi-square test, the Fisher exact test |
| Iwata et al. [42], 2021 | Myocardial ischemia | Classification | Decision tree | |||
| Wang et al. [43], 2021 | Congenital heart defects | The study described in the article is a methodological study that proposes a myocardial segmentation algorithm based on adversarial learning; the authors conducted experiments using the HVSMR 2016 dataset to evaluate the performance of their proposed method | Not mentioned | Not mentioned | Segmentation | Generative adversarial networks (GAN), Gaussian mixture model, expectation maximization |
| Park et al. [35], 2020 | Acute myocardial infarction | A retrospective study used machine learning algorithms to predict acute myocardial infarction in 4070 patients with chest pain, analyzing data from coronary angiography between 2004 and 2014 | The study included a total of 4070 consecutive patients who had undergone CAG; the training set consisted of 3044 patients, while the test set included 1026 patients | Not mentioned | Classification | Using logistic regression, linear discriminant analysis, decision tree, K-nearest neighbor, support vector machine, and ensemble bagged tree |
| Sander et al. [31], 2020 | Heart failure resulting from myocardial infarction, dilated cardiomyopathy, hypertrophic cardiomyopathy, and abnormalities in the right ventricle | The study utilizes automatic segmentation and uncertainty assessment in CMRI to detect local segmentation failures in cardiac structures, using publicly available scans from the MICCAI 2017 ACDC challenge | The article mentions that the complete set of scans of 100 patients was used for simulating manual correction of the detected regions; additionally, a random subset of scans from 50 patients was manually corrected | Not mentioned | Segmentation | Convolutional neural networks |
| Uthoff et al. [44], 2020 | Pulmonary hypertension | The study developed a geodesically smoothed tensor (GST) feature-learning method for predicting pulmonary arterial hypertension using CMRI; it evaluated 150 individuals with confirmed PAH and 1-year mortality census, comparing GST method performance with RVESVi measurement | The study used CMRI scans from 150 individuals with confirmed PAH for evaluation; the article does not provide information on the comorbidities of the patients | Not mentioned | Classification | Geodesically smoothed tensor feature-learning method |
| Alis et al. [45], 2020 | Patients diagnosed with hypertrophic cardiomyopathy may experience ventricular tachyarrhythmia | A retrospective study of 64 hypertrophic cardiomyopathy patients used machine learning classifiers to predict ventricular tachyarrhythmia (VT) presence using extracted features; the results were assessed using 24 h Holter monitoring and the synthetic minority over-sampling technique (SMOTE) | The study included a total of 64 patients with hypertrophic cardiomyopathy | Not mentioned | Classification | Support vector machines, naive Bayes, k-nearest neighbors, and random forest |
| Painchaud et al. [34], 2019 | Delineation of the LV cavity, myocardium, and right ventricle from cardiac magnetic resonance images | The article presents a framework for cardiac image segmentation maps using CNN and cVAE, ensuring pre-defined criteria and inter-expert variability | Not mentioned | Not mentioned | Segmentation | Adversarial variational autoencoder, convolutional neural networks |
| Duan et al. [33], 2019 | Pulmonary hypertension | The study utilizes multi-task deep learning and atlas propagation to develop a segmentation pipeline using a fully convolutional network architecture, incorporating 2.5D representation and refinement steps | The article states that the pipeline was validated on 1831 healthy subjects and 649 subjects with pulmonary hypertension; these numbers represent the patient cohorts used to evaluate the proposed method | Not mentioned | Segmentation | Shape-refined multi-task deep learning approach |
| Sparapani et al. [46], 2019 | LV hypertrophy | The study used MESA cohort data to develop a new left ventricular hypertrophy criterion using Bayesian additive regression trees, comparing its diagnostic and prognostic performance with traditional ECG and imaging criteria | The analysis included 4714 participants from the MESA cohort | The study involved participants from MESA (Multi-Ethnic Study of Atherosclerosis), which is a multi-ethnic study conducted in the United States | Classification | Bayesian additive regression trees |
| Oktay et al. [32], 2017 | UK Digital Heart Project dataset | The study introduces anatomically constrained neural networks (ACNN) as a training strategy, incorporating anatomical prior knowledge into convolutional neural networks; the approach is evaluated on multi-modal cardiac datasets and public benchmarks | Not mentioned | Not mentioned | Segmentation | Anatomically constrained neural networks, convolutional neural networks |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zareiamand, H.; Darroudi, A.; Mohammadi, I.; Moravvej, S.V.; Danaei, S.; Alizadehsani, R. Cardiac Magnetic Resonance Imaging (CMRI) Applications in Patients with Chest Pain in the Emergency Department: A Narrative Review. Diagnostics 2023, 13, 2667. https://doi.org/10.3390/diagnostics13162667
Zareiamand H, Darroudi A, Mohammadi I, Moravvej SV, Danaei S, Alizadehsani R. Cardiac Magnetic Resonance Imaging (CMRI) Applications in Patients with Chest Pain in the Emergency Department: A Narrative Review. Diagnostics. 2023; 13(16):2667. https://doi.org/10.3390/diagnostics13162667
Chicago/Turabian StyleZareiamand, Hossein, Amin Darroudi, Iraj Mohammadi, Seyed Vahid Moravvej, Saba Danaei, and Roohallah Alizadehsani. 2023. "Cardiac Magnetic Resonance Imaging (CMRI) Applications in Patients with Chest Pain in the Emergency Department: A Narrative Review" Diagnostics 13, no. 16: 2667. https://doi.org/10.3390/diagnostics13162667






