Abstract
Purpose: Magnetic resonance elastography (MRE) has been established as the most accurate noninvasive technique for diagnosing liver fibrosis. Recent publications have suggested that the measurement of splenic stiffness is useful in setting where portal hypertension may be present. The goal of the current study was to compile normative data for MRE-assessed stiffness measurements of the spleen in young adults. Materials and Methods: A total of 100 healthy young Caucasian volunteers (65 females and 35 males) in the age range of 20 to 32 years were enrolled in this study. The participants reported no history of chronic spleen and liver disease, normal alcohol consumption, and a normal diet. The MRE data were acquired by using a 1.5 T whole-body scanner and a 2D GRE pulse sequence with 60 Hz excitation. Spleen stiffness was calculated as a weighted mean of stiffness values in the regions of interest manually drawn by the radiologist on three to five spleen slices. Results: Mean spleen stiffness was 5.09 ± 0.65 kPa for the whole group. Male volunteers had slightly higher splenic stiffness compared to females: 5.28 ± 0.78 vs. 4.98 ± 0.51 kPa, however, this difference was not statistically significant (p = 0.12). Spleen stiffness did not correlate with spleen fat content and liver stiffness but a statistically significant correlation with spleen volume was found. Conclusions: The findings of this study provide normative values for 2D MRE-based measurement of spleen stiffness in young adults, a basis for assessing the value of this biomarker in young patients with portal system pathologies.
1. Introduction
Magnetic resonance elastography (MRE) is an innovative diagnostic technique used to assess the stiffness of soft tissues in a non-invasive manner. MRE was developed by researchers at the Mayo Clinic in Rochester, USA, during the 1990s [1,2]. Initial scientific papers presenting the theoretical foundations of MRE, along with results from examinations using phantom and tissue samples, were published in 1995 in Science and in 1996 in Nature Medicine [1,2]. The first human studies on measuring liver stiffness using MRE were presented at the Annual Meetings of the International Society for Magnetic Resonance in Medicine (ISMRM) in 2004 and 2005 [3,4]. Subsequent research publications have further confirmed the effectiveness of MRE in diagnosing liver fibrosis [5,6,7,8,9].
From a physical perspective, MRE involves three steps. The first step is to induce shear waves in the targeted area. This is achieved by applying vibrations generated by an active acoustic driver located outside the diagnostic room. Typically, the vibration frequency ranges from 20 to 100 Hz [10]. The acoustic waves produced are transmitted to a disc-shaped passive acoustic driver in contact with the tissue via a connecting polyvinyl chloride tubing.
The second step is to image the shear waves propagating through the human body using MRI. In MRE, tissue movement induced by the driver is measured using a specific phase-contrast MRI sequence, such as gradient recalled echo or spin-echo echo-planar imaging. Motion-encoding gradient pairs (MEGs) are used to encode tissue displacements [10]. The MEGs can be applied in any direction.
MRE typically involves multiple image acquisitions at different time points of the vibration cycle to obtain 3 to 8 phase offsets between MEGs and mechanical oscillation equally distributed over the course of the motion cycle [10]. This allows for the visualization of shear waves propagating through the body and enables MRE to encode tissue displacements at the nanometer or micrometer level.
The final component of MRE is the application of an appropriate inversion algorithm to the images depicting shear wave propagation in the tissues. In this step, stiffness maps, also known as elastograms, are generated. Stiffness is usually reported as a shear modulus magnitude (|G*| or µ) or shear wave speed (SWS) [11].
The MRI scanner equipped with a commercially available, FDA-approved MRE setup automatically applies a multimodel direct inversion (MMDI) algorithm to the wave propagation images and generates elastograms. The scanner software also allows for the overlaying of the stiffness maps onto the corresponding magnitude anatomical images to facilitate the exam reading process.
Another crucial step in evaluating tissue mechanical properties using MRE is selecting a region of interest (ROI) on the stiffness map. The ROI is used to calculate the average stiffness of the assessed tissue. The ROI drawn by a radiologist can have various shapes, such as oval, round, or irregular. It is also recommended to avoid organ edges, focal lesions, vessels with a diameter exceeding 3 mm, and areas with wave interference and pulsation-related artifacts when drawing ROIs to ensure reliable inversion [12]. The final stiffness measurement result is usually reported as the mean value of stiffness in the region of interest, expressed in kilopascals (kPa) or meters per second (m/s).
Currently, magnetic resonance elastography is considered the most accurate and reliable non-invasive method for diagnosing and staging liver fibrosis in patients with chronic liver disease. It has the potential to serve as an alternative to expensive and invasive liver biopsy. Moreover, MRE enables the diagnosis of liver fibrosis even at early stages, when it is still reversible [13,14,15,16,17,18,19].
With ongoing research and advancements in technology, MRE holds promise for expanding its applications to a wide range of organs and conditions, ultimately improving patient care and outcomes. Researchers are investigating the clinical utility of MRE in evaluating other organs and tissues, such as the pancreas, kidney, prostate, uterus, brain, breast, and muscles [20,21,22,23,24,25,26,27,28,29].
MRE may also be of significant help in tumor diagnostics, as the mechanical properties of healthy tissue and different types of tumors usually vary. Its ability to provide quantitative measurements of tissue properties opens up new possibilities for diagnosing and monitoring various pathologies and diseases. These potential applications of MRE are supported by evidence demonstrating excellent reproducibility and repeatability [30,31,32,33,34].
An active area of research focuses on determining the usefulness of measuring spleen stiffness using MRE for detecting portal hypertension [35,36], staging liver cirrhosis [19,37], or predicting esophageal varices in chronic liver disease [38,39,40]. According to the study of Ronot et al., spleen stiffness may be the best biomarker for identifying patients with severe portal hypertension or high-risk esophageal varices [35]. Other research revealed that the combination of spleen stiffness and liver stiffness can increase sensitivity in the prediction of advanced liver fibrosis [37]. However, there is a need to establish reference data for these studies since information on MRE-based splenic stiffness in healthy individuals is currently limited [33,38]. Therefore, the aim of this work is to compile normative data for MRE-based spleen stiffness in a group of healthy young subjects.
2. Methods
The Ethics Committee of the Medical Department of the University of Rzeszow approved the study protocol (Resolution No 8/10/2016) and each participant provided written informed consent before being enrolled in this study. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008).
According to a priori power analysis, a sample size of 99 participants was required for this study (α = 0.05, power of 80%; estimated effect size of 0.4). However, more volunteers were recruited since, based on our experience, we expected 15 to 25% of participants not to meet the inclusion criteria. Finally, 100 out of 115 volunteers met the eligibility criteria and were involved in this study.
The following factors were taken into account when choosing the target group:
- Potential contraindications to MRI
Volunteers with pacemakers, drug infusion devices, metallic foreign bodies, or metallic ink tattoos were excluded from the study;
- History of liver and spleen disease of the volunteer and his or her familyOnly participants with no history of liver and spleen disease were selected;
- Liver function tests results
ASPAT: 17–59 U/Lfor male and 14–36 U/L for female, ALAT: <50 U/L for male and <35 U/L for female, Lipase: 23–300 U/L, liver iron: 49–181 µg/dL for male and 37–170 µg/dL for female;
- Alcohol consumption, diet type, and medicines taken by the individual
Each subject confirmed following a normal diet, consuming no more than 30 g of alcohol per day, and taking no medication;
- Liver stiffness and fat fraction values
Only volunteers with normal liver stiffness < 2.9 kPa and liver fat fraction < 5% were enrolled in this study;
- Body Mass Index (BMI)
Obese and severely obese participants were excluded from this study.
The MRE data were acquired at the University of Rzeszow’s Center for Medical and Natural Sciences Research and Innovation during the period from December 2018 until July 2019.
2.1. Study Protocol
A 1.5-T whole-body unit MR OPTIMA MR360 Advance (GE Healthcare, Milwaukee, Wisconsin, United States) was used in this study. The foot-first supine position was chosen for the examination with a drum-like passive driver placed approximately at the spleen level over the subject’s left side of the abdomen. The actuator was secured with an elastic strap and the 8-channel torso phased array coil was positioned above.
To acquire the spleen MRE data, a 2D modified gradient recalled echo-based MR Touch pulse sequence (GE Healthcare, Waukesha, WI, USA) with the following imaging parameters was applied: 60 Hz vibrations, 40 cm FOV, 33.3 ms TR, 20.5 ms TE, 224 × 64 acquisition matrix, 256 × 256 recon matrix size, 10 mm slice thickness, 30° flip angle, and 4 time offsets.
Eight to nine slices were imaged through the spleen during three to four breath holds at the end of expiration. The stiffness maps generated on the scanner were transferred offline for further analysis and measurements.
2.2. Image Analysis
The MRE data were analyzed simultaneously by an MRE technician with more than five years of experience and a radiologist with more than twenty years of experience. One or two irregular polygon ROI masks of the maximum possible area were manually drawn on three to five spleen slices on the stiffness maps (Figure 1). The weighted mean spleen stiffness value was then calculated for each subject. The spleen borders and large vessels were avoided when placing the ROIs to increase the inversion accuracy. The average size of the ROI for the spleen was 3 to 5 cm2.
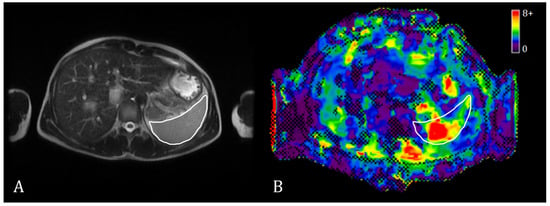
Figure 1.
Exemplary T2w MR image (A) and a stiffness map with a confidence mask (B) in spleen MRE. The spleen is outlined with a white contour.
2.3. Statistical Analysis
The mean (SD) values of the spleen stiffness were calculated based on the values from each slice. The Shapiro-Wilk test was performed to investigate the normality of the distribution of measurable variables. The correlation between spleen stiffness and other parameters, including spleen fat content and spleen volume was assessed by calculating Spearman rank correlation coefficients. A Mann-Whitney U-test was performed to determine whether the difference in spleen stiffness values in males and females was statistically significant.
Statistical significance was set at a p value < 0.05. The open-source JASP Statistical Software version 0.16.4 (University of Amsterdam, Amsterdam, The Netherlands; https://jasp-stats.org, accessed on 5 May 2023) was used for statistical modeling.
3. Results
A total of 15 subjects initially selected for the study did not meet all inclusion criteria, reducing the total cohort from 115 to 100 subjects. All 100 volunteers (65 females and 35 males) had successful MRE examinations without reporting any discomfort during the data acquisition.
The calculated mean [SD] spleen stiffness value in the cohort was 5.09 [0.65] kPa (95% CI, 4.97–5.23), ranging from 3.89 to 7.24 kPa.
The BMI was in the range of 16.85–25.76 kg/m2 with a mean [SD] of 21.33 [2.16] kg/m2. The spleen volume ranged from 123.94 to 422.43 cm3 while the mean [SD] value was 231.13 [58.44] cm3.
The difference in splenic stiffness between female (mean [SD], 4.98 [0.51] kPa; range 3.89–6.03 kPa) and male volunteers (mean [SD], 5.28 [0.78] kPa; range 3.99–7.24 kPa) was not significant statistically (p = 0.12) (Figure 2).
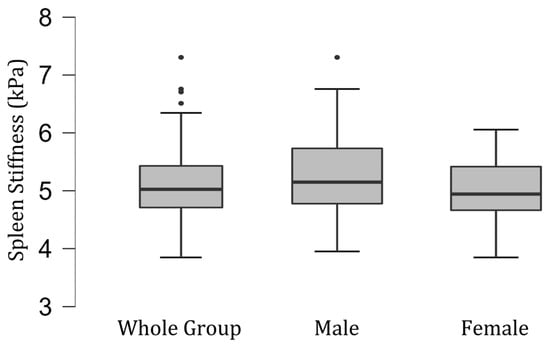
Figure 2.
Box and whisker plots of the distribution of measured spleen stiffness values (in kilopascals) in the group of 100 healthy volunteers—35 men and 65 women.
The mean [SD] spleen fat content value was 2.04 [0.39]%, ranging from 1.15% to 3.27%, and no correlation with spleen stiffness was found (rho= −0.13; p = 0.20) (Figure 3). Spleen stiffness did not correlate with spleen R2* (rho = 0.03, p = 0.74), BMI (rho = 0.09; p = 0.36) (Figure 4), and liver stiffness (rho = 0.01; p = 0.95) (Figure 5). However, spleen stiffness was found to be positively correlated with spleen volume, and this relationship was statistically significant (rho = 0.40, p < 0.001) (Figure 6). The results are summarized in the Table 1.
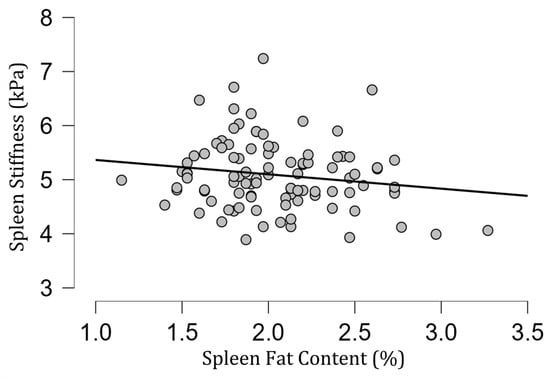
Figure 3.
A scatter plot showing the correlation between spleen stiffness (in kilopascals) and spleen fat content.
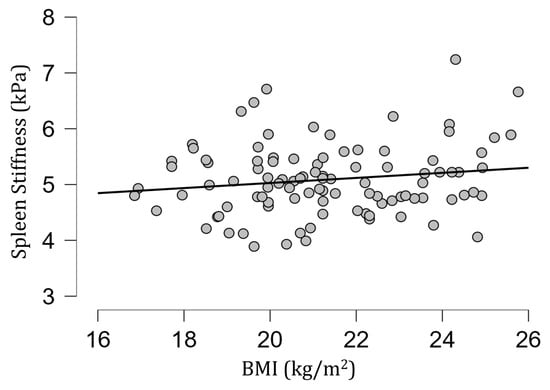
Figure 4.
A scatter plot showing the correlation between spleen stiffness (in kilopascals) and BMI. No significant correlation was observed.
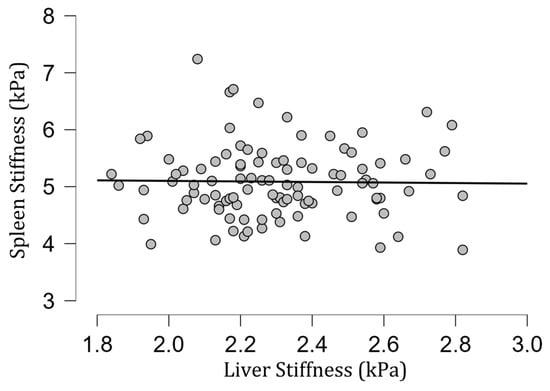
Figure 5.
A scatter plot showing the correlation between spleen and liver stiffness (in kilopascals).
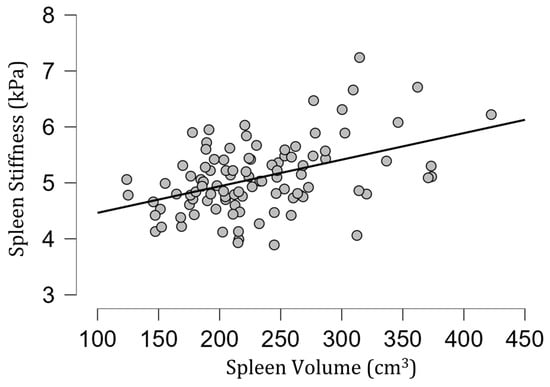
Figure 6.
A scatter plot of spleen stiffness (in kilopascals) versus spleen volume. A statistically significant positive correlation between these two parameters was found (rho = 0.40, p < 0.001).

Table 1.
A brief summary of the key results of this work.
4. Discussion
The spleen stiffness obtained in the group of healthy volunteers ranged from 3.89 to 7.24 kPa with a mean value of 5.09 kPa. These results show that the normal spleen is stiffer than the normal liver, pancreas, prostate, and uterus [20,41,42,43,44,45].
The spleen stiffness value from our study was higher than in a group of 12 volunteers recruited by Talwalkar et al.: 3.6 ± 0.3 kPa [46]. Other research, involving sixteen volunteers, reports a mean splenic stiffness of 4.255 ± 0.625 kPa [47]. In another study, Yasar et al., examined 12 subjects (5 healthy volunteers and 7 patients with chronic liver disease) to assess inter-platform reproducibility of liver and spleen stiffness measurement by MRE [33]. The mean value of spleen stiffness was reported for the whole group and reached 7.54 to 7.91 kPa. There was no differentiation between healthy and ill participants, so the findings cannot be compared to the current results. Another retrospective study reported a mean splenic stiffness value of 5.2 ± 1.5 kPa in a sub-group of 115 patients not displaying symptoms of chronic liver disease [38]. This value is comparable to our own findings but was obtained based on a retrospective analysis of medical records.
The splenic stiffness was higher in men than in women but this difference did not show statistical significance. Prior reports have been mixed in this regard [46,47].
In contrast to the results of Talwalkar et al. [46], the spleen stiffness obtained in the group under investigation did not correlate with liver stiffness.
The estimated fat content in the spleen and spleen R2* also did not impact its stiffness in healthy volunteers. On the other hand, we observed a statistically significant correlation between spleen stiffness and spleen volume in healthy volunteers.
Alternative shear wave-based methods for elasticity assessment of the spleen are ultrasound-based transient elastography (TE), point shear wave elastography (pSWE), and 2D and 3D shear wave elastography (SWE) [48,49]. The available data on splenic stiffness in healthy volunteers derived from US-based elastography are mixed. In a study by Arda et al., a mean SWE-based spleen stiffness value of 2.9 ± 1.8 kPa was revealed [50]. Takuma et al., obtained spleen stiffness of 2.16 (IQR, 1.99–2.26) m/s measured by acoustic radiation force impulse shear wave imaging in a group of 16 healthy volunteers [51]. Leung et al., reported a mean value of SWE-based spleen stiffness of 17.3 ± 2.6 kPa in a group of 171 healthy individuals [52]. The mean value of splenic stiffness measured by Pawluś et al., was determined as 16.6 ± 2.5 kPa [53]. Giuffrè et al., using point shear wave elastography, obtained a mean splenic stiffness value of 18.14 ± 3.08 kPa [54]. There were no statistically significant differences in stiffness values between sexes [50,52,53,54]. Also, no correlation between splenic stiffness and spleen size was found [53,54].
It is worth emphasizing that ultrasound-based elastography techniques differ from each other and MRE in terms of technological and physical details as well as methods of data acquisition [53]. Hence, there is no possibility to compare or extrapolate the findings obtained by using different diagnostic modalities.
The presented study has several limitations. In this project, we focused on assessing the spleen stiffness of healthy young adults, therefore, the age range of the subjects was relatively narrow. Future studies including individuals within a wider age range should be conducted to verify the results. The study included exclusively Caucasian volunteers, which might be also perceived as a limitation. Finally, the study was conducted with a 2D MRE technique, which has known limitations compared with 3D MRE for splenic stiffness measurement but the latter technique is not yet widely available.
The current research is the largest prospective study compared with all other reports on this topic. Another strength of the presented study is the meticulous selection of participants, which increases the credibility of the obtained findings.
The findings of this study provide normative values for 2D MRE-based measurement of spleen stiffness, a basis for assessing the value of this biomarker in young patients with portal system pathologies.
Author Contributions
Conceptualization, M.O., M.C., K.G. and B.O.; Methodology, M.O. and R.L.E.; Software, V.A. and D.O.; Validation, M.O., R.L.E., M.Y., W.D. and B.O.; Formal analysis, M.O. and R.L.E.; Investigation, M.O. and V.A.; Resources, M.O. and K.G.; Data curation, V.A.; Writing—original draft, M.O., V.A., D.O. and B.O.; Writing—review & editing, M.O., M.Y., M.C., K.G., W.D. and B.O.; Visualization, V.A.; Supervision, R.L.E. and B.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Ethics Committee of the Medical Department of the University of Rzeszow approved the study protocol (Resolution No 8/10/2016). The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The Mayo Clinic, R.L.E. and M.Y. have intellectual property rights and a financial interest in magnetic resonance technology. The remaining authors declare no conflict of interest.
References
- Muthupillai, R.; Ehman, R.L. Magnetic resonance elastography. Nat. Med. 1996, 2, 601–603. [Google Scholar] [CrossRef]
- Muthupillai, R.; Lomas, D.J.; Rossman, P.J.; Greenleaf, J.F.; Manduca, A.; Ehman, R.L. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science 1995, 269, 1854–1857. [Google Scholar] [CrossRef]
- Dresner, M.A.; Fidler, J.L.; Ehman, R.L. MR elastography of in vivo human liver. In Proceedings of the 12th Annual Meeting of the International Society for Magnetic Resonance in Medicine, Kyoto, Japan, 12–17 May 2004; International Society for Magnetic Resonance in Medicine: Berkeley, CA, USA, 2004; p. 502. [Google Scholar]
- Rouvière, O.; Yin, M.; Dresner, M.A.; Rossman, P.J.; Burgart, L.J.; Fidler, J.L.; Ehman, R.L. In Vivo MR Elastography of the Liver: Preliminary Results. In Proceedings of the 13th Annual Meeting of the International Society for Magnetic Resonance in Medicine, Miami, FL, USA, 7–13 May 2005; International Society for Magnetic Resonance in Medicine: Berkeley, CA, USA, 2005; p. 340. [Google Scholar]
- Huwart, L.; Peeters, F.; Sinkus, R.; Annet, L.; Salameh, N.; ter Beek, L.C.; Horsmans, Y.; Van Beers, B.E. Liver fibrosis: Non-invasive assessment with MR elastography. NMR Biomed. 2006, 19, 173–179. [Google Scholar] [CrossRef]
- Klatt, D.; Asbach, P.; Rump, J.; Papazoglou, S.; Somasundaram, R.; Modrow, J.; Braun, J.; Sack, I. In vivo determination of hepatic stiffness using steady-state free precession magnetic resonance elastography. Investig. Radiol. 2006, 41, 841–848. [Google Scholar] [CrossRef]
- Rouvière, O.; Yin, M.; Dresner, M.A.; Rossman, P.J.; Burgart, L.J.; Fidler, J.L.; Ehman, R.L. MR elastography of the liver: Preliminary results. Radiology 2006, 240, 440–448. [Google Scholar] [CrossRef]
- Serai, S.D.; Obuchowski, N.A.; Venkatesh, S.K.; Sirlin, C.B.; Miller, F.H.; Ashton, E.; Cole, P.E.; Ehman, R.L. Repeatability of MR Elastography of Liver: A Meta-Analysis. Radiology 2017, 285, 92–100. [Google Scholar] [CrossRef]
- Singh, S.; Venkatesh, S.K.; Wang, Z.; Miller, F.H.; Motosugi, U.; Low, R.N.; Hassanein, T.; Asbach, P.; Godfrey, E.M.; Yin, M.; et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: A systematic review and meta-analysis of individual participant data. Clin. Gastroenterol. Hepatol. 2015, 13, 440–451.e6. [Google Scholar] [CrossRef]
- Manduca, A.; Bayly, P.V.; Ehman, R.L.; Kolipaka, A.; Royston, T.J.; Sack, I.; Sinkus, R.; Van Beers, B.E. MR elastography: Principles, guidelines, and terminology. Magn. Reson. Med. 2021, 85, 2377–2390. [Google Scholar] [CrossRef]
- Reeder, S.B. Emergence of 3D MR Elastography–based Quantitative Markers for Diffuse Liver Disease. Radiology 2021, 301, 163–165. [Google Scholar] [CrossRef]
- Venkatesh, S.K.; Ehman, R.L. Magnetic resonance elastography of liver. Magn. Reson. Imaging Clin. N. A. 2014, 22, 433–446. [Google Scholar] [CrossRef]
- Ehman, R.L. Magnetic resonance elastography: From invention to standard of care. Abdom. Radiol. 2022, 47, 3028–3036. [Google Scholar] [CrossRef]
- Hoodeshenas, S.; Yin, M.; Venkatesh, S.K. Magnetic Resonance Elastography of Liver: Current Update. Top. Magn. Reson. Imaging 2018, 27, 319–333. [Google Scholar] [CrossRef]
- Li, J.; Venkatesh, S.K.; Yin, M. Advances in Magnetic Resonance Elastography of Liver. Magn. Reson. Imaging Clin. N. A. 2020, 28, 331–340. [Google Scholar] [CrossRef]
- Liang, Y.; Li, D. Magnetic resonance elastography in staging liver fibrosis in non-alcoholic fatty liver disease: A pooled analysis of the diagnostic accuracy. BMC Gastroenterol. 2020, 20, 89. [Google Scholar] [CrossRef]
- Morisaka, H.; Motosugi, U.; Ichikawa, S.; Nakazawa, T.; Kondo, T.; Funayama, S.; Matsuda, M.; Ichikawa, T.; Onishi, H. Magnetic resonance elastography is as accurate as liver biopsy for liver fibrosis staging. J. Magn. Reson. Imaging 2018, 47, 1268–1275. [Google Scholar] [CrossRef]
- Nielsen, J.; Kjær, M.S.; Rasmussen, A.; Chiranth, D.; Willemoe, G.L.; Henriksen, B.M.; Borgwardt, L.; Grand, M.K.; Borgwardt, L.; Christensen, V.B. Noninvasive Prediction of Advanced Fibrosis in Pediatric Liver Disease-Discriminatory Performance of 2D Shear Wave Elastography, Transient Elastography and Magnetic Resonance Elastography in Comparison to Histopathology. Diagnostics 2022, 12, 2785. [Google Scholar] [CrossRef]
- Selvaraj, E.A.; Mózes, F.E.; Jayaswal, A.N.A.; Zafarmand, M.H.; Vali, Y.; Lee, J.A.; Levick, C.K.; Young, L.A.J.; Palaniyappan, N.; Liu, C.-H.; et al. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: A systematic review and meta-analysis. J. Hepatol. 2021, 75, 770–785. [Google Scholar] [CrossRef]
- Bae, J.S.; Lee, J.M.; Park, S.J.; Lee, K.B.; Han, J.K. Magnetic resonance elastography of healthy livers at 3.0 T: Normal liver stiffness measured by SE-EPI and GRE. Eur. J. Radiol. 2018, 107, 46–53. [Google Scholar] [CrossRef]
- Svensson, S.F.; Fuster-Garcia, E.; Latysheva, A.; Fraser-Green, J.; Nordhøy, W.; Darwish, O.I.; Hovden, I.T.; Holm, S.; Vik-Mo, E.O.; Sinkus, R.; et al. Decreased tissue stiffness in glioblastoma by MR elastography is associated with increased cerebral blood flow. Eur. J. Radiol. 2022, 147, 110136. [Google Scholar] [CrossRef]
- Hsieh, T.J.; Chou, M.C.; Chen, Y.C.; Chou, Y.C.; Lin, C.H.; Chen, C.K. Reliability of Gradient-Echo Magnetic Resonance Elastography of Lumbar Muscles: Phantom and Clinical Studies. Diagnostics 2022, 12, 1385. [Google Scholar] [CrossRef]
- Jondal, D.E.; Wang, J.; Chen, J.; Gorny, K.R.; Felmlee, J.; Hesly, G.; Laughlin-Tommaso, S.; Stewart, E.A.; Ehman, R.; Woodrum, D.A. Uterine fibroids: Correlations between MRI appearance and stiffness via magnetic resonance elastography. Abdom. Radiol. 2018, 43, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.C.; Huston, J., 3rd; Ehman, R.L. MR elastography of the brain and its application in neurological diseases. Neuroimage 2019, 187, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Obrzut, M.; Obrzut, B.; Zmuda, M.; Baran, J.; Cholewa, M.; Ehman, R.; Darmochwal-Kolarz, D. Uterine leiomyomas: Correlation between histologic composition and stiffness via magnetic resonance elastography—A Pilot Study. Ginekol. Pol. 2020, 91, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Plaikner, M.; Kremser, C.; Zoller, H.; Kannengiesser, S.; Henninger, B. MR elastography in patients with suspected diffuse liver disease at 1.5T: Intraindividual comparison of gradient-recalled echo versus spin-echo echo-planar imaging sequences and investigation of potential confounding factors. Eur. J. Radiol. 2021, 142, 109898. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Y.; Jondal, D.; Woodrum, D.M.; Shi, Y.; Yin, M.; Venkatesh, S.K. New and Emerging Applications of Magnetic Resonance Elastography of Other Abdominal Organs. Top. Magn. Reson. Imaging 2018, 27, 335–352. [Google Scholar] [CrossRef]
- Yang, J.Y.; Qiu, B.S. The Advance of Magnetic Resonance Elastography in Tumor Diagnosis. Front. Oncol. 2021, 11, 722703. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Jiang, H.; Rong, D.; Guo, N.; Yang, H.; Zhu, J.; Hu, B.; He, B.; Yin, M.; et al. MR elastography as a biomarker for prediction of early and late recurrence in HBV-related hepatocellular carcinoma patients before hepatectomy. Eur. J. Radiol. 2022, 152, 110340. [Google Scholar] [CrossRef]
- Pagé, G.; Garteiser, P.; Van Beers, B.E. Magnetic resonance elastography of malignant tumors. Front. Phys. 2022, 10, 910036. [Google Scholar] [CrossRef]
- Pepin, K.M.; Ehman, R.L.; McGee, K.P. Magnetic resonance elastography (MRE) in cancer: Technique, analysis, and applications. Prog. Nucl. Magn. Reson. Spectrosc. 2015, 90–91, 32–48. [Google Scholar] [CrossRef]
- Runge, J.H.; Bohte, A.E.; Verheij, J.; Terpstra, V.; Nederveen, A.J.; van Nieuwkerk, K.M.J.; de Knegt, R.J.; Baak, B.C.; Jansen, P.L.M.; Sinkus, R.; et al. Comparison of interobserver agreement of magnetic resonance elastography with histopathological staging of liver fibrosis. Abdom. Imaging 2014, 39, 283–290. [Google Scholar] [CrossRef]
- Yasar, T.K.; Wagner, M.; Bane, O.; Besa, C.; Babb, J.S.; Kannengiesser, S.; Fung, M.; Ehman, R.L.; Taouli, B. Interplatform reproducibility of liver and spleen stiffness measured with MR elastography. J. Magn. Reson. Imaging 2016, 43, 1064–1072. [Google Scholar] [CrossRef]
- Zhang, L.; Long, X.; Nijiati, M.; Zhang, T.; Li, M.; Deng, Y.; Kuang, S.; Xiao, Y.; Zhu, J.; He, B.; et al. Tumor stiffness measured by 3D magnetic resonance elastography can help predict the aggressiveness of endometrial carcinoma: Preliminary findings. Cancer Imaging 2021, 21, 50. [Google Scholar] [CrossRef] [PubMed]
- Ronot, M.; Lambert, S.; Elkrief, L.; Doblas, S.; Rautou, P.-E.; Castera, L.; Vilgrain, V.; Sinkus, R.; Van Beers, B.E.; Garteiser, P. Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. Eur. Radiol. 2014, 24, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Kolipaka, A.; Woodrum, D.A.; Glaser, K.J.; Romano, A.J.; Manduca, A.; Talwalkar, J.A.; Araoz, P.A.; McGee, K.P.; Anavekar, N.S.; et al. Hepatic and splenic stiffness augmentation assessed with MR elastography in an in vivo porcine portal hypertension model. J. Magn. Reson. Imaging 2013, 38, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.W.; Chang, Y.C.; Chen, Y.L.; Chen, R.C.; Chou, C.T. Feasibility of measuring spleen stiffness with MR elastography and splenic volume to predict hepatic fibrosis stage. PLoS ONE 2019, 14, e0217876. [Google Scholar] [CrossRef]
- Jhang, Z.E.; Wu, K.L.; Chen, C.B.; Chen, Y.L.; Lin, P.Y.; Chou, C.T. Diagnostic value of spleen stiffness by magnetic resonance elastography for prediction of esophageal varices in cirrhotic patients. Abdom. Radiol. 2021, 46, 526–533. [Google Scholar] [CrossRef]
- Morisaka, H.; Motosugi, U.; Ichikawa, S.; Sano, K.; Ichikawa, T.; Enomoto, N. Association of splenic MR elastographic findings with gastroesophageal varices in patients with chronic liver disease. J. Magn. Reson. Imaging 2015, 41, 117–124. [Google Scholar] [CrossRef]
- Shin, S.U.; Yu, M.H.; Yoon, J.H.; Han, J.K.; Choi, B.-I.; Glaser, K.J.; Ehman, R.L.; Lee, J.M.; Kang, H.-J.; Ahn, S.J.; et al. Prediction of esophageal varices in patients with cirrhosis: Usefulness of three-dimensional MR elastography with echo-planar imaging technique. Radiology 2014, 272, 143–153. [Google Scholar] [CrossRef]
- Jiang, X.; Asbach, P.; Streitberger, K.-J.; Thomas, A.; Hamm, B.; Braun, J.; Sack, I.; Guo, J. In vivo high-resolution magnetic resonance elastography of the uterine corpus and cervix. Eur. Radiol. 2014, 24, 3025–3033. [Google Scholar] [CrossRef]
- Kemper, J.; Sinkus, R.; Lorenzen, J.; Nolte-Ernsting, C.; Stork, A.; Adam, G. MR elastography of the prostate: Initial in-vivo application. Rofo 2004, 176, 1094–1099. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.M.; Han, J.K.; Choi, B.I. MR elastography of healthy liver parenchyma: Normal value and reliability of the liver stiffness value measurement. J. Magn. Reson. Imaging 2013, 38, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Obrzut, M.; Atamaniuk, V.; Obrzut, B.; Ehman, R.; Cholewa, M.; Rzucidło, M.; Pozaruk, A.; Gutkowski, K. Normative values for magnetic resonance elastography-based liver stiffness in a healthy population. Pol. Arch. Intern. Med. 2019, 129, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Glaser, K.J.; Venkatesh, S.K.; Ben-Abraham, E.I.; Ehman, R.L. Feasibility of using 3D MR elastography to determine pancreatic stiffness in healthy volunteers. J. Magn. Reson. Imaging 2015, 41, 369–375. [Google Scholar] [CrossRef]
- Talwalkar, J.A.; Yin, M.; Venkatesh, S.K.; Rossman, P.J.; Grimm, R.C.; Manduca, A.; Romano, A.; Kamath, P.S.; Ehman, R.L. Feasibility of in vivo MR elastographic splenic stiffness measurements in the assessment of portal hypertension. Am. J. Roentgenol. 2009, 193, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, L.; Godfrey, E.; Joubert, I.; Patterson, A.J.; Graves, M.J.; Gallagher, F.A.; Lomas, D.J. MR elastography: Spleen stiffness measurements in healthy volunteers—Preliminary experience. Am. J. Roentgenol. 2010, 195, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Bamber, J.; Cosgrove, D.; Dietrich, C.F.; Fromageau, J.; Bojunga, J.; Calliada, F.; Cantisani, V.; Correas, J.-M.; D’Onofrio, M.; Drakonaki, E.E.; et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013, 34, 169–184. [Google Scholar] [CrossRef]
- Cosgrove, D.; Piscaglia, F.; Bamber, J.; Bojunga, J.; Correas, J.-M.; Gilja, O.H.; Klauser, A.S.; Sporea, I.; Calliada, F.; Cantisani, V.; et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013, 34, 238–253. [Google Scholar] [CrossRef]
- Arda, K.; Ciledag, N.; Aktas, E.; Aribas, B.K.; Kose, K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. Am. J. Roentgenol. 2011, 197, 532–536. [Google Scholar] [CrossRef]
- Takuma, Y.; Nouso, K.; Morimoto, Y.; Tomokuni, J.; Sahara, A.; Toshikuni, N.; Takabatake, H.; Shimomura, H.; Doi, A.; Sakakibara, I.; et al. Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology 2013, 144, 92–101.e2. [Google Scholar] [CrossRef]
- Leung, V.Y.-F.; Shen, J.; Wong, V.W.-S.; Abrigo, J.; Wong, G.L.-H.; Chim, A.M.-L.; Chu, S.H.-T.; Chan, A.W.-H.; Choi, P.C.-L.; Ahuja, A.T.; et al. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: Comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology 2013, 269, 910–918. [Google Scholar] [CrossRef]
- Pawluś, A.; Inglot, M.S.; Szymańska, K.; Kaczorowski, K.; Markiewicz, B.D.; Kaczorowska, A.; Gąsiorowski, J.; Szymczak, A.; Inglot, M.; Bladowska, J.; et al. Shear wave elastography of the spleen: Evaluation of spleen stiffness in healthy volunteers. Abdom. Radiol. 2016, 41, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, M.; Macor, D.; Masutti, F.; Abazia, C.; Tinè, F.; Patti, R.; Buonocore, M.R.; Colombo, A.; Visintin, A.; Campigotto, M.; et al. Evaluation of spleen stiffness in healthy volunteers using point shear wave elastography. Ann. Hepatol. 2019, 18, 736–741. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).