Second Opinion for Non-Surgical Root Canal Treatment Prognosis Using Machine Learning Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample
- A general and dental clinical history with reports of general, facial, and oral inspection, as well as dental inspection, percussion and palpation;
- Results of a complementary thermal test with an ice pencil and periapical radiography; and
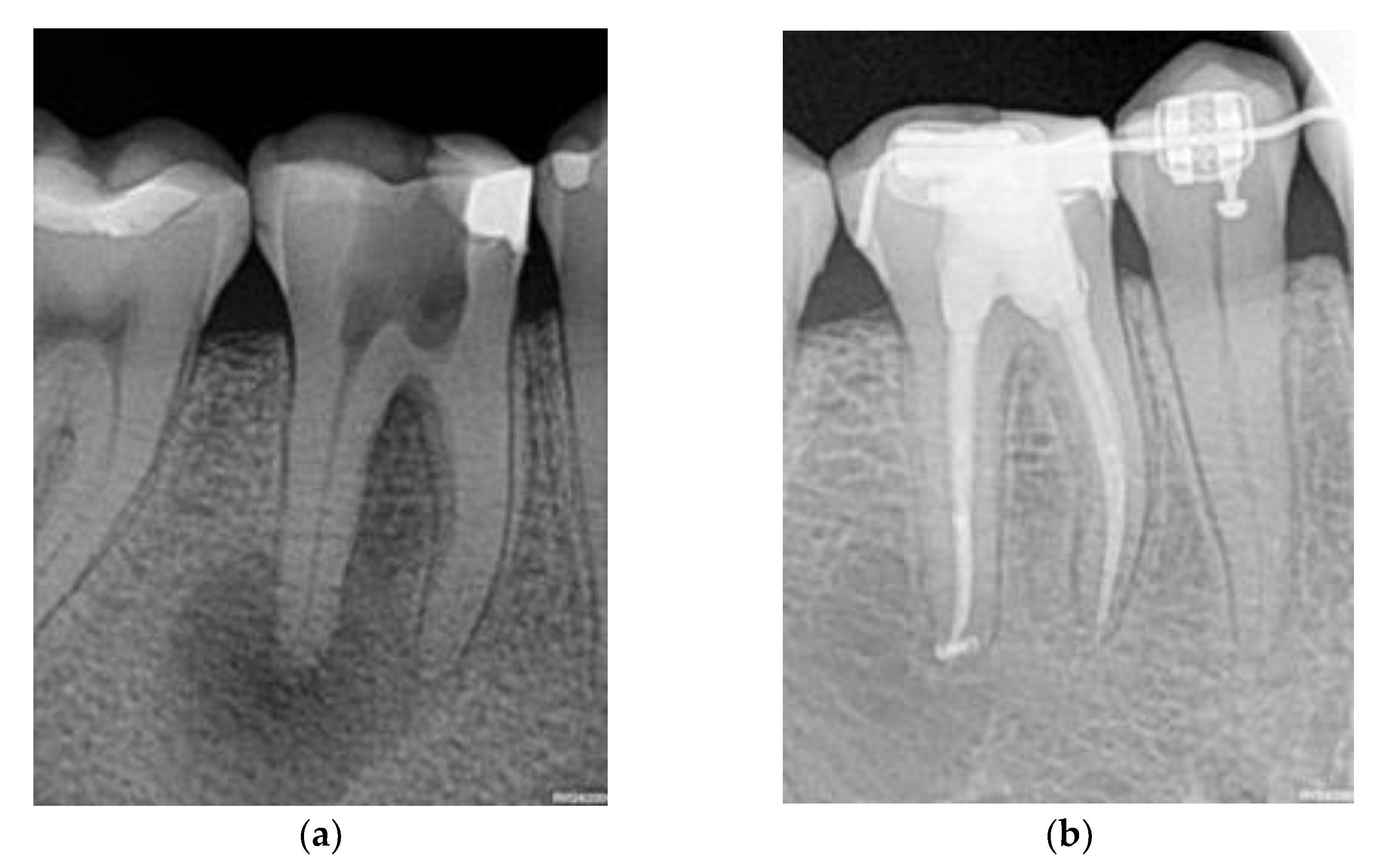
- At least nine years of follow-up data for each patient, during which the dentist recorded the cases with a favorable (or unfavorable) recovery process towards recovery after performing the following procedure: a clinical examination measuring suppuration or functional incapacity and comparison of the diagnostic periapical radiography with a control one, to determine whether there had been a lessening in the lesion’s size.
2.2. Intervention
2.3. Variables and Outcome
2.4. Statistical Analysis and ML Models
3. Results
3.1. Association Analysis
3.2. Outcome Prediction
4. Discussion
- Would a dentist who regularly performs NSRCT for AP cases, following the same protocol, using the same materials, and having a database where all the variables included in the DCT have been recorded, benefit from using ML algorithms as a second opinion on treatment prognosis?
- Would it be feasible to integrate this second opinion tool in a clinical setting?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tiburcio-Machado, C.S.; Michelon, C.; Zanatta, F.B.; Gomes, M.S.; Marin, J.A.; Bier, C.A. The global prevalence of apical periodontitis: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 712–735. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwenhuysen, J.P.; D’Hoore, W.; Leprince, J.G. What ultimately matters in root canal treatment success and tooth preservation: A 25- year cohort study. Int. Endod. J. 2023, 56, 544–557. [Google Scholar] [CrossRef]
- Segura-Egea, J.J.; Cabanillas-Balsera, D.; Martín-González, J.; Cintra, L.T.A. Impact of systemic health on treatment outcomes in endodontics. Int. Endod. J. 2022, 56, 219–235. [Google Scholar] [CrossRef]
- Jakovljevic, A.; Nikolic, N.; Jacimovic, J.; Pavlovic, O.; Milicic, B.; Beljic-Ivanovic, K.; Miletic, M.; Andric, M.; Milasin, J. Prevalence of Apical Periodontitis and Conventional Nonsurgical Root Canal Treatment in General Adult Population: An Updated Systematic Review and Meta-analysis of Cross-sectional Studies Published between 2012–2020. J. Endod. 2020, 46, 1371.e8–1386.e8. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Lin, Z.; Yang, Z.; Lin, H.; Huang, X.; Gu, L. Machine learning models for prognosis prediction in endodontic microsurgery. J. Dent. 2022, 118, 103947. [Google Scholar] [CrossRef]
- Cui, Q.; Chen, Q.; Liu, P.; Liu, D.; Wen, Z. Clinical decision support model for tooth extraction therapy derived from electronic dental records. J. Prosthet. Dent. 2021, 126, 83–90. [Google Scholar] [CrossRef]
- Shen, S.; Liu, Z.; Wang, J.; Fan, L.; Ji, F.; Tao, J. Machine learning assisted Cameriere method for dental age estimation. BMC Oral Health 2021, 21, 64. [Google Scholar] [CrossRef]
- Bura, A.M.; Holcomba, A.; Goodwina, S.; Woodroof, J.; Karadaghy, O.; Shnayder, Y.; Kakarala, K.; Brant, J.; Shew, M. Machine learning to predict occult nodal metastasis in early oral squamous cell carcinoma. Oral Oncol. 2019, 92, 20–25. [Google Scholar] [CrossRef]
- Elani, H.W.; Batista, A.F.M.; Thomson, W.M.; Kawachi, I.; Chiavegatto Filho, A.D.P. Predictors of tooth loss: A machine learning approach. PLoS ONE 2021, 16, e0252873. [Google Scholar] [CrossRef]
- Lee, S.J.; Chung, D.; Asano, A.; Sasaki, D.; Maeno, M.; Ishida, Y.; Kobayashi, T.; Kuwajima, Y.; Da Silva, J.D.; Nagai, S. Diagnosis of Tooth Prognosis Using Artificial Intelligence. Diagnostics 2022, 12, 1422. [Google Scholar] [CrossRef]
- Huang, H.Y.; Broughton, M.; Mohseni, M.; Babbush, R.; Boixo, S.; Neven, H.; McClean, J.R. Power of data in quantum machine learning. Nat. Commun. 2021, 12, 2631. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; De Filippo, O.; Gallone, G.; Mittone, G.; Deriu, M.A.; Iannaccone, M.; Ariza-Solé, A.; Liebetrau, C.; Manzano-Fernández, S.; Quadri, G.; et al. Machine learning-based prediction of adverse events following an acute coronary syndrome (PRAISE): A modelling study of pooled datasets. Lancet 2021, 397, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Mentis, A.F.A.; Garcia, I.; Jiménez, J.; Paparoupa, M.; Xirogianni, A.; Papandreou, A.; Tzanakaki, G. Artificial intelligence in differential diagnostics of meningitis: A nationwide study. Diagnostics 2021, 11, 602. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Liu, Y.; Xiao, Y.; Yuan, X.; Xu, X.; Zhang, S.; Zhou, S. A machine-learning-based prediction method for hypertension outcomes based on medical data. Diagnostics 2019, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Santana, C.P.; de Carvalho, E.A.; Rodrigues, I.D.; Sousa Bastos, G.; de Souza, A.D.; de Brito, L. rs-fMRI and machine learning for ASD diagnosis: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 6030. [Google Scholar] [CrossRef] [PubMed]
- Herbst, C.S.; Schwendicke, F.; Krois, J.; Herbst, S.R. Association between patient-, tooth- and treatment-level factors and root canal treatment failure: A retrospective longitudinal and machine learning study. J. Dent. 2022, 117, 103937. [Google Scholar] [CrossRef] [PubMed]
- Herbst, S.R.; Herbst, C.S.; Schwendicke, F. Preoperative risk assessment does not allow to predict root filling length using machine learning: A longitudinal study. J. Dent. 2023, 128, 104378. [Google Scholar] [CrossRef] [PubMed]
- Azarpazhooh, A.; Khazaei, S.; Jafarzadeh, H.; Malkhassian, G.; Sgro, A.; Elbarbary, M.; Cardoso, E.; Oren, A.; Kishen, A.; Shah, P.S. A Scoping Review of Four Decades of Outcomes in Nonsurgical Root Canal Treatment, Nonsurgical Retreatment, and Apexification Studies: Part 3: Proposed Data Collection Template and Reporting Guideline for Endodontic Outcome Studies. J Endod. 2022, 48, 40–54. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning, 2nd ed.; Springer Texts in Statistics; Springer: Berlin/Heidelberg, Germany, 2021; pp. 229–232. [Google Scholar]
- Zhang, D.; Maslej, N.; Brynjolfsson, E.; Etchemendy, J.; Lyons, T.; Manyika, J.; Ngo, H.; Niebles, J.C.; Sellitto, M.; Sakhaee, E.; et al. “The AI Index 2022 Annual Report”. AI Index Steering Committee, Stanford Institute for Human-Centered AI, Stanford University. March 2022. Available online: https://aiindex.stanford.edu/ (accessed on 16 January 2023).
- Fan, W.; Zhang, J.; Wang, N.; Li, J.; Hu, L. The Application of Deep Learning on CBCT in Dentistry. Diagnostics 2023, 13, 2056. [Google Scholar] [CrossRef]
- Ezhov, M.; Gusarev, M.; Golitsyna, M.; Yates, J.M.; Kushnerev, E.; Tamimi, D.; Aksoy, S.; Shumilov, E.; Sanders, A.; Orhan, K. Clinically applicable artificial intelligence system for dental diagnosis with CBCT. Sci. Rep. 2021, 11, 15006. [Google Scholar] [CrossRef]
- Asiri, A.F.; Altuwalah, A.S. The role of neural artificial intelligence for diagnosis and treatment planning in endodontics: A qualitative review. Saudi Dent. J. 2022, 34, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Tay, F.R.; Gu, L. Application of Artificial Intelligence in Dentistry. J. Dent. Res. 2021, 100, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Thurzo, A.; Strunga, M.; Urban, R.; Surovková, J.; Afrashtehfar, K.I. Impact of Artificial Intelligence on Dental Education: A Review and Guide for Curriculum Update. Educ. Sci. 2023, 13, 150. [Google Scholar] [CrossRef]
- Eschert, T.; Schwendicke, F.; Krois, J.; Bohner, L.; Vinayahalingam, S.; Hanisch, M. A Survey on the Use of Artificial Intelligence by Clinicians in Dentistry and Oral and Maxillofacial Surgery. Medicina 2022, 58, 1059. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Garcia-Godoy, F.; Gutmann, J.L.; Lotfi, M.; Asgar, K. The reliability of artificial neural network in locating minor apical foramen: A cadaver study. J. Endod. 2012, 38, 1130–1134. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Asgar, K.; Boukani, K.K.; Lotfi, M. A new approach for locating the minor apical foramen using an artificial neural network. Int. Endod. J. 2012, 45, 257–265. [Google Scholar] [CrossRef]
- Johari, M.; Esmaeili, F.; Andalib, A.; Garjani, S. Detection of vertical root fractures in intact and endodontically treated premolar teeth by designing a probabilistic neural network: An ex vivo study. Dentomaxillofac. Radiol. 2017, 46, 20160107. [Google Scholar] [CrossRef]
- Fukuda, M.; Inamoto, K.; Shibata, N.; Arii, Y.; Yanashita, Y.; Kutsuna, S.; Nakata, K.; Katsumata, A.; Fujita, H.; Ariji, E. Evaluation of an artificial intelligence system for detecting vertical root fracture on panoramic radiography. Oral Radiol. 2019, 36, 337–343. [Google Scholar] [CrossRef]
| Domain | Variables |
|---|---|
| Demographic data | Gender (Male, Female, Other), Age (≤15, 15–24, 25–34, 35–44, 45–54, 55–64, ≥65), Highest level of education (Primary, Secondary, Post-secondary), Treated tooth number (1–32), Tooth type (Incisor, Canine, Premolar, Molar), Arch (Mandible, Maxilla) |
| Preoperative patient-related data (medical history) | ASA category, Allergies (No, Yes-Latex-Penicillin-Other), Premedication for endodontic treatment (Analgesic, Antibiotic, Other), Smoking (No, Everyday, Someday, Former), Recreational drugs/products (No, Everyday, Someday, Former), Patient co-operation (No, Yes), Anxiety (No, Yes), Sedation required (None, GA, IV, N2O:O2, Oral) |
| Preoperative clinical signs and symptoms | Spontaneous pain (No, Yes), Chronic pain in the orofacial region (No, Yes), Chronic pain outside the orofacial region (No, Yes), Pain triggered by (None, Sweet, Cold, Heat, Bite, Touch), Pain relieved by (None, Cold, Heat, Medication), Intensity of pain (Mild, Moderate, Severe), Time-lasting of the pain (Sec, Min, Continuous), Nature of pain (Sharp, Dull, Burning), Swelling (Absent, Present), Sinus tract (Absent, Present) |
| Preoperative clinical findings (intraoral and extraoral examination) | Soft tissue appearance (Normal, Abnormal), Lymphadenopathy (Absent, Present), Discoloration (No, Yes) |
| Preoperative diagnostic data (clinical) | Cold test (Negative, Positive), Percussion (Not sensitive, Sensitive), Palpation (Not tender, Tender) |
| Preoperative radiographic techniques and findings | Periapical index (No, PAI 1–2, PAI 3–5), Periapical rarefying osteitis (2–4 mm, 5–7 mm, ≥8 mm), Location of radiolucency (Apical, Furcal, Lateral), Canal curvature (<10°, 10°–30°, >30°) |
| Preoperative diagnosis | Pulp (Normal, Reversible pulpitis, Asymptomatic irreversible pulpitis, Symptomatic irreversible, Necrosis), Periapical (Normal, Asymptomatic AP, Symptomatic AP, Chronic Apical Abscess, Acute Apical Abscess), Number of roots |
| Estimated prognosis | Prognosis (Hopeless, Questionable, Fair, Good, Excellent) |
| Variable | Levels | p-Value | Effect Size |
|---|---|---|---|
| Age | 15–24; 25–34; 35–44; 45–54; 55–64; ≥65 | 0.0056 | 0.372 |
| Highest level of education | Primary; Secondary; Post secondary | 0.0016 | 0.33 |
| Arch | Mandible; Maxilla | 0.02 | 0.21 |
| Smoking | No; Everyday; Someday; Former | 0.046 | 0.26 |
| Patient co-operation | No; Yes | 0.028 | 0.21 |
| Pain relieved by | None; Cold; Medication | 0.003 | 0.31 |
| Time-lasting of the pain | Sec; Min; Continuous | 0.027 | 0.245 |
| Periapical | Asymptomatic AP; Symptomatic AP; Chronic Apical Abscess; Acute Apical Abscess | 0.01 | 0.31 |
| Estimated Prognosis by clinician | Hopeless; Questionable; Fair; Good; Excellent | 0.034 | 0.29 |
| Metric | DP | LR | RF | NB | KNN |
|---|---|---|---|---|---|
| TP | 42 | 53 | 57 | 53 | 55 |
| FN | 27 | 16 | 12 | 16 | 14 |
| FP | 21 | 17 | 15 | 20 | 17 |
| TN | 29 | 33 | 35 | 30 | 33 |
| Sensitivity |
0.61 [0.48, 0.72] |
0.77 [0.65, 0.86] |
0.83 [0.72, 0.91] |
0.77 [0.65, 0.86] |
0.8 [0.68, 0.88] |
| Specificity |
0.58 [0.43, 0.72] |
0.66 [0.51, 0.79] |
0.7 [0.55, 0.82] |
0.6 [0.45, 0.74] |
0.66 [0.51, 0.79] |
| PPV |
0.67 [0.54, 0.78] |
0.77 [0.65, 0.86] |
0.79 [0.68, 0.88] |
0.73 [0.61, 0.82] |
0.76 [0.65, 0.86] |
| NPV |
0.52 [0.38, 0.65] |
0.67 [0.52, 0.8] |
0.74 [0.6, 0.86] |
0.65 [0.5, 0.79] |
0.7 [0.55, 0.83] |
| Accuracy |
0.6 [0.5, 0.69] |
0.72 [0.63, 0.8] |
0.77 [0.69, 0.84] |
0.7 [0.61, 0.78] |
0.74 [0.65, 0.82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennasar, C.; García, I.; Gonzalez-Cid, Y.; Pérez, F.; Jiménez, J. Second Opinion for Non-Surgical Root Canal Treatment Prognosis Using Machine Learning Models. Diagnostics 2023, 13, 2742. https://doi.org/10.3390/diagnostics13172742
Bennasar C, García I, Gonzalez-Cid Y, Pérez F, Jiménez J. Second Opinion for Non-Surgical Root Canal Treatment Prognosis Using Machine Learning Models. Diagnostics. 2023; 13(17):2742. https://doi.org/10.3390/diagnostics13172742
Chicago/Turabian StyleBennasar, Catalina, Irene García, Yolanda Gonzalez-Cid, Francesc Pérez, and Juan Jiménez. 2023. "Second Opinion for Non-Surgical Root Canal Treatment Prognosis Using Machine Learning Models" Diagnostics 13, no. 17: 2742. https://doi.org/10.3390/diagnostics13172742
APA StyleBennasar, C., García, I., Gonzalez-Cid, Y., Pérez, F., & Jiménez, J. (2023). Second Opinion for Non-Surgical Root Canal Treatment Prognosis Using Machine Learning Models. Diagnostics, 13(17), 2742. https://doi.org/10.3390/diagnostics13172742








