Detecting Bone Marrow Edema of the Extremities on Spectral Computed Tomography Using a Three-Material Decomposition
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Data
- True/false positive/negative results using the TMD approach
- Calculated diagnostic performance using the TMD approach
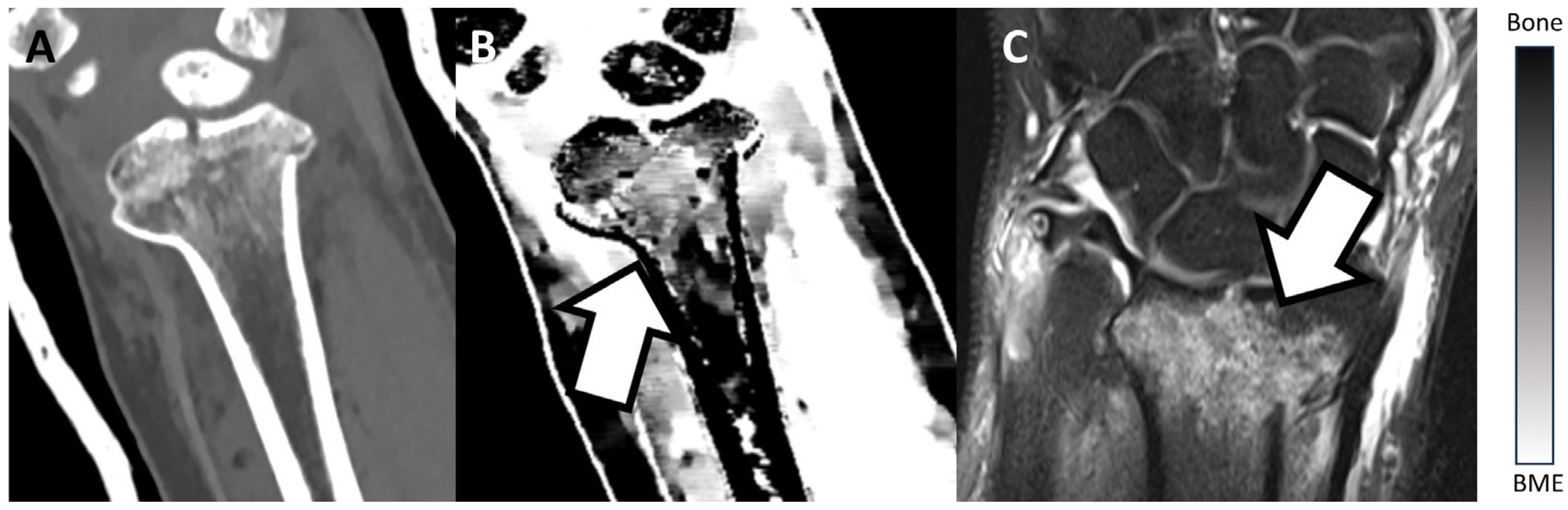
3.2. Examples

| Sensitivity | Specificity | PPV | NPV | AUC | |
|---|---|---|---|---|---|
| Distal radius | 93.8% | 85.7% | 88.2% | 92.3% | 90.0% |
| Proximal femur | 86.7% | 94.1% | 92.9% | 88.9% | 90.6% |
| Proximal tibia | 90.0% | 93.3% | 94.7% | 87.5% | 91.4% |
| Distal tibia | 87.5% | 84.2% | 82.4% | 88.9% | 85.7% |
| Long bone diaphysis | 92.9% | 93.3% | 92.9% | 93.3% | 93.1% |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Angelo, T.; Albrecht, M.H.; Caudo, D.; Mazziotti, S.; Vogl, T.J.; Wichmann, J.L.; Martin, S.; Yel, I.; Ascenti, G.; Koch, V.; et al. Virtual non-calcium dual-energy CT: Clinical applications. Eur. Radiol. Exp. 2021, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, U.; Greggi, C.; Cariati, I.; Manenti, G.; Primavera, M.; Ferrante, P.; Iundusi, R.; Gasbarra, E.; Gatti, A. Reviewing Bone Marrow Edema in Athletes: A Difficult Diagnostic and Clinical Approach. Medicina 2021, 57, 1143. [Google Scholar] [CrossRef] [PubMed]
- Baumbach, S.F.; Pfahler, V.; Bechtold-Dalla Pozza, S.; Feist-Pagenstert, I.; Fürmetz, J.; Baur-Melnyk, A.; Stumpf, U.C.; Saller, M.M.; Straube, A.; Schmidmaier, R.; et al. How We Manage Bone Marrow Edema-An Interdisciplinary Approach. J. Clin. Med. 2020, 9, 551. [Google Scholar] [CrossRef]
- Kellock, T.T.; Nicolaou, S.; Kim, S.S.Y.; Al-Busaidi, S.; Louis, L.J.; O’Connell, T.W.; Ouellette, H.A.; McLaughlin, P.D. Detection of bone marrow edema in nondisplaced hip fractures: Utility of a virtual noncalcium dual-energy CT application. Radiology 2017, 284, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Reddy, T.; McLaughlin, P.D.; Mallinson, P.I.; Reagan, A.C.; Munk, P.L.; Nicolaou, S.; Ouellette, H.A. Detection of occult, undisplaced hip fractures with a dual-energy CT algorithm targeted to detection of bone marrow edema. Emerg. Radiol. 2015, 22, 25–29. [Google Scholar] [CrossRef]
- Mandalia, V.; Henson, J.H.L. Traumatic bone bruising—A review article. Eur. J. Radiol. 2008, 67, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, B.J.; Gersing, A.S.; Hammel, J.; Mei, K.; Kopp, F.K.; Kirschke, J.S.; Rummeny, E.J.; Wörtler, K.; Baum, T.; Noël, P.B. Three-material decomposition with dual-layer spectral CT compared to MRI for the detection of bone marrow edema in patients with acute vertebral fractures. Skelet. Radiol. 2018, 47, 1533–1540. [Google Scholar] [CrossRef]
- Abbassi, M.; Jain, A.; Shin, D.; Arasa, C.A.; Li, B.; Anderson, S.W.; LeBedis, C.A. Quantification of bone marrow edema using dual-energy CT at fracture sites in trauma. Emerg. Radiol. 2022, 29, 691–696. [Google Scholar] [CrossRef]
- de Bakker, C.M.J.; Walker, R.E.A.; Besler, B.A.; Tse, J.J.; Manske, S.L.; Martin, C.R.; French, S.J.; Dodd, A.E.; Boyd, S.K. A quantitative assessment of dual energy computed tomography-based material decomposition for imaging bone marrow edema associated with acute knee injury. Med. Phys. 2021, 48, 1792–1803. [Google Scholar] [CrossRef]
- Mallinson, P.I.; Coupal, T.M.; McLaughlin, P.D.; Nicolaou, S.; Munk, P.L.; Ouellette, H.A. Dual-energy CT for the musculoskeletal system. Radiology 2016, 281, 690–707. [Google Scholar] [CrossRef]
- Kaup, M.; Wichmann, J.L.; Scholtz, J.-E.; Beeres, M.; Kromen, W.; Albrecht, M.H.; Lehnert, T.; Boettcher, M.; Vogl, T.J.; Bauer, R.W. Dual-energy CT–based display of bone marrow edema in osteoporotic vertebral compression fractures: Impact on diagnostic accuracy of radiologists with varying levels of experience in correlation to MR imaging. Radiology 2016, 280, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Rassouli, N.; Etesami, M.; Dhanantwari, A.; Rajiah, P. Detector-based spectral CT with a novel dual-layer technology: Principles and applications. Insights Imaging 2017, 8, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, V.; Lennartz, S.; Abdullayev, N.; Hokamp, N.G.; Shapira, N.; Kafri, G.; Holz, J.A.; Krug, B.; Hellmich, M.; Maintz, D.; et al. Bone marrow edema in traumatic vertebral compression fractures: Diagnostic accuracy of dual-layer detector CT using calcium suppressed images. Eur. J. Radiol. 2018, 105, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Pache, G.; Krauss, B.; Strohm, P.; Saueressig, U.; Blanke, P.; Bulla, S.; Schäfer, O.; Helwig, P.; Kotter, E.; Langer, M.; et al. Dual-energy CT virtual noncalcium technique: Detecting posttraumatic bone marrow lesions—Feasibility study. Radiology 2010, 256, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Wortman, J.R.; Uyeda, J.W.; Fulwadhva, U.P.; Sodickson, A.D. Dual-energy CT for abdominal and pelvic trauma. Radiographics 2018, 38, 586–602. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef]
- Grajo, J.R.; Sahani, D.V. Dual-Energy CT of the Abdomen and Pelvis: Radiation Dose Considerations. J. Am. Coll. Radiol. 2018, 15, 1128–1132. [Google Scholar] [CrossRef]
- Langguth, P.; Aludin, S.; Horr, A.; Campbell, G.M.; Lebenatus, A.; Ravesh, M.S.; Schunk, D.; Austein, F.; Larsen, N.; Syrek, H.; et al. Iodine uptake of adrenal glands: A novel and reliable spectral dual-layer computed tomographic-derived biomarker for acute septic shock. Eur. J. Radiol. 2022, 156, 110492. [Google Scholar] [CrossRef]
- Sedaghat, S.; Langguth, P.; Larsen, N.; Campbell, G.; Both, M.; Jansen, O. Diagnostische Wertigkeit der Dual-Layer-Spektral-CT zur Detektion von posttraumatischen prävertebralen Hämatomen der Halswirbelsäule unter Verwendung von Elektronendichtebildern. Rofo 2021, 193, 1445–1450. [Google Scholar]
- Wang, C.-K.; Tsai, J.-M.; Chuang, M.-T.; Wang, M.-T.; Huang, K.-Y.; Lin, R.-M. Bone marrow edema in vertebral compression fractures: Detection with dual-energy CT. Radiology 2013, 269, 525–533. [Google Scholar] [CrossRef]
- Petritsch, B.; Kosmala, A.; Weng, A.M.; Krauss, B.; Heidemeier, A.; Wagner, R.; Heintel, T.M.; Gassenmaier, T.; Bley, T.A. Vertebral Compression Fractures: Third-Generation Dual-Energy CT for Detection of Bone Marrow Edema at Visual and Quantitative Analyses. Radiology 2017, 284, 161–168. [Google Scholar] [CrossRef]
- Yadav, H.; Khanduri, S.; Yadav, P.; Pandey, S.; Yadav, V.K.; Khan, S. Diagnostic accuracy of dual energy CT in the assessment of traumatic bone marrow edema of lower limb and its correlation with MRI. Indian J. Radiol. Imaging 2020, 30, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Jeon, J.Y.; Lee, S.-W.; Jeong, Y.M. Added value of color-coded virtual non-calcium dual-energy CT in the detection of acute knee fractures in non-radiology inexpert readers. Eur. J. Radiol. 2020, 129, 109112. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.W.; Olson, P.R.; Rosenwasser, M.P. The Epidemiology of Upper Extremity Fractures in the United States, 2009. J. Orthop. Trauma 2015, 29, e242–e244. [Google Scholar] [CrossRef] [PubMed]
- Beerekamp, M.S.H.; de Muinck Keizer, R.J.O.; Schep, N.W.L.; Ubbink, D.T.; Panneman, M.J.M.; Goslings, J.C. Epidemiology of extremity fractures in the Netherlands. Injury 2017, 48, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Palvanen, M.; Kannus, P.; Parkkari, J.; Pitkäjärvi, T.; Pasanen, M.; Vuori, I.; Järvinen, M. The injury mechanisms of osteoporotic upper extremity fractures among older adults: A controlled study of 287 consecutive patients and their 108 controls. Osteoporos. Int. 2000, 11, 822–831. [Google Scholar] [CrossRef]
- Milner, C.E.; Hamill, J.; Davis, I. Are knee mechanics during early stance related to tibial stress fracture in runners? Clin. Biomech. 2007, 22, 697–703. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Cameron, K.L.; Bojescul, J.A. Lower extremity stress fractures in the military. Clin. Sports Med. 2014, 33, 591–613. [Google Scholar] [CrossRef]
- Zbijewski, W.; Sisniega, A.; Stayman, J.W.; Thawait, G.; Packard, N.; Yorkston, J.; Demehri, S.; Fritz, J.; Siewerdsen, J.H. Dual-Energy Imaging of Bone Marrow Edema on a Dedicated Multi-Source Cone-Beam CT System for the Extremities. Proc. SPIE Int. Soc. Opt. Eng. 2015, 9412, 94120V. [Google Scholar]
- Boks, S.S.; Vroegindeweij, D.; Koes, B.W.; Hunink, M.G.M.; Bierma-Zeinstra, S.M.A. Follow-up of occult bone lesions detected at MR imaging: Systematic review. Radiology 2006, 238, 853–862. [Google Scholar] [CrossRef]
- Dubreuil, T.; Mouly, J.; Ltaief-Boudrigua, A.; Martinon, A.; Tilhet-Coartet, S.; Tazarourte, K.; Pialat, J.B. Comparison of Cone-Beam Computed Tomography and Multislice Computed Tomography in the Assessment of Extremity Fractures. J. Comput. Assist Tomogr. 2019, 43, 372–378. [Google Scholar] [CrossRef]
- Falkowski, A.L.; Kovacs, B.K.; Schwartz, F.R.; Benz, R.M.; Stieltjes, B.; Hirschmann, A. Comparison of 3D X-ray tomography with computed tomography in patients with distal extremity fractures. Skelet. Radiol. 2020, 49, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Prosser, I.; Maguire, S.; Harrison, S.K.; Mann, M.; Sibert, J.R.; Kemp, A.M. How old is this fracture? Radiologic dating of fractures in children: A systematic review. AJR Am. J. Roentgenol. 2005, 184, 1282–1286. [Google Scholar] [CrossRef]
- Pinto, A.; Brunese, L. Spectrum of diagnostic errors in radiology. World J. Radiol. 2010, 2, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-J.; Tsai, W.-C.; Tiu, C.-M.; Wu, H.-T.; Chiou, H.-J.; Chang, C.-Y. Systematic analysis of missed extremity fractures in emergency radiology. Acta Radiol. 2006, 47, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Gosangi, B.; Mandell, J.C.; Weaver, M.J.; Uyeda, J.W.; Smith, S.E.; Sodickson, A.D.; Khurana, B. Bone Marrow Edema at Dual-Energy CT: A Game Changer in the Emergency Department. Radiographics 2020, 40, 859–874. [Google Scholar] [CrossRef]
- Cavallaro, M.; D’Angelo, T.; Albrecht, M.H.; Yel, I.; Martin, S.S.; Wichmann, J.L.; Lenga, L.; Mazziotti, S.; Blandino, A.; Ascenti, G.; et al. Comprehensive comparison of dual-energy computed tomography and magnetic resonance imaging for the assessment of bone marrow edema and fracture lines in acute vertebral fractures. Eur. Radiol. 2022, 32, 561–571. [Google Scholar] [CrossRef]
- Kalender, W.A.; Perman, W.H.; Vetter, J.R.; Klotz, E. Evaluation of a prototype dual-energy computed tomographic apparatus. I. Phantom studies. Med. Phys. 1986, 13, 334–339. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Zhang, X.-Y.; Xu, L.; Feng, Y.; Xu, Y.-C.; Qi, L.; Zou, Y.F. Detection of bone marrow oedema in knee joints using a dual-energy CT virtual non-calcium technique. Clin. Radiol. 2019, 74, 815.e1–815.e7. [Google Scholar] [CrossRef]
- Court-Brown, C.M.; Caesar, B. Epidemiology of adult fractures: A review. Injury 2006, 37, 691–697. [Google Scholar] [CrossRef]
- Melton, L.J. Epidemiology of Fractures. In Osteoporosis in Men; Elsevier: Amsterdam, The Netherlands, 1999; pp. 1–13. [Google Scholar]
- Sedaghat, S. Success Through Simplicity: What Other Artificial Intelligence Applications in Medicine Should Learn from History and ChatGPT. Ann. Biomed. Eng. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
| Location | BME (n) | No BME (n) | Total (n) |
|---|---|---|---|
| Distal radius | 16 | 14 | 30 |
| Proximal femur | 15 | 17 | 32 |
| Proximal tibia | 20 | 15 | 35 |
| Distal tibia | 16 | 19 | 35 |
| Long bone diaphysis | 14 | 15 | 29 |
| Total | 81 | 80 | 161 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schierenbeck, M.; Grözinger, M.; Reichardt, B.; Jansen, O.; Kauczor, H.-U.; Campbell, G.M.; Sedaghat, S. Detecting Bone Marrow Edema of the Extremities on Spectral Computed Tomography Using a Three-Material Decomposition. Diagnostics 2023, 13, 2745. https://doi.org/10.3390/diagnostics13172745
Schierenbeck M, Grözinger M, Reichardt B, Jansen O, Kauczor H-U, Campbell GM, Sedaghat S. Detecting Bone Marrow Edema of the Extremities on Spectral Computed Tomography Using a Three-Material Decomposition. Diagnostics. 2023; 13(17):2745. https://doi.org/10.3390/diagnostics13172745
Chicago/Turabian StyleSchierenbeck, Marie, Martin Grözinger, Benjamin Reichardt, Olav Jansen, Hans-Ulrich Kauczor, Graeme M. Campbell, and Sam Sedaghat. 2023. "Detecting Bone Marrow Edema of the Extremities on Spectral Computed Tomography Using a Three-Material Decomposition" Diagnostics 13, no. 17: 2745. https://doi.org/10.3390/diagnostics13172745





