Plasma Glial Fibrillary Acidic Protein and N-Terminal Pro B-Type Natriuretic Peptide: Potential Biomarkers to Differentiate Ischemic and Hemorrhagic Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Cohorts
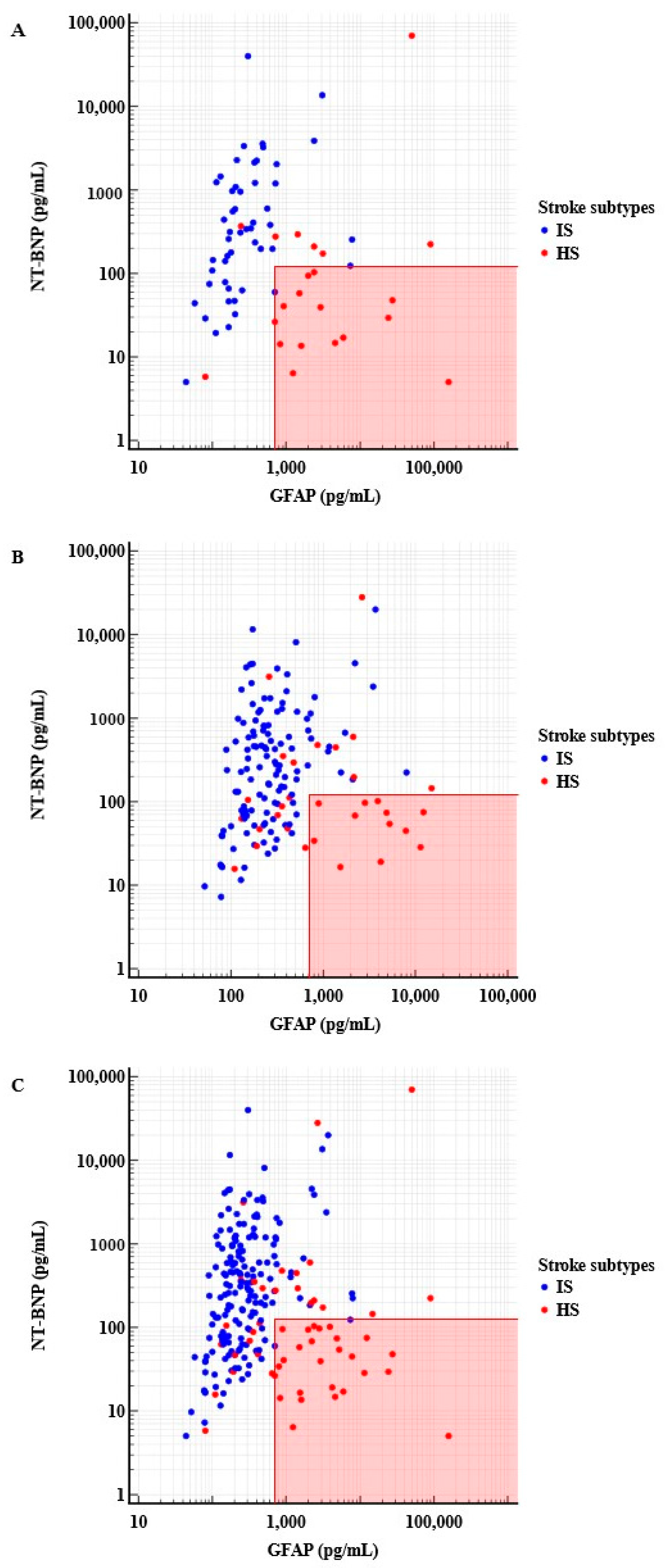
2.2.1. Derivation Cohort
2.2.2. Validation Cohort
2.3. Measurement of the Biomarkers
2.4. Statistical Analysis
3. Results
3.1. Derivation Cohort
3.2. Validation Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ekker, M.S.; Verhoeven, J.I.; Vaartjes, I.; Jolink, W.M.T.; Klijn, C.J.M.; de Leeuw, F.E. Association of Stroke Among Adults Aged 18 to 49 Years with Long-term Mortality. JAMA 2019, 321, 2113–2123. Available online: https://jamanetwork.com/journals/jama/articlepdf/2734509/jama_ekker_2019_oi_190049.pdf (accessed on 23 May 2019). [CrossRef] [PubMed]
- Liu, Z.; Yang, C.; Wang, X.; Xiang, Y. Blood-Based Biomarkers: A Forgotten Friend of Hyperacute Ischemic Stroke. Front. Neurol. 2021, 12, 634717. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kang, K.; Kang, J.; Koo, J.; Kim, D.H.; Kim, B.J.; Kim, W.J.; Kim, E.G.; Kim, J.G.; Kim, J.M.; et al. Executive Summary of Stroke Statistics in Korea 2018: A Report from the Epidemiology Research Council of the Korean Stroke Society. J. Stroke 2019, 21, 42–59. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6372894/pdf/jos-2018-03125.pdf (accessed on 23 May 2019). [CrossRef] [PubMed]
- GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. Available online: https://www.sciencedirect.com/science/article/pii/S0140673618323353?via%3Dihub (accessed on 20 June 2019). [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Hand, P.J.; Kwan, J.; Lindley, R.I.; Dennis, M.S.; Wardlaw, J.M. Distinguishing between stroke and mimic at the bedside: The brain attack study. Stroke 2006, 37, 769–775. [Google Scholar] [CrossRef]
- Brott, T.; Adams, H.P., Jr.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V.; et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef]
- Li, D.; Mielke, M.M. An Update on Blood-Based Markers of Alzheimer’s Disease Using the SiMoA Platform. Neurol. Ther. 2019, 8, 73–82. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6908531/pdf/40120_2019_Article_164.pdf (accessed on 16 July 2019). [CrossRef]
- Eng, L.F.; Ghirnikar, R.S.; Lee, Y.L. Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000). Neurochem. Res. 2000, 25, 1439–1451. Available online: https://link.springer.com/article/10.1023/A:1007677003387 (accessed on 11 April 2000). [CrossRef]
- Dvorak, F.; Haberer, I.; Sitzer, M.; Foerch, C. Characterisation of the diagnostic window of serum glial fibrillary acidic protein for the differentiation of intracerebral haemorrhage and ischaemic stroke. Cerebrovasc. Dis. 2009, 27, 37–41. [Google Scholar] [CrossRef]
- Foerch, C.; Niessner, M.; Back, T.; Bauerle, M.; De Marchis, G.M.; Ferbert, A.; Grehl, H.; Hamann, G.F.; Jacobs, A.; Kastrup, A.; et al. Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin. Chem. 2012, 58, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Luger, S.; Witsch, J.; Dietz, A.; Hamann, G.F.; Minnerup, J.; Schneider, H.; Sitzer, M.; Wartenberg, K.E.; Niessner, M.; Foerch, C. Glial Fibrillary Acidic Protein Serum Levels Distinguish between Intracerebral Hemorrhage and Cerebral Ischemia in the Early Phase of Stroke. Clin. Chem. 2017, 63, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Brunkhorst, R.; Pfeilschifter, W.; Foerch, C. Astroglial proteins as diagnostic markers of acute intracerebral hemorrhage-pathophysiological background and clinical findings. Transl. Stroke Res. 2010, 1, 246–251. Available online: https://link.springer.com/article/10.1007/s12975-010-0040-6 (accessed on 28 August 2010). [CrossRef] [PubMed]
- De Marchis, G.M.; Katan, M.; Barro, C.; Fladt, J.; Traenka, C.; Seiffge, D.J.; Hert, L.; Gensicke, H.; Disanto, G.; Sutter, R.; et al. Serum neurofilament light chain in patients with acute cerebrovascular events. Eur. J. Neurol. 2018, 25, 562–568. [Google Scholar] [CrossRef]
- Hviid, C.V.B.; Gyldenholm, T.; Lauridsen, S.V.; Hjort, N.; Hvas, A.M.; Parkner, T. Plasma neurofilament light chain is associated with mortality after spontaneous intracerebral hemorrhage. Clin. Chem. Lab. Med. 2020, 58, 261–267. [Google Scholar] [CrossRef]
- Singh, P.; Yan, J.; Hull, R.; Read, S.; O’Sullivan, J.; Henderson, R.D.; Rose, S.; Greer, J.M.; McCombe, P.A. Levels of phosphorylated axonal neurofilament subunit H (pNfH) are increased in acute ischemic stroke. J. Neurol. Sci. 2011, 304, 117–121. [Google Scholar] [CrossRef]
- Traenka, C.; Disanto, G.; Seiffge, D.J.; Gensicke, H.; Hert, L.; Grond-Ginsbach, C.; Peters, N.; Regeniter, A.; Kloss, M.; De Marchis, G.M.; et al. Serum Neurofilament Light Chain Levels Are Associated with Clinical Characteristics and Outcome in Patients with Cervical Artery Dissection. Cerebrovasc. Dis. 2015, 40, 222–227. Available online: https://www.karger.com/Article/Pdf/440774 (accessed on 1 September 2015). [CrossRef]
- Levin, E.R.; Gardner, D.G.; Samson, W.K. Natriuretic peptides. N. Engl. J. Med. 1998, 339, 321–328. Available online: https://www-nejm-org-ssl.proxy.cuk.ac.kr/doi/full/10.1056/NEJM199807303390507 (accessed on 30 July 1998).
- Kerr, B.; Brandon, L. Atrial Fibrillation, thromboembolic risk, and the potential role of the natriuretic peptides, a focus on BNP and NT-proBNP—A narrative review. Int. J. Cardiol. Heart Vasc. 2022, 43, 101132. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9562601 (accessed on 10 October 2022). [CrossRef]
- Montaner, J.; Perea-Gainza, M.; Delgado, P.; Ribó, M.; Chacón, P.; Rosell, A.; Quintana, M.; Palacios, M.E.; Molina, C.A.; Alvarez-Sabín, J. Etiologic diagnosis of ischemic stroke subtypes with plasma biomarkers. Stroke 2008, 39, 2280–2287. [Google Scholar] [CrossRef]
- Fonseca, A.C.; Matias, J.S.; Pinho e Melo, T.; Falcão, F.; Canhão, P.; Ferro, J.M. N-terminal probrain natriuretic peptide as a biomarker of cardioembolic stroke. Int. J. Stroke 2011, 6, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Kunz, A.; Ebinger, M.; Geisler, F.; Rozanski, M.; Waldschmidt, C.; Weber, J.E.; Wendt, M.; Winter, B.; Zieschang, K.; Fiebach, J.B.; et al. Functional outcomes of pre-hospital thrombolysis in a mobile stroke treatment unit compared with conventional care: An observational registry study. Lancet Neurol. 2016, 15, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, A.; López-Cancio, E.; Pich, S.; Penalba, A.; Giralt, D.; García-Berrocoso, T.; Ferrer-Costa, C.; Gasull, T.; Hernández-Pérez, M.; Millan, M.; et al. Blood Biomarkers for the Early Diagnosis of Stroke: The Stroke-Chip Study. Stroke 2017, 48, 2419–2425. [Google Scholar] [CrossRef] [PubMed]
| Derivation Cohort | ||||
|---|---|---|---|---|
| All | IS | HS | p Value | |
| Number of patients | 73 | 51 | 22 | |
| Age (years), median (IQR) | 72 (59–79) | 73 (68–83) | 55 (48–72) | <0.001 † |
| Female, n (%) | 39 (53.4) | 31 (60.8) | 8 (36.4) | 0.218 ‡ |
| Medical history, n (%) | ||||
| Atrial fibrillation, n (%) | 18 (24.7) | 17 (33.3) | 1 (4.5) | 0.001 ‡ |
| Diabetes, n (%) | 17 (23.3) | 15 (29.4) | 2 (9.1) | 0.061 ‡ |
| Dyslipidemia, n (%) | 16 (21.9) | 13 (25.5) | 3 (13.6) | 0.265 ‡ |
| Hypertension, n (%) | 42 (57.5) | 35 (68.6) | 7 (31.8) | 0.004 ‡ |
| Ischemic heart disease, N (%) | 5 (6.8) | 5 (9.8) | 0 (0) | 0.130 ‡ |
| IS history, n (%) | 15 (20.5) | 13 (25.5) | 2 (0.8) | 0.114 ‡ |
| ICH history, n (%) | 3 (4.1) | 2 (3.9) | 1 (4.5) | 0.907 ‡ |
| LKW to sample time (min), median (IQR) | 188 (85–597) | 278 (101–633) | 131 (60–527) | 0.114 † |
| <1 h, n (%) | 9 (12.3) | 4 (7.8) | 5 (22.7) | 0.078 ‡ |
| <3 h, n (%) | 36 (49.3) | 23 (45.1) | 13 (59.1) | 0.276 ‡ |
| Ischemic Stroke | Hemorrhagic Stroke | ||||||
|---|---|---|---|---|---|---|---|
| Biomarker | Number of Patients | Median | IQR | Number of Patients | Median | IQR | p Value † |
| GFAP (pg/mL) | 51 | 213.8 | 161.8–439.4 | 22 | 2171.0 | 922.8–5883 | <0.001 |
| NT-proBNP (pg/mL) | 51 | 312 | 85.8–1206 | 22 | 44.2 | 14.8–209 | <0.001 |
| NF-L (pg/mL) | 51 | 26.6 | 17.0–35.7 | 22 | 26.2 | 12.3–49.8 | 0.848 |
| Copeptin (pg/mL) | 49 | 41.1 | 26.4–81.2 | 21 | 41.9 | 22.0–64.7 | 0.715 |
| Neutrophil % | 51 | 65.6 | 57.9–76.1 | 22 | 60.1 | 46.4–71.7 | 0.161 |
| NLR | 51 | 2.7 | 1.7–4.8 | 22 | 2.0 | 1.1–3.6 | 0.136 |
| Platelet (109/L) | 51 | 226 | 185.3–278.3 | 22 | 257.0 | 222.0–283.0 | 0.102 |
| Derivation Cohort | Whole Cohort | |||
|---|---|---|---|---|
| Coefficient | p Value | Coefficient | p Value | |
| Age | 0.01517 | 0.359 | 0.01313 | 0.490 |
| Atrial fibrillation | −0.5149 | 0.204 | −0.38441 | 0.375 |
| Hypertension | −0.34524 | 0.151 | −0.14318 | 0.607 |
| GFAP | −0.00057 | <0.001 | −0.00064 | <0.001 |
| NT-proBNP | 0.00033 | 0.030 | 0.00038 | 0.016 |
| Biomarker | Derivation Cohort | Validation Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | p | Ideal Cut-Off | Sensitivity% (95% CI) | Specificity% (95% CI) | PPV (95% CI) | NPV (95% CI) | Sensitivity% (95% CI) | Specificity% (95% CI) | PPV (95% CI) | NPV (95% CI) | |
| GFAP | 0.886 (0.790–0.948) | <0.001 | >703 | 90.9 (70.8–98.9) | 88.2 (76.1–95.6) | 76.9 (60.9–87.7) | 95.8 (85.7–98.8) | 59.4 (40.6–76.3) | 90.2 (83.4–94.8) | 61.3 (46.3–74.4) | 89.4 (84.7–92.8) |
| NT-BNP | 0.775 (0.662–0.864) | <0.001 | ≤292 | 90.9 (70.8–98.9) | 52.9 (38.5–67.1) | 45.5 (37.7–53.4) | 93.1 (77.8–98.1) | 77.4 (58.9–90.4) | 46.7 (37.6–56.0) | 27.0 (22.3–32.2) | 89.1 (80.5–94.1) |
| GFAP and NT-BNP | 0.781 (0.669–0.870) | <0.0001 | >703 (GFAP) and ≤125 (NT-BNP) | 95.5 (77.2–99.9) | 60.8 (46.11–74.16) | 93.3 (66.2–99.0) | 86.2 (78.2–91.6) | 38.7 (21.7–57.8) | 100 (97.0–100) | 100 (73.5–100) | 86.5 (82.9–89.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, E.; Kim, H.; Cho, B.; Lee, J.-J.; Shin, S.; Oh, E.-J.; Chae, H. Plasma Glial Fibrillary Acidic Protein and N-Terminal Pro B-Type Natriuretic Peptide: Potential Biomarkers to Differentiate Ischemic and Hemorrhagic Stroke. Diagnostics 2023, 13, 2757. https://doi.org/10.3390/diagnostics13172757
Han E, Kim H, Cho B, Lee J-J, Shin S, Oh E-J, Chae H. Plasma Glial Fibrillary Acidic Protein and N-Terminal Pro B-Type Natriuretic Peptide: Potential Biomarkers to Differentiate Ischemic and Hemorrhagic Stroke. Diagnostics. 2023; 13(17):2757. https://doi.org/10.3390/diagnostics13172757
Chicago/Turabian StyleHan, Eunhee, Hyejeong Kim, Bongrae Cho, Jeong-Joong Lee, Soyoung Shin, Eun-Jee Oh, and Hyojin Chae. 2023. "Plasma Glial Fibrillary Acidic Protein and N-Terminal Pro B-Type Natriuretic Peptide: Potential Biomarkers to Differentiate Ischemic and Hemorrhagic Stroke" Diagnostics 13, no. 17: 2757. https://doi.org/10.3390/diagnostics13172757






