Exploring the Paradox of Bone Mineral Density in Type 2 Diabetes: A Comparative Study Using Opportunistic Chest CT Texture Analysis and DXA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Selection Criteria
2.2. CT and DXA Scanning Procedures
2.3. Defining Regions of Interest
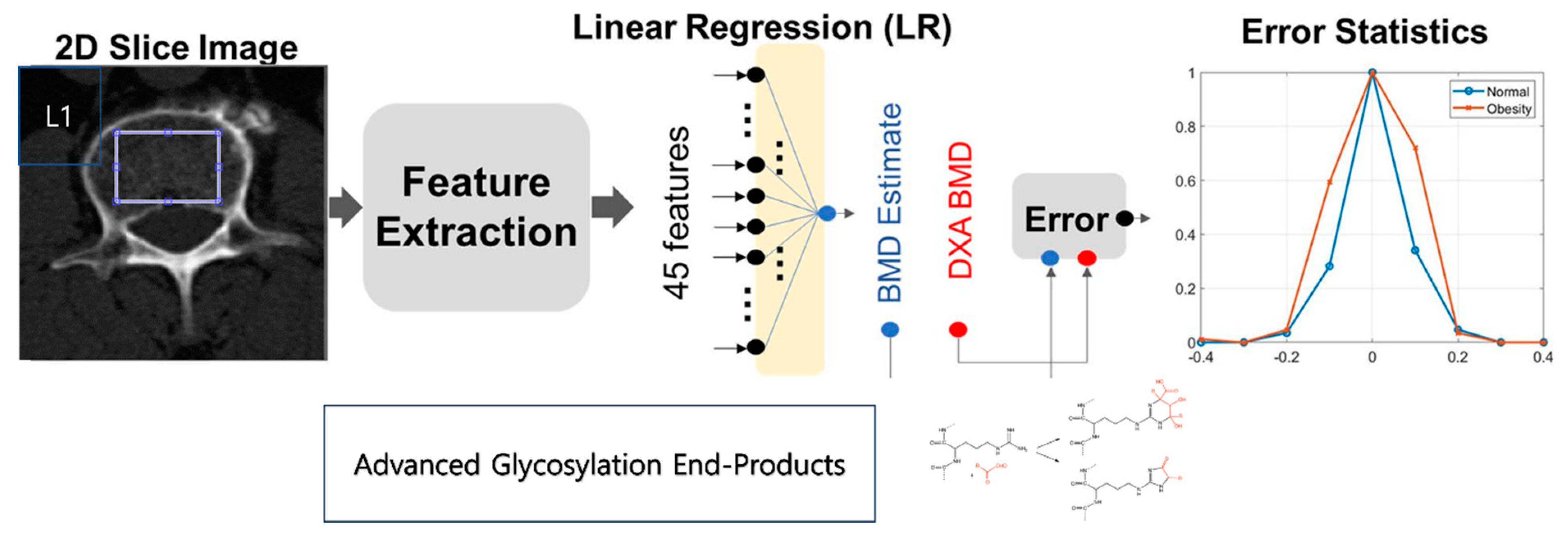
2.4. Feature Derivation and Analysis
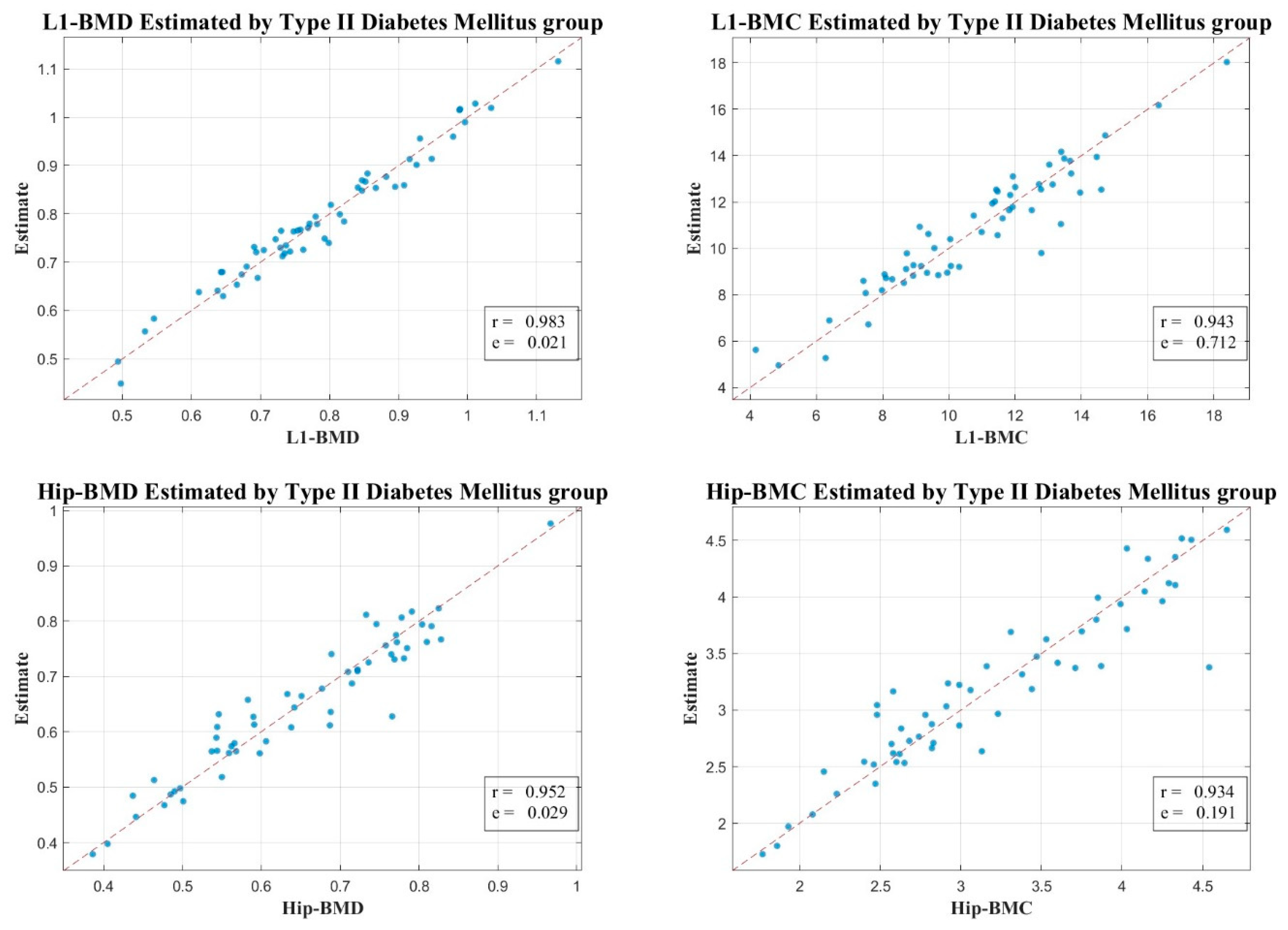
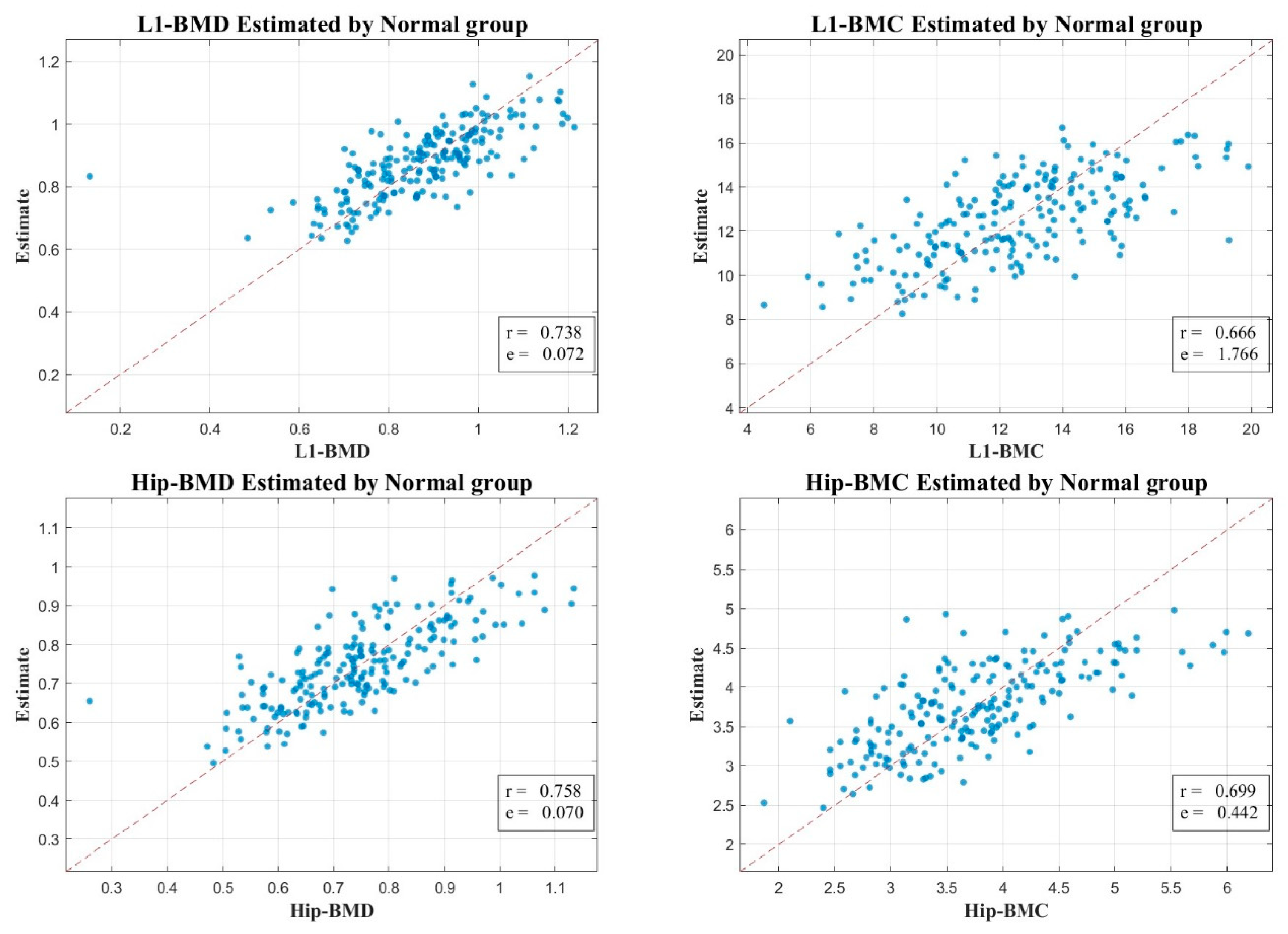
2.5. Correlation Examination
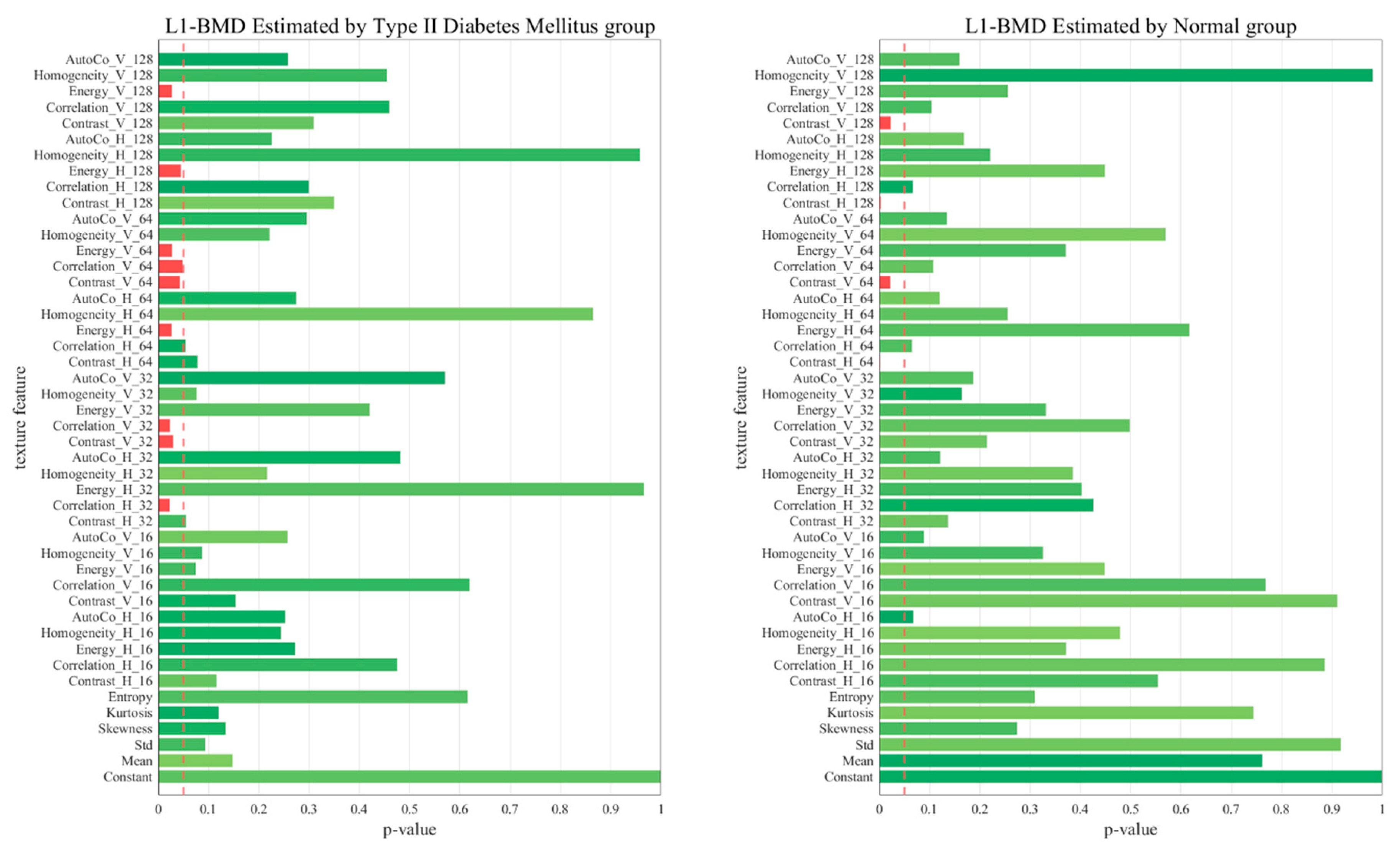
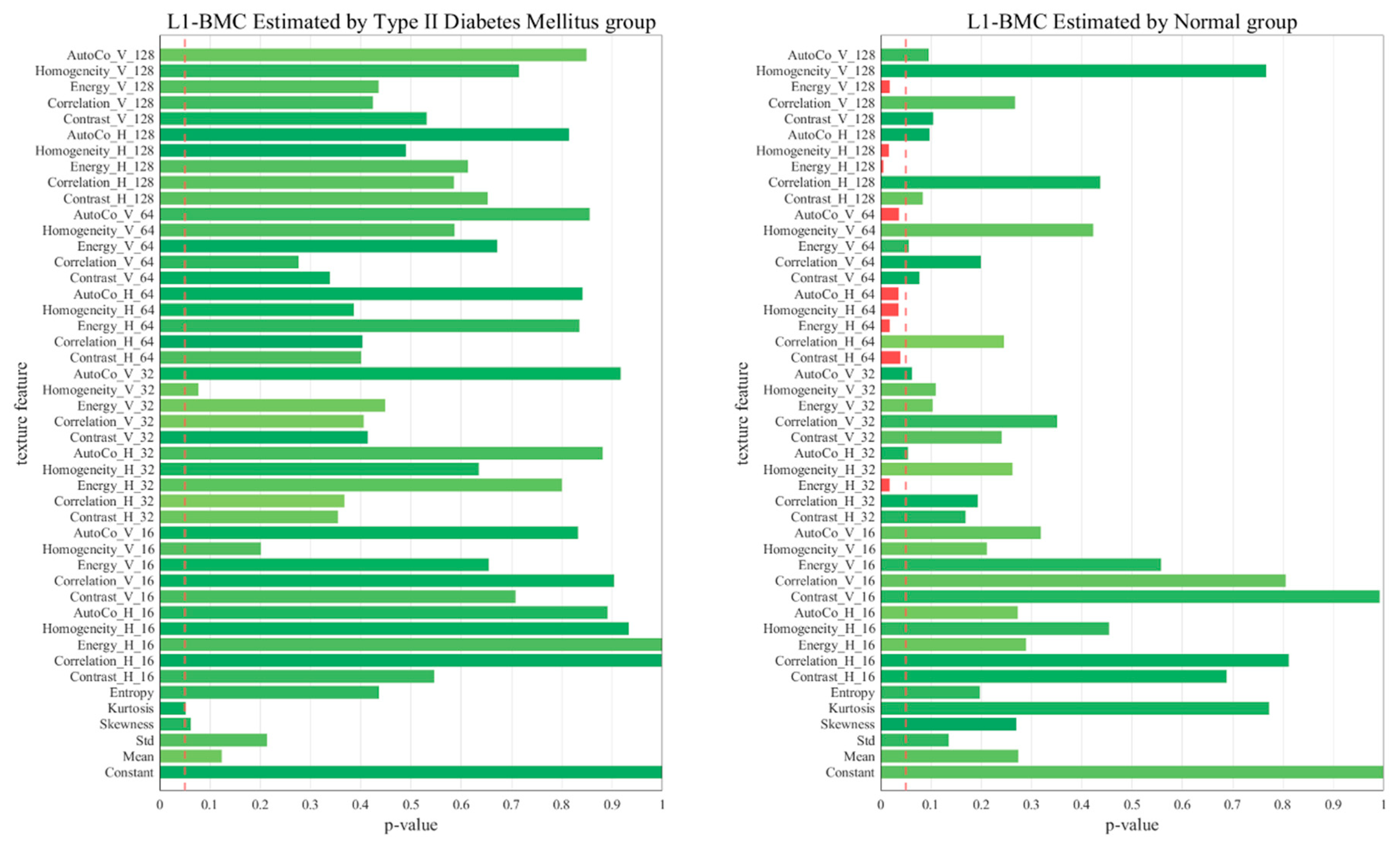
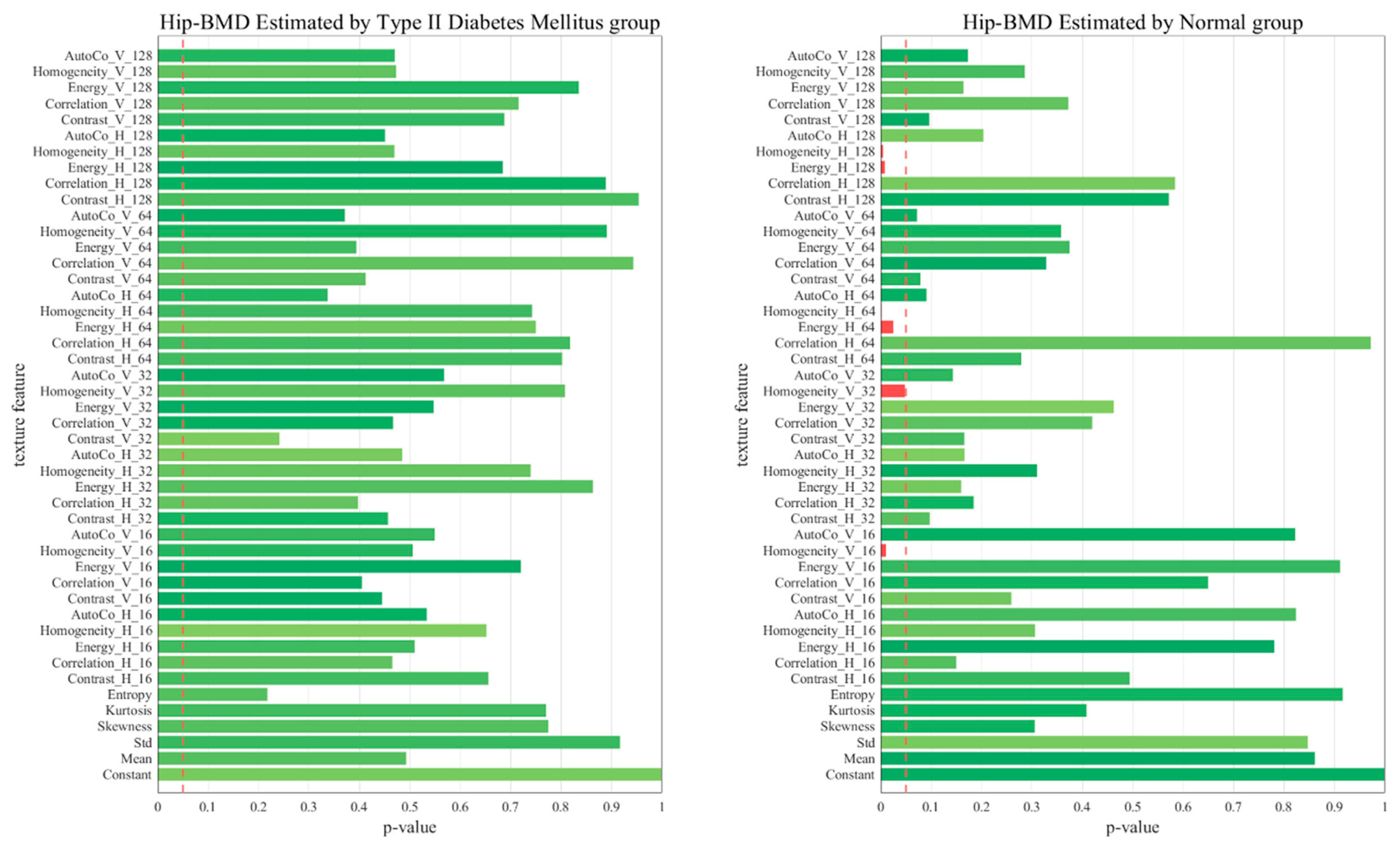
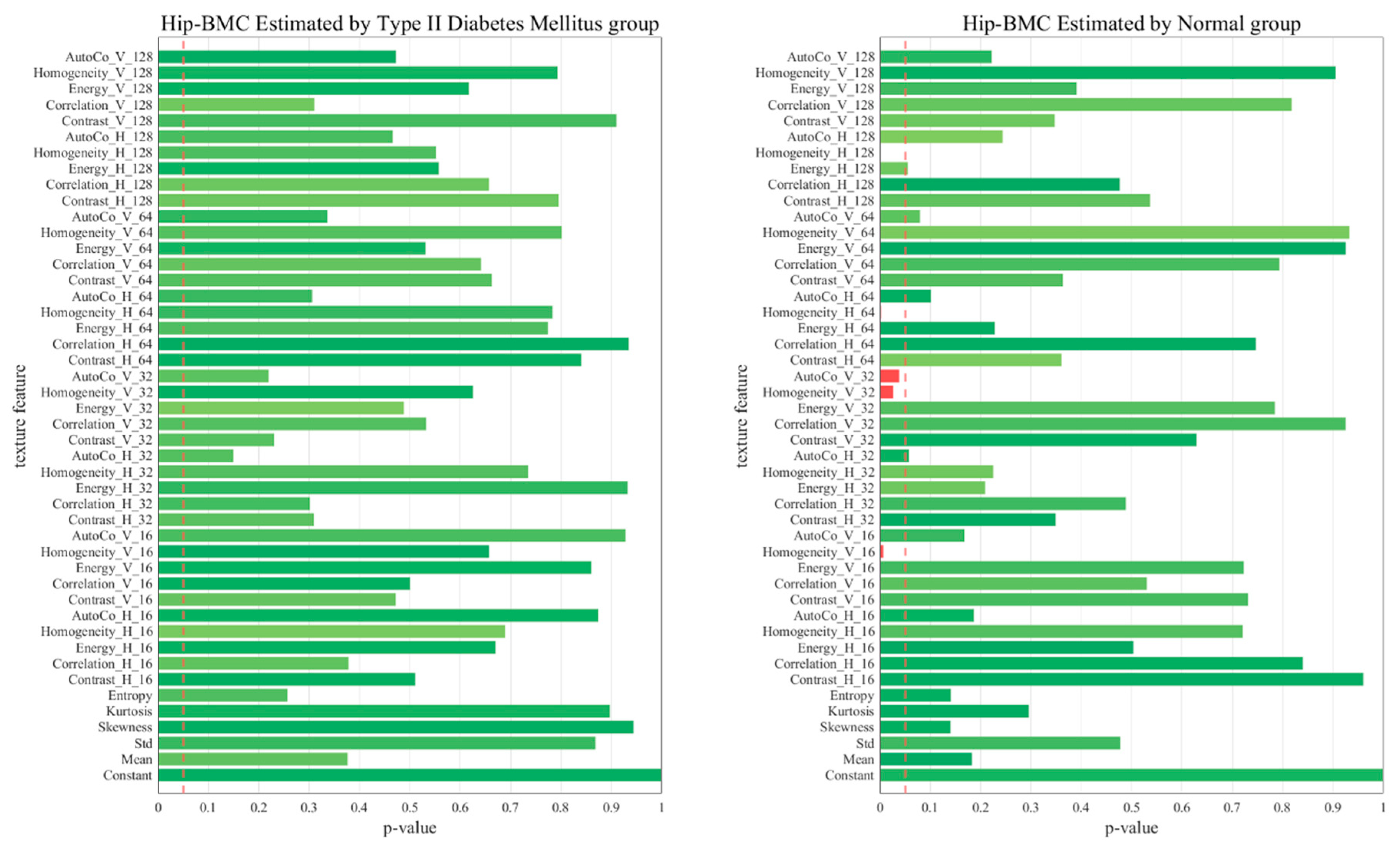
2.6. p-Value of the 45 Texture Feature Analysis between the Two Groups
3. Results
Patient Demographics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lane, N.E. Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol. 2006, 194, S3–S11. [Google Scholar] [CrossRef]
- Melton, L.J., III; Chrischilles, E.A.; Cooper, C.; Lane, A.W.; Riggs, B.L. Perspective how many women have osteoporosis? J. Bone Miner. Res. 1992, 7, 1005–1010. [Google Scholar] [CrossRef]
- Jang, H.-D.; Kim, E.-H.; Lee, J.C.; Choi, S.-W.; Kim, H.S.; Cha, J.-S.; Shin, B.-J. Management of Osteoporotic Vertebral Fracture: Review Update 2022. Asian Spine J. 2022, 16, 934–946. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johnell, O.; Oden, A.; Sernbo, I.; Redlund-Johnell, I.; Dawson, A.; De Laet, C.; Jonsson, B. Long-Term Risk of Osteoporotic Fracture in Malmö. Osteoporos. Int. 2000, 11, 669–674. [Google Scholar] [CrossRef]
- Mills, E.S.; Ton, A.T.; Bouz, G.; Alluri, R.K.; Hah, R.J. Acute Operative Management of Osteoporotic Vertebral Compression Fractures Is Associated with Decreased Morbidity. Asian Spine J. 2022, 16, 634–642. [Google Scholar] [CrossRef]
- Muraki, S.; Yamamoto, S.; Ishibashi, H.; Horiuchi, T.; Hosoi, T.; Orimo, H.; Nakamura, K. Impact of degenerative spinal diseases on bone mineral density of the lumbar spine in elderly women. Osteoporos. Int. 2004, 15, 724–728. [Google Scholar] [CrossRef]
- World Health Organization. Prevention and management of Osteoporosis: Report of a WHO Scientific Group; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Blanchard, J.F.; Ludwig, S.; Wajda, A.; Dean, H.; Anderson, K.; Kendall, O.; Depew, N. Incidence and Prevalence of Diabetes in Manitoba, 1986–1991. Diabetes Care 1996, 19, 807–811. [Google Scholar] [CrossRef]
- Bonds, D.E.; Larson, J.C.; Schwartz, A.V.; Strotmeyer, E.S.; Robbins, J.; Rodriguez, B.L.; Johnson, K.C.; Margolis, K.L. Risk of Fracture in Women with Type 2 Diabetes: The Women’s Health Initiative Observational Study. J. Clin. Endocrinol. Metab. 2006, 91, 3404–3410. [Google Scholar] [CrossRef] [PubMed]
- Janghorbani, M.; Feskanich, D.; Willett, W.C.; Hu, F. Prospective study of diabetes and risk of hip fracture: The Nurses’ Health Study. Diabetes Care 2006, 29, 1573–1578. [Google Scholar] [CrossRef]
- de Liefde, I.I.; van der Klift, M.; de Laet, C.E.D.H.; van Daele, P.L.A.; Hofman, A.; Pols, H.A.P. Bone mineral density and fracture risk in type-2 diabetes mellitus: The Rotterdam Study. Osteoporos. Int. 2005, 16, 1713–1720. [Google Scholar] [CrossRef]
- Janghorbani, M.; Van Dam, R.M.; Willett, W.C.; Hu, F.B. Systematic Review of Type 1 and Type 2 Diabetes Mellitus and Risk of Fracture. Am. J. Epidemiol. 2007, 166, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Strotmeyer, E.S.; Cauley, J.A.; Schwartz, A.V.; Nevitt, M.C.; Resnick, H.E.; Bauer, D.C.; Tylavsky, F.A.; de Rekeneire, N.; Harris, T.B.; Newman, A.B. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: The health, aging, and body composition study. Arch. Intern. Med. 2005, 165, 1612–1617. [Google Scholar] [CrossRef]
- Unwin, N.; Whiting, D.; Gan, D.; Jacqmain, O.; Ghyoot, G. International Diabetes Federation Diabetes Atlas. IDF Diabetes Atlas 2009, 4, 23–25. [Google Scholar]
- Wild, S.H.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030. Diabetes Care 2004, 27, 2569. [Google Scholar] [CrossRef]
- Andreoli, A.; Scalzo, G.; Masala, S.; Tarantino, U.; Guglielmi, G. Body composition assessment by dual-energy X-ray absorptiometry (DXA). Radiol. Med. 2009, 114, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-W.; Lee, D.-H.; Huh, J.-W.; Bai, J.-W. The impact of obesity on the accuracy of DXA BMD for DXA-equivalent BMD estimation. BMC Musculoskelet. Disord. 2022, 23, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Xuan, M.; Ji, P.; Zhang, X. Assessment of fracture risk by FRAX model in older adults with type 2 diabetes: A cross-sectional study in China. Int. J. Clin. Exp. Med. 2016, 9, 20432–20438. [Google Scholar]
- Hou, D.J.; Tso, D.K.; Davison, C.; Inacio, J.; Louis, L.J.; Nicolaou, S.; Reimann, A.J. Clinical utility of ultra high pitch dual source thoracic CT imaging of acute pulmonary embolism in the emergency department: Are we one step closer towards a non-gated triple rule out? Eur. J. Radiol. 2013, 82, 1793–1798. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Gordon, C.M.; Baim, S.; Leonard, M.B.; Bishop, N.J.; Bianchi, M.-L.; Kalkwarf, H.J.; Langman, C.B.; Plotkin, H.; Rauch, F.; et al. International Society for Clinical Densitometry 2007 Adult and Pediatric Official Positions. Bone 2008, 43, 1115–1121. [Google Scholar] [CrossRef]
- Mardia, K.V.; Hainsworth, T. A spatial thresholding method for image segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 1988, 10, 919–927. [Google Scholar] [CrossRef]
- Leslie, W.D.; Rubin, M.R.; Schwartz, A.V.; Kanis, J.A. Type 2 diabetes and bone. J. Bone Miner. Res. 2012, 27, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Zubair, A.R.; Alo, O.A. Grey level co-occurrence matrix (GLCM) based second order statistics for image texture analysis. Int. J. Sci. Eng. Investig. 2019, 8, 64–73. [Google Scholar]
- Chen, S.M.; Bauer, D.J.; Belzak, W.M.; Brandt, H. Advantages of Spike and Slab Priors for Detecting Differential Item Functioning Relative to Other Bayesian Regularizing Priors and Frequentist Lasso. Struct. Equ. Model. A Multidiscip. J. 2022, 29, 122–139. [Google Scholar] [CrossRef]
- Huang, H.-H.; Rao, H.; Miao, R.; Liang, Y. A novel meta-analysis based on data augmentation and elastic data shared lasso regularization for gene expression. BMC Bioinform. 2022, 23, 353. [Google Scholar] [CrossRef]
- Han, L.; Pinson, P.; Kazempour, J. Trading data for wind power forecasting: A regression market with lasso regularization. Electr. Power Syst. Res. 2022, 212, 108442. [Google Scholar] [CrossRef]
- Ofori, I.K.; Obeng, C.K.; Asongu, S.A. What Really Drives Economic Growth in Sub-Saharan Africa? Evidence from the Lasso Regularization and Inferential Techniques. J. Knowl. Econ. 2022, 1–36. [Google Scholar] [CrossRef]
- Dong, X.N.; Yeni, Y.N.; Zhang, B.; Les, C.M.; Gibson, G.J.; Fyhrie, D.P. Matrix Concentration of Insulin-like Growth Factor I (IGF-I) is Negatively Associated with Biomechanical Properties of Human Tibial Cancellous Bone Within Individual Subjects. Calcif. Tissue Int. 2005, 77, 37–44. [Google Scholar] [CrossRef]
- Hsieh, C.-I.; Zheng, K.; Lin, C.; Mei, L.; Lu, L.; Li, W.; Chen, F.-P.; Wang, Y.; Zhou, X.; Wang, F.; et al. Automated bone mineral density prediction and fracture risk assessment using plain radiographs via deep learning. Nat. Commun. 2021, 12, 5472. [Google Scholar] [CrossRef]
- Ma, B.; Li, C.; Pan, J.; Zhang, S.; Dong, H.; Wu, Y.; Lv, J. Causal Associations of Anthropometric Measurements With Fracture Risk and Bone Mineral Density: A Mendelian Randomization Study. J. Bone Miner. Res. 2021, 36, 1281–1287. [Google Scholar] [CrossRef]
- Shevroja, E.; Cafarelli, F.P.; Guglielmi, G.; Hans, D. DXA parameters, Trabecular Bone Score (TBS) and Bone Mineral Density (BMD), in fracture risk prediction in endocrine-mediated secondary osteoporosis. Endocrine 2021, 74, 20–28. [Google Scholar] [CrossRef]
- Larsson, B.A.M.; Johansson, L.; Johansson, H.; Axelsson, K.F.; Harvey, N.; Vandenput, L.; Magnusson, P.; McCloskey, E.; Liu, E.; Kanis, J.A.; et al. The timed up and go test predicts fracture risk in older women independently of clinical risk factors and bone mineral density. Osteoporos. Int. 2021, 32, 75–84. [Google Scholar] [CrossRef]
- Ginsberg, C.; Hoofnagle, A.N.; Katz, R.; Hughes-Austin, J.; Miller, L.M.; Becker, J.O.; Kritchevsky, S.B.; Shlipak, M.G.; Sarnak, M.J.; Ix, J.H. The vitamin D metabolite ratio is associated with changes in bone density and fracture risk in older adults. J. Bone Miner. Res. 2021, 36, 2343–2350. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Back, G.-Y.; Lee, S.-H. Relationship between Type 2 Diabetes Mellitus and Lumbar Bone Mineral Density in Postmenopausal Women. Asian Spine J. 2021, 15, 721. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.N.; Shirvaikar, M.; Wang, X. Biomechanical properties and microarchitecture parameters of trabecular bone are correlated with stochastic measures of 2D projection images. Bone 2013, 56, 327–336. [Google Scholar] [CrossRef] [PubMed]
- van Staa, T.; Dennison, E.; Leufkens, H.; Cooper, C. Epidemiology of fractures in England and Wales. Bone 2001, 29, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, D.K.; Soliman, M.; Elfar, J.C.; Lewis, G.S.; Abraham, T.; Kamal, F.; Elbarbary, R.A. Aberrant structure of fibrillar collagen and elevated levels of advanced glycation end products typify delayed fracture healing in the diet-induced obesity mouse model. Bone 2020, 137, 115436. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.W.; Thomas, B.J.; Brown, J.K.; Finkelstein, J.S. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J. Bone Miner. Res. 2012, 27, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; He, D.; Ai, B.; Wang, J.; Qi, H.; Guan, K.; Zhong, Z. Artificial Neural Network Based Path Loss Prediction for Wireless Communication Network. IEEE Access 2020, 8, 199523. [Google Scholar] [CrossRef]
- Smys, D.S.; Basar, D.A.; Wang, D.H. Artificial neural network based power management for smart street lighting systems. J. Artif. Intell. Capsul. Netw. 2020, 2, 42–52. [Google Scholar]
- Toraman, S.; Alakus, T.B.; Turkoglu, I. Convolutional capsnet: A novel artificial neural network approach to detect COVID-19 disease from X-ray images using capsule networks. Chaos Solitons Fractals 2020, 140, 110122. [Google Scholar] [CrossRef]
- Herzog, S.; Tetzlaff, C.; Wörgötter, F. Evolving artificial neural networks with feedback. Neural Netw. 2020, 123, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Heyl, M. Quantum Many-Body Dynamics in Two Dimensions with Artificial Neural Networks. Phys. Rev. Lett. 2020, 125, 100503. [Google Scholar] [CrossRef] [PubMed]
- Rasamoelina, A.D.; Adjailia, F.; Sinčák, P. A Review of Activation Function for Artificial Neural Network. In Proceedings of the 2020 IEEE 18th World Symposium on Applied Machine Intelligence and Informatics (SAMI), Kassa, Slovakia, 23–25 January 2020. [Google Scholar]
- Jordan, M.I.; Mitchell, T.M. Machine learning: Trends, perspectives, and prospects. Science 2015, 349, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Hall-Beyer, M. GLCM Texture: A Tutorial; National Council on Geographic Information and Analysis Remote Sensing Core Curriculum: Calgary, AB, Canada, 2000; Volume 3, p. 75. [Google Scholar]
- Mohanaiah, P.; Sathyanarayana, P.; GuruKumar, L. Image texture feature extraction using GLCM approach. Int. J. Sci. Res. Publ. 2013, 3, 5. [Google Scholar]
- Yanti, C.P.; Andika, I.G. Klasifikasi citra HSV aksara kuno pada prasasti tembaga Kintamani menggunakan GLRCM dan SVM. J. Teknol. Dan Sist. Komput. 2020, 8, 94–99. [Google Scholar]
- Tan, J.; Gao, Y.; Liang, Z.; Cao, W.; Pomeroy, M.J.; Huo, Y.; Li, L.; Barish, M.A.; Abbasi, A.F.; Pickhardt, P.J. 3D-GLCM CNN: A 3-Dimensional Gray-Level Co-Occurrence Matrix-Based CNN Model for Polyp Classification via CT Colonography. IEEE Trans. Med. Imaging 2019, 39, 2013–2024. [Google Scholar] [CrossRef]
- Mall, P.K.; Singh, P.K.; Yadav, D. GLCM Based Feature Extraction and Medical X-RAY Image Classification Using Machine Learning Techniques. In Proceedings of the 2019 IEEE Conference on Information and Communication Technology, Xi’an, China, 16–19 October 2019. [Google Scholar]
- Park, Y.; Guldmann, J.-M. Measuring continuous landscape patterns with Gray-Level Co-Occurrence Matrix (GLCM) indices: An alternative to patch metrics? Ecol. Indic. 2020, 109, 105802. [Google Scholar] [CrossRef]

| Analytical Tool | Parameter | Value/Name/Function | Feature # |
|---|---|---|---|
| Histogram | Statistics (k) | mean (k = 1), standard deviation (k = 2), skewness (k = 3), kurtosis (k = 4) entropy (k = 5) | 5 |
| Texture (GLCM) | Directions (l) | horizontal (l = 1), vertical (l = 2) | 2 × 4 × 5 = 40 |
| Levels (m) | 16 (m = 1), 32 (m = 2), 64 (m = 3), 128 (m = 4) | ||
| Statistics (n) | contrast (n = 1), correlation (n = 2), energy (n = 3), homogeneity (n = 4), variance (n = 5) |
| Case (number) (T2D/Normal) | 510 (145/365) |
| Mean age (years) for T2D | 59.52 ± 11.26 |
| The time between CT and DXA dates (days) for T2D | 1.57 ± 3.82 |
| Sex (male/female) for T2D | 65/80 |
| BMI (kg/m2) for T2D | 24.79 ± 3.35 |
| Mean age (years) for normal | 57.12 ± 12.46 |
| The time between CT and DXA dates (days) for normal | 1.38 ± 4.17 |
| Sex (male/female) for normal | 171/194 |
| BMI (kg/m2) for normal | 23.15 ± 4.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.W.; Huh, J.W.; Noh, Y.M.; Seo, H.E.; Lee, D.H. Exploring the Paradox of Bone Mineral Density in Type 2 Diabetes: A Comparative Study Using Opportunistic Chest CT Texture Analysis and DXA. Diagnostics 2023, 13, 2784. https://doi.org/10.3390/diagnostics13172784
Kim MW, Huh JW, Noh YM, Seo HE, Lee DH. Exploring the Paradox of Bone Mineral Density in Type 2 Diabetes: A Comparative Study Using Opportunistic Chest CT Texture Analysis and DXA. Diagnostics. 2023; 13(17):2784. https://doi.org/10.3390/diagnostics13172784
Chicago/Turabian StyleKim, Min Woo, Jung Wook Huh, Young Min Noh, Han Eol Seo, and Dong Ha Lee. 2023. "Exploring the Paradox of Bone Mineral Density in Type 2 Diabetes: A Comparative Study Using Opportunistic Chest CT Texture Analysis and DXA" Diagnostics 13, no. 17: 2784. https://doi.org/10.3390/diagnostics13172784
APA StyleKim, M. W., Huh, J. W., Noh, Y. M., Seo, H. E., & Lee, D. H. (2023). Exploring the Paradox of Bone Mineral Density in Type 2 Diabetes: A Comparative Study Using Opportunistic Chest CT Texture Analysis and DXA. Diagnostics, 13(17), 2784. https://doi.org/10.3390/diagnostics13172784






