New Non-Invasive Imaging Technologies in Cardiac Transplant Follow-Up: Acquired Evidence and Future Options
Abstract
1. Introduction
2. Echocardiographic Follow-Up in Transplant Recipients
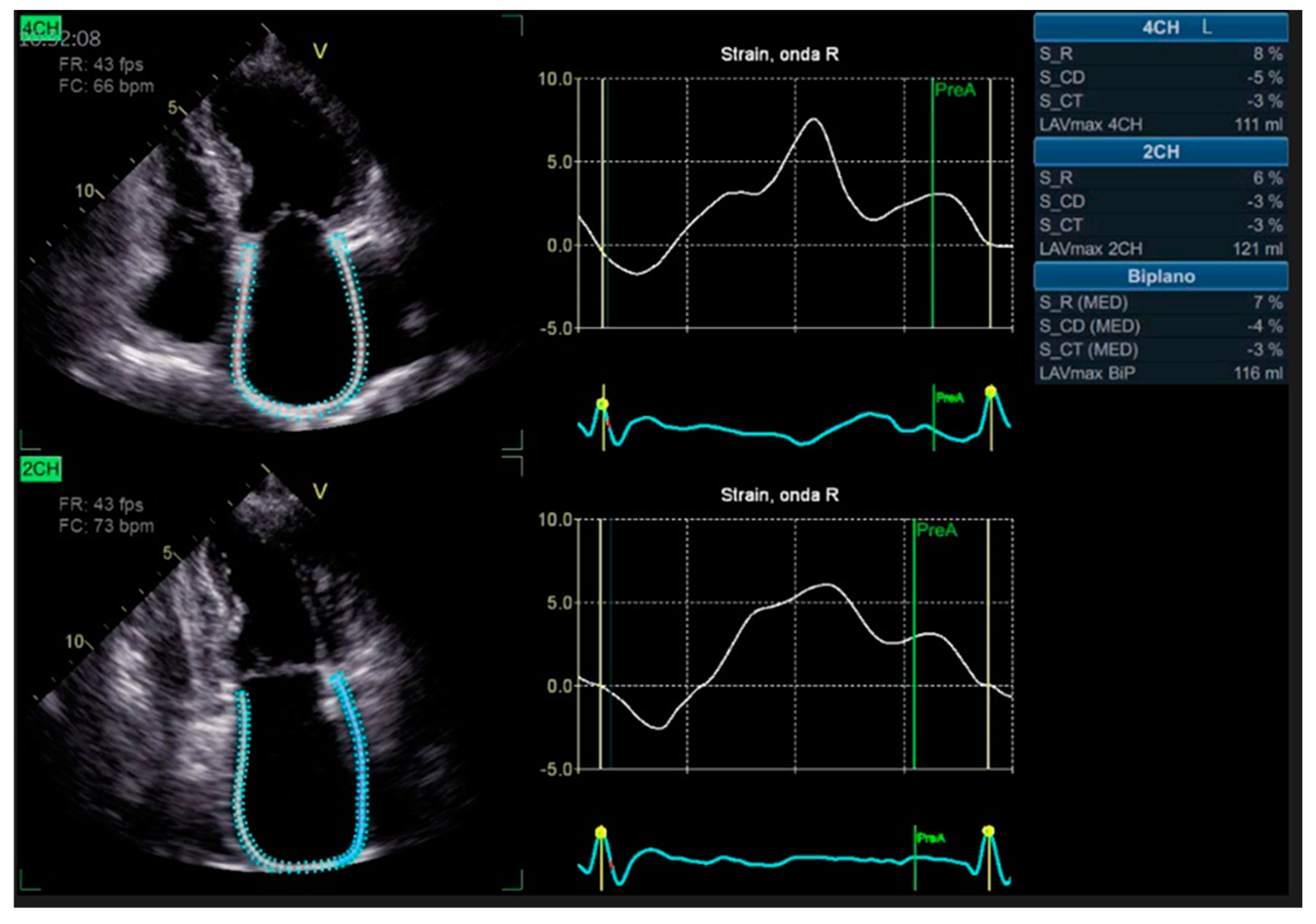
3. Echocardiography: What New Technologies Add
4. Assessment of Right Ventricle and Tricuspid Valve
5. Stress Echocardiography
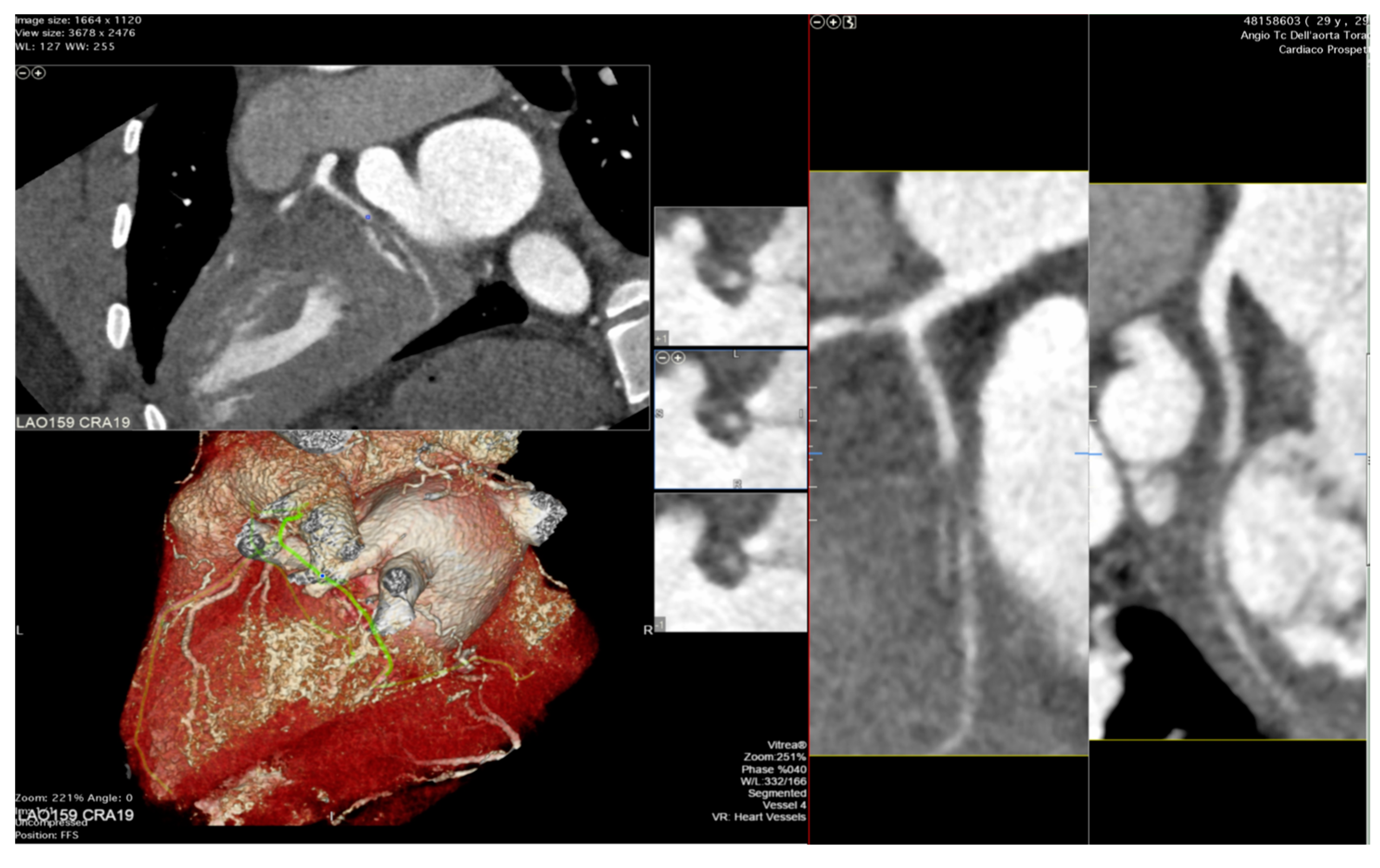
6. The Role of Coronary Computed Tomography Angiography and Nuclear Imaging in the Follow-Up of Heart Transplant Recipients
7. Diagnostic Challenges and Potential Benefits of CMR in the Follow-Up of Heart Transplant Recipients
8. Conclusions
9. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mehra, M.R.; Crespo-Leiro, M.G.; Dipchand, A.; Ensminger, S.M.; Hiemann, N.E.; Kobashigawa, J.A.; Madsen, J.; Parameshwar, J.; Starling, R.C.; Uber, P.A. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy—2010. J. Heart Lung Transplant. 2010, 29, 717–727. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Badano, L.P.; Miglioranza, M.H.; Edvardsen, T.; Colafranceschi, A.S.; Muraru, D.; Bacal, F.; Nieman, K.; Zoppellaro, G.; Marcondes Braga, F.G.; Binder, T.; et al. European Association of Cardiovascular Imaging/Cardiovascular Imaging Department of the Brazilian Society of Cardiology recommendations for the use of cardiac imaging to assess and follow pa-tients after heart transplantation. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 919–948. [Google Scholar] [CrossRef] [PubMed]
- Sciaccaluga, C.; Ghionzoli, N.; Mandoli, G.; Sisti, N.; D’ascenzi, F.; Focardi, M.; Bernazzali, S.; Vergaro, G.; Emdin, M.; Valente, S.; et al. The role of non-invasive imaging modalities in cardiac allograft vasculopathy: An updated focus on current evidences. Heart Fail. Rev. 2022, 27, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Velleca, A.; Shullo, M.A.; Dhital, K.; Azeka, E.; Colvin, M.; DePasquale, E.; Farrero, M.; García-Guereta, L.; Jamero, G.; Khush, K.; et al. The International Society for Heart and Lung Transplantation (ISHLT) guidelines for the care of heart transplant recipients. J. Heart Lung Transplant. 2023, 42, e1–e141. [Google Scholar] [CrossRef] [PubMed]
- Valantine, H.A.; Hatle, L.K.; Appleton, C.P.; Gibbons, R.; Popp, R.L. Variability of Doppler Echocardiographic Indexes of Left Ventricular Filling in Transplant Recipients and in Normal Subjects. J. Am. Soc. Echocardiogr. 1990, 3, 276–284. [Google Scholar] [CrossRef]
- Gorcsan, J., 3rd; Snow, F.R.; Paulsen, W.; Arrowood, J.A.; Thompson, J.A.; Nixon, J. Echocardiographic profile of the transplanted human heart in clinically well recipients. J. Heart Lung Transplant. 1992, 11 Pt 1, 80–89. [Google Scholar] [PubMed]
- Clemmensen, T.S.; Løgstrup, B.B.; Eiskjær, H.; Poulsen, S.H. Evaluation of longitudinal myocardial deformation by 2-dimensional speckle-tracking echocardiography in heart transplant recipients: Relation to coronary allograft vasculopathy. J. Heart Lung Transplant. 2015, 34, 195–203. [Google Scholar] [CrossRef]
- Sade, L.; Sezgin, A.; Uluçam, M.; Taymaz, S.; Şimşek, V.; Tayfun, E.; Tokel, K.; Aşlamaci, S.; Müderrisoğlu, H. Evaluation of the potential role of echocardiography in the detection of allograft rejection in heart transplant recipients. Transplant. Proc. 2006, 38, 636–638. [Google Scholar] [CrossRef]
- Bech-Hanssen, O.; Al-Habeeb, W.; Ahmed, W.; Di Salvo, G.; Pergola, V.; Al-Admawi, M.; Al-Amri, M.; Al-Shahid, M.; Al-Buraiki, J.; Fadel, B.M. Echocardiography detects elevated left ventricular filling pressures in heart transplant recipients. Echocardiography 2014, 32, 411–419. [Google Scholar] [CrossRef]
- LeJemtel, T.H. Review of a controlled trial of exercise rehabilitation after heart transplantation. Curr. Cardiol. Rep. 1999, 1, 32. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, E.W.; Epstein, M.; Ota, D.; Hoagland, P.M.; Gordon, J.B.; Adamson, R.M.; McDaniel, M.; Peterson, K.L.; Smith, S.C., Jr.; Jaski, B.E. Right and left ventricular function after cardiac transplantation: Changes during and after rejection. Circulation 1991, 84, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Tona, F.; Caforio, A.L.P.; Piaserico, S.; Bontorin, M.; De Simone, G.; Leone, M.G.; Fortina, A.B.; Gambino, A.; Feltrin, G.; Calzolari, D.; et al. Abnormal total ejection isovolume index as early noninvasive marker of chronic rejection in heart transplantation*. Transpl. Int. 2005, 18, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Antończyk, K.; Niklewski, T.; Antończyk, R.; Zakliczyński, M.; Zembala, M.; Kukulski, T. Speckle-Tracking Echocardiography for Monitoring Acute Rejection in Transplanted Heart. Transplant. Proc. 2018, 50, 2090–2094. [Google Scholar] [CrossRef] [PubMed]
- Eleid, M.F.; Caracciolo, G.; Cho, E.J.; Scott, R.L.; Steidley, D.E.; Wilansky, S.; Arabia, F.A.; Khandheria, B.K.; Sengupta, P.P. Natural History of Left Ventricular Mechanics in Transplanted Hearts: Relationships With Clinical Variables and Genetic Expression Profiles of Allograft Rejection. JACC Cardiovasc. Imaging 2010, 3, 989–1000. [Google Scholar] [CrossRef]
- Saleh, H.K.; Villarraga, H.R.; Kane, G.C.; Pereira, N.L.; Raichlin, E.; Yu, Y.; Koshino, Y.; Kushwaha, S.S.; Miller, F.A.; Oh, J.K.; et al. Normal left ventricular mechanical function and synchrony values by speckle-tracking echocardiography in the transplanted heart with normal ejection fraction. J. Heart Lung Transplant. 2011, 30, 652–658. [Google Scholar] [CrossRef]
- Sarvari, S.I.; Gjesdal, O.; Gude, E.; Arora, S.; Andreassen, A.K.; Gullestad, L.; Geiran, O.; Edvardsen, T. Early postoperative left ventricular function by echocardiographic strain is a predictor of 1-year mortality in heart transplant recipients. J. Am. Soc. Echocardiogr. 2012, 25, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Dell'Aquila, A.M.; Mastrobuoni, S.; Bastarrika, G.; Praschker, B.L.; Agüero, P.A.; Castaño, S.; Herreros, J.; Rabago, G. Bicaval versus standard technique in orthotopic heart transplant: Assessment of atrial performance at magnetic resonance and transthoracic echocardiography. Interact. Cardiovasc. Thorac. Surg. 2012, 14, 457–462. [Google Scholar] [CrossRef]
- Bech-Hanssen, O.; Pergola, V.; Al-Admawi, M.; Fadel, B.M.; Di Salvo, G. Atrial function in heart transplant recipients operated with the bicaval technique. Scand. Cardiovasc. J. 2015, 50, 42–51. [Google Scholar] [CrossRef]
- Zhu, S.; Xie, Y.; Qiao, W.; Tian, F.; Sun, W.; Wang, Y.; Wu, C.; Li, H.; Yi, L.; Zhong, Y.; et al. Impaired left atrial function in clinically well heart transplant patients. Int. J. Cardiovasc. Imaging 2021, 37, 1937–1945. [Google Scholar] [CrossRef]
- Bhatia, S.J.; Kirshenbaum, J.M.; Shemin, R.J.; Cohn, L.H.; Collins, J.J.; Di Sesa, V.J.; Young, P.J.; Mudge, G.H., Jr.; Sutton, M.G. Time course of resolution of pulmonary hypertension and right ventricular remodeling after orthotopic cardiac transplantation. Circulation 1987, 76, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, R.; Edwards, N.F.A.; Scalia, G.M.; Chan, J. Novel left and right ventricular strain analysis to detect subclinical myocardial dysfunction in cardiac allograft rejection. Int. J. Cardiovasc. Imaging 2021, 38, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.B.B.V.; Hajjar, L.A.; Bacal, F.; Lofrano-Alves, M.S.; Lima, M.S.M.; Abduch, M.C.; Vieira, M.L.C.; Chiang, H.P.; Salviano, J.B.C.; da Silva Costa, I.B.S.; et al. Usefulness of speckle tracking echocardiography and biomarkers for detecting acute cellular rejection after heart transplantation. Cardiovasc. Ultrasound 2021, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Atzenhoefer, M.; Jahangir, A.; Payne, A.; Hendawi, M.; Dakwar, O.; Ali, M.; Thohan, V.; Muthukumar, L. Echocardiographic assessment of radial right ventricular function in heart transplant recipients. ESC Heart Fail. 2021, 8, 5613–5616. [Google Scholar] [CrossRef] [PubMed]
- Wartig, M.; Tesan, S.; Gäbel, J.; Jeppsson, A.; Selimovic, N.; Holmberg, E.; Dellgren, G. Tricuspid regurgitation influences outcome after heart transplantation. J. Heart Lung. Transplant. 2014, 33, 829–835. [Google Scholar] [CrossRef]
- Wong, R.C.-C.; Abrahams, Z.; Hanna, M.; Pangrace, J.; Gonzalez-Stawinski, G.; Starling, R.; Taylor, D. Tricuspid regurgitation after cardiac transplantation: An old problem revisited. J. Heart Lung Transplant. 2008, 27, 247–252. [Google Scholar] [CrossRef]
- De Simone, R.; Lange, R.; Sack, R.U.; Mehmanesh, H.; Hagl, S. Atrioventricular valve insufficiency and atrial geometry after or-thotopic heart transplantation. Ann. Thorac. Surg. 1995, 60, 1686–1693. [Google Scholar] [CrossRef]
- Kwon, M.H.; Shemin, R.J. Tricuspid valve regurgitation after heart transplantation. Ann. Cardiothorac. Surg. 2017, 6, 270–274. [Google Scholar] [CrossRef]
- Aziz, T.M.; Burgess, M.I.; Rahman, A.N.; Campbell, C.S.; Deiraniya, A.K.; Yonan, N.A. Risk factors for tricuspid valve regurgitation after orthotopic heart transplantation. Ann. Thorac. Surg. 1999, 68, 1247–1251. [Google Scholar] [CrossRef]
- Kim, H.R.; Kim, H.J.; Lee, S.E.; Jung, S.-H.; Yun, T.-J.; Kim, J.J.; Lee, J.W. Prevalence and Risk Factors of Post–heart Transplant Tricuspid Regurgitation. Transplantation 2022, 106, e297–e303. [Google Scholar] [CrossRef]
- Veen, K.M.; Papageorgiou, G.; Zijderhand, C.F.; Mokhles, M.M.; Brugts, J.J.; Manintveld, O.C.; Constantinescu, A.A.; Bekkers, J.A.; Takkenberg, J.J.M.; Bogers, A.J.J.C.; et al. The clinical impact of tricuspid regurgitation in patients with a biatrial orthotopic heart transplant. Front. Med. 2023, 17, 527–533. [Google Scholar] [CrossRef]
- Tan, Z.; Roscoe, A.; Rubino, A. Transesophageal Echocardiography in Heart and Lung Transplantation. J. Cardiothorac. Vasc. Anesthesia 2019, 33, 1548–1558. [Google Scholar] [CrossRef]
- Wells, C.M.; Rangasetty, U.; Subramaniam, K. Imaging in heart failure: Role of preoperative imaging and intraoperative transesophageal echocardiography for heart failure surgery. Int. Anesthesiol. Clin. 2012, 50, 55–82. [Google Scholar] [CrossRef]
- Hahn, R.T.; Abraham, T.; Adams, M.S.; Bruce, C.J.; Glas, K.E.; Lang, R.M.; Reeves, S.T.; Shanewise, J.S.; Siu, S.C.; Stewart, W.; et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: Recommendations from the American society of echocardiography and the society of cardiovascular anesthesiologists. J. Am. Soc. Echocardiogr. 2013, 26, 921–964. [Google Scholar] [CrossRef]
- Shou, B.L.; Halub, M.E.; Zhou, A.L.; Tompkins, B.A.; Choi, C.W. Massive left atrial thrombus evades multimodality imaging as a myxoma in a bicaval heart transplant recipient. J. Card. Surg. 2022, 37, 2884–2887. [Google Scholar] [CrossRef]
- Derumeaux, G.; Redonnet, M.; Mouton-Schleifer, D.; Bessou, J.P.; Cribier, A.; Saoudi, N.; Koning, R.; Soyer, R.; Letac, B. Dobutamine stress echocardiography in orthotopic heart transplant recipients. J. Am. Coll. Cardiol. 1995, 25, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Akosah, K.O.; Mohanty, P.K. Role of dobutamine stress echocardiography in heart transplant patients. Chest 1998, 113, 809–815. [Google Scholar] [CrossRef]
- Elkaryoni, A.; Abu-Sheasha, G.; Altibi, A.M.; Hassan, A.; Ellakany, K.; Nanda, N.C. Diagnostic accuracy of dobutamine stress echocardiography in the detection of cardiac allograft vasculopathy in heart transplant recipients: A systematic review and meta-analysis study. Echocardiography 2019, 36, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodurrahman, M.; Marek, J.; Al Otaibi, T.; Salemi, V.M.C.; Echahidi, N.; Al Buraiki, J.; Fadel, B.M.; Mohty, D. Diagnostic Accuracy of Dobutamine Stress Echocardiography for Detection of Cardiac Allograft Vasculopathy in Orthotopic Heart Transplant Patients. J. Saudi Heart Assoc. 2021, 33, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, E.; D'Hooge, J.; Sutherland, G.R.; Marciniak, A.; Thijs, D.; Droogne, W.; Herbots, L.; Van Cleemput, J.; Claus, P.; Bijnens, B.; et al. Quantitative dobutamine stress echocardiography for the early detection of cardiac allograft vasculopathy in heart transplant recipients. Heart 2008, 94, e3. [Google Scholar] [CrossRef]
- Voigt, J.-U.; Exner, B.; Schmiedehausen, K.; Huchzermeyer, C.; Reulbach, U.; Nixdorff, U.; Platsch, G.; Kuwert, T.; Daniel, W.G.; Flachskampf, F.A.; et al. Strain-rate imaging during dobutamine stress echocardiography provides objective evidence of inducible ischemia. Circulation 2003, 107, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Ingul, C.B.; Stoylen, A.; Slordahl, S.A.; Wiseth, R.; Burgess, M.; Marwick, T.H. Automated Analysis of Myocardial Deformation at Dobutamine Stress Echocardiography: An Angiographic Validation. J. Am. Coll. Cardiol. 2007, 49, 1651–1659. [Google Scholar] [CrossRef]
- Tona, F.; Caforio, A.L.; Montisci, R.; Gambino, A.; Angelini, A.; Ruscazio, M.; Toscano, G.; Feltrin, G.; Ramondo, A.; Gerosa, G.; et al. Coronary flow velocity pattern and coronary flow reserve by contrast-enhanced transthoracic echocardiography predict long-term outcome in heart transplantation. Circulation 2006, 114 (Suppl. 1), I-49–I–55. [Google Scholar] [CrossRef]
- Cecere, A.; Kerkhof, P.L.M.; Civieri, G.; Angelini, A.; Gambino, A.; Fraiese, A.; Bottio, T.; Osto, E.; Famoso, G.; Fedrigo, M.; et al. Coronary Flow Evaluation in Heart Transplant Patients Compared to Healthy Controls Documents the Superiority of Coronary Flow Velocity Reserve Companion as Diagnostic and Prognostic Tool. Front. Cardiovasc. Med. 2022, 9, 887370. [Google Scholar] [CrossRef]
- Shah, N.R.; Blankstein, R.; Villines, T.; Imran, H.; Morrison, A.R.; Cheezum, M.K. Coronary CTA for Surveillance of Cardiac Allograft Vasculopathy. Curr. Cardiovasc. Imaging Rep. 2018, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Nous, F.M.A.; Roest, S.; Dijkman, E.D.; Attrach, M.; Caliskan, K.; Brugts, J.J.; Nieman, K.; Hirsch, A.; Constantinescu, A.A.; Manintveld, O.C.; et al. Clinical implementation of coronary computed tomography angiography for routine detection of cardiac allograft vasculopathy in heart transplant patients. Transpl. Int. 2021, 34, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Günther, A.; Aaberge, L.; Abildgaard, A.; Ragnarsson, A.; Edvardsen, T.; Jakobsen, J.; Andersen, R. Coronary computed tomography in heart transplant patients: Detection of significant stenosis and cardiac allograft vasculopathy, image quality, and radiation dose. Acta Radiol. 2018, 59, 1066–1073. [Google Scholar] [CrossRef]
- Bartykowszki, A.; Kolossváry, M.; Jermendy, Á.L.; Karády, J.; Szilveszter, B.; Károlyi, M.; Balogh, O.; Sax, B.; Merkely, B.; Maurovich-Horvat, P. Image quality of prospectively ECG-triggered coronary CT angiography in heart transplant recipients. Am. J. Roentgenol. 2018, 210, 314–319. [Google Scholar] [CrossRef]
- Stolzmann, P.; Leschka, S.; Scheffel, H.; Krauss, T.; Desbiolles, L.; Plass, A.; Genoni, M.; Flohr, T.G.; Wildermuth, S.; Marincek, B.; et al. Dual-source CT in step-and-shoot mode: Noninvasive coronary angiography with low radiation dose. Radiology 2008, 249, 71–80. [Google Scholar] [CrossRef]
- Budde, R.P.J.; Nous, F.M.A.; Roest, S.; Constantinescu, A.A.; Nieman, K.; Brugts, J.J.; Koweek, L.M.; Hirsch, A.; Leipsic, J.; Manintveld, O.C. CT-derived fractional flow reserve (FFRct) for functional coronary artery evaluation in the follow-up of patients after heart transplantation. Eur. Radiol. 2022, 32, 1843–1852. [Google Scholar] [CrossRef]
- Yang, H.-M.; Khush, K.; Luikart, H.; Okada, K.; Lim, H.-S.; Kobayashi, Y.; Honda, Y.; Yeung, A.C.; Valantine, H.; Fearon, W.F. Invasive assessment of coronary physiology predicts late mortality after heart transplantation. Circulation 2016, 133, 1945–1950. [Google Scholar] [CrossRef]
- Narula, J.; Chandrashekhar, Y.; Ahmadi, A.; Abbara, S.; Berman, D.S.; Blankstein, R.; Leipsic, J.; Newby, D.; Nicol, E.D.; Nieman, K.; et al. SCCT 2021 Expert Consensus Document on Coronary Computed Tomographic Angiography: A Report of the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2021, 15, 192–217. [Google Scholar] [CrossRef] [PubMed]
- Rohnean, A.; Houyel, L.; Sigal-Cinqualbre, A.; To, N.-T.; Elfassy, E.; Paul, J.-F. Heart Transplant Patient Outcomes: 5-Year Mean Follow-Up by Coronary Computed Tomography Angiography. Transplantation 2011, 91, 583–588. [Google Scholar] [CrossRef]
- Bogot, N.R.; Durst, R.; Shaham, D.; Admon, D. Cardiac CT of the Transplanted Heart: Indications, Technique, Appearance, and Complications. Radiographics 2007, 27, 1297–1309. [Google Scholar] [CrossRef]
- Pflugfelder, P.W.; Boughner, D.R.; Rudas, L.; Kostuk, W.J. Enhanced detection of cardiac allograft arterial disease with intracoronary ultrasonographic imaging. Am. Heart J. 1993, 125, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.N.; Narula, J.; Starling, R.C.; Towbin, J.; et al. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation 2007, 116, 2216–2233. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Kindermann, I.; Kindermann, M.; Mahfoud, F.; Ukena, C.; Athanasiadis, A.; Hill, S.; Mahrholdt, H.; Voehringer, M.; Schieber, M.; et al. Comparative evaluation of left and right ventricular endomyocardial biopsy: Differences in complication rate and diagnostic performance. Circulation 2010, 122, 900–909. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A.; Hoe, J. Quantification of coronary arterial stenoses by multidetector CT angiography in comparison with conventional angiography: Methods, caveats, and implications. JACC Cardiovasc. Imaging 2011, 4, 191–202. [Google Scholar] [CrossRef]
- Károlyi, M.; Kolossváry, M.; Bartykowszki, A.; Kocsmár, I.; Szilveszter, B.; Karády, J.; Merkely, B.; Maurovich-Horvat, P. Quantitative CT assessment identifies more heart transplanted patients with progressive coronary wall thickening than standard clinical read. J. Cardiovasc. Comput. Tomogr. 2019, 13, 128–133. [Google Scholar] [CrossRef]
- de Graaf, M.A.; Broersen, A.; Kitslaar, P.H.; Roos, C.J.; Dijkstra, J.; Lelieveldt, B.P.F.; Jukema, J.W.; Schalij, M.J.; Delgado, V.; Bax, J.J.; et al. Automatic quantification and characterization of coronary atherosclerosis with computed tomography coronary angiography: Cross-correlation with intravascular ultrasound virtual histology. Int. J. Cardiovasc. Imaging 2013, 29, 1177–1190. [Google Scholar] [CrossRef]
- Feher, A.; Sinusas, A.J. Evaluation of cardiac allograft vasculopathy by positron emission tomography. J. Nucl. Cardiol. 2021, 28, 2616–2628. [Google Scholar] [CrossRef] [PubMed]
- Feher, A.; Miller, E.J. PET Myocardial Blood Flow for Post-transplant Surveillance and Cardiac Allograft Vasculopathy in Heart Transplant Recipients. Curr. Cardiol. Rep. 2022, 24, 1865–1871. [Google Scholar] [CrossRef]
- Wiefels, C.; Almufleh, A.; Yao, J.; Dekemp, R.A.; Chong, A.-Y.; Mielniczuk, L.M.; Stadnick, E.; Davies, R.A.; Beanlands, R.S.; Chih, S. Prognostic utility of longitudinal quantification of PET myocardial blood flow early post heart transplantation. J. Nucl. Cardiol. 2022, 29, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, U.M.; Sciammarella, M.; Pampaloni, M.H.; Botvinick, E.H.; Gullberg, G.T.; DeMarco, T.; Seo, Y. Assessment of late-term progression of cardiac allograft vasculopathy in patients with orthotopic heart transplantation using quantitative cardiac 82Rb PET. Int. J. Cardiovasc. Imaging 2020, 37, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Abadie, B.Q.; Chan, N.; Sharalaya, Z.; Bhat, P.; Harb, S.; Jacob, M.; Starling, R.C.; Tang, W.W.; Cremer, P.C.; Jaber, W.A. Negative Predictive Value and Prognostic Associations of Rb-82 PET/CT with Myocardial Blood Flow in CAV. JACC Heart Fail. 2023, 11, 555–565. [Google Scholar] [CrossRef]
- Ko, K.-Y.; Ko, C.-L.; Lee, C.-M.; Cheng, J.-S.; Wu, Y.-W.; Hsu, R.-B.; Chen, Y.-S.; Wang, S.-S.; Yen, R.-F.; Cheng, M.-F. Myocardial Flow Assessment After Heart Transplantation Using Dynamic Cadmium-Zinc-Telluride Single-Photon Emission Computed Tomography With 201Tl and 99mTc Tracers and Validated by 13N-NH3 Positron Emission Tomography. Circ. Cardiovasc. Imaging 2023, 16, e015034. [Google Scholar] [CrossRef]
- Bellenger, N.; Burgess, M.; Ray, S.; Lahiri, A.; Coats, A.; Cleland, J.; Pennell, D. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance. Are they interchangeable? Eur. Heart J. 2000, 21, 1387–1396. [Google Scholar] [CrossRef]
- Eitel, I.; Friedrich, M.G. T2-weighted cardiovascular magnetic resonance in acute cardiac disease. J. Cardiovasc. Magn. Reson. 2011, 13, 13. [Google Scholar] [CrossRef]
- Vermes, E.; Pantaléon, C.; Auvet, A.; Cazeneuve, N.; Machet, M.C.; Delhommais, A.; Bourguignon, T.; Aupart, M.; Brunereau, L. Cardiovascular magnetic resonance in heart transplant patients: Diagnostic value of quantitative tissue markers: T2 mapping and extracellular volume fraction, for acute rejection diagnosis. J. Cardiovasc. Magn. Reson. 2018, 20, 59. [Google Scholar] [CrossRef]
- Moon, J.C.; Messroghli, D.R.; Kellman, P.; Piechnik, S.K.; Robson, M.D.; Ugander, M.; Gatehouse, P.D.; Arai, A.E.; Friedrich, M.G.; Neubauer, S.; et al. Myocardial T1 mapping and extracellular volume quantification: A Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J. Cardiovasc. Magn. Reson. 2013, 15, 92. [Google Scholar] [CrossRef]
- Taylor, A.J.; Vaddadi, G.; Pfluger, H.; Butler, M.; Bergin, P.; Leet, A.; Richardson, M.; Cherayath, J.; Iles, L.; Kaye, D.M. Diagnostic performance of multisequential cardiac magnetic resonance imaging in acute cardiac allograft rejection. Eur. J. Heart Fail. 2010, 12, 45–51. [Google Scholar] [CrossRef]
- Shenoy, C.; Romano, S.; Hughes, A.; Okasha, O.; Nijjar, P.S.; Velangi, P.; Martin, C.M.; Akçakaya, M.; Farzaneh-Far, A. Cardiac Magnetic Resonance Feature Tracking Global Longitudinal Strain and Prognosis After Heart Transplantation. JACC Cardiovasc. Imaging 2020, 13, 1934–1942. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Cao, J.; Li, X.; Lin, L.; Chen, W.; Wang, Y.-N.; Jin, Z.-Y. Cardiovascular Magnetic Resonance Mapping and Strain Assessment for the Diagnosis of Cardiac Involvement in Idiopathic Inflammatory Myopathy Patients With Preserved Left Ventricular Ejection Fraction. J. Thorac. Imaging 2021, 36, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Anthony, C.; Imran, M.; Pouliopoulos, J.; Emmanuel, S.; Iliff, J.; Liu, Z.; Moffat, K.; Qiu, M.R.; McLean, C.A.; Stehning, C.; et al. Cardiovascular Magnetic Resonance for Rejection Surveillance After Cardiac Transplantation. Circulation 2022, 145, 1811–1824. [Google Scholar] [CrossRef]
- Braunlin, E.A.; Shumway, S.J.; Bolman, R.M.; McDonald, K.M.; Ring, W.S.; Olivari, M.T.; Nakhleh, R.E. Usefulness of surveillance endomyocardial biopsy after pediatric cardiac transplantation. Clin. Transplant. 1998, 12, 184–189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cornicelli, M.D.; Rigsby, C.K.; Rychlik, K.; Pahl, E.; Robinson, J.D. Diagnostic performance of cardiovascular magnetic resonance native T1 and T2 mapping in pediatric patients with acute myocarditis. J. Cardiovasc. Magn. Reson. 2019, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.; Oliver, M.C.; Boyle, G.J.; Miller, S.A.; Law, Y.M.; Pigula, F.; Webber, S.A. Endomyocardial biopsy in pediatric heart transplant recipients: A useful exercise? (Analysis of 1169 biopsies). Pediatr. Transplant. 2000, 4, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.B.; Waldron, N.; Schmitt, M.; Miller, C.A. The Value of Cardiovascular Magnetic Resonance in Heart Transplant Patients. Curr. Cardiol. Rep. 2015, 17, 58. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pergola, V.; Mattesi, G.; Cozza, E.; Pradegan, N.; Tessari, C.; Dellino, C.M.; Savo, M.T.; Amato, F.; Cecere, A.; Perazzolo Marra, M.; et al. New Non-Invasive Imaging Technologies in Cardiac Transplant Follow-Up: Acquired Evidence and Future Options. Diagnostics 2023, 13, 2818. https://doi.org/10.3390/diagnostics13172818
Pergola V, Mattesi G, Cozza E, Pradegan N, Tessari C, Dellino CM, Savo MT, Amato F, Cecere A, Perazzolo Marra M, et al. New Non-Invasive Imaging Technologies in Cardiac Transplant Follow-Up: Acquired Evidence and Future Options. Diagnostics. 2023; 13(17):2818. https://doi.org/10.3390/diagnostics13172818
Chicago/Turabian StylePergola, Valeria, Giulia Mattesi, Elena Cozza, Nicola Pradegan, Chiara Tessari, Carlo Maria Dellino, Maria Teresa Savo, Filippo Amato, Annagrazia Cecere, Martina Perazzolo Marra, and et al. 2023. "New Non-Invasive Imaging Technologies in Cardiac Transplant Follow-Up: Acquired Evidence and Future Options" Diagnostics 13, no. 17: 2818. https://doi.org/10.3390/diagnostics13172818
APA StylePergola, V., Mattesi, G., Cozza, E., Pradegan, N., Tessari, C., Dellino, C. M., Savo, M. T., Amato, F., Cecere, A., Perazzolo Marra, M., Tona, F., Guaricci, A. I., De Conti, G., Gerosa, G., Iliceto, S., & Motta, R. (2023). New Non-Invasive Imaging Technologies in Cardiac Transplant Follow-Up: Acquired Evidence and Future Options. Diagnostics, 13(17), 2818. https://doi.org/10.3390/diagnostics13172818








