An Overview of Circulating Biomarkers in Neuroendocrine Neoplasms: A Clinical Guide
Abstract
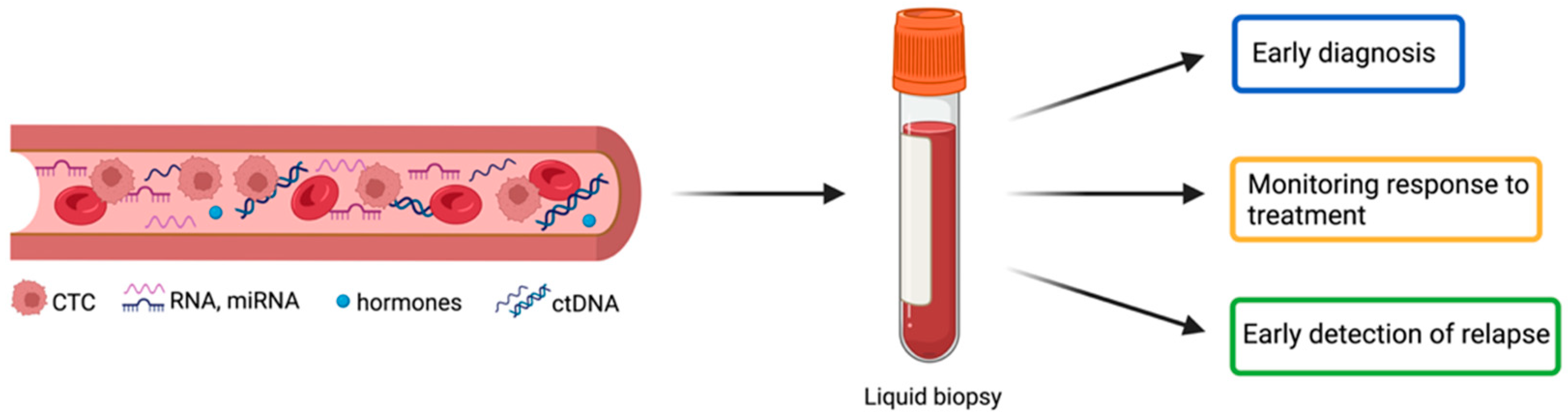
:1. Introduction
2. Circulating Biomarkers in NENs: Mono-Analytes versus Multi-Analytes
3. Circulating Biomarkers in NENs
3.1. Available Mono-Analyte Circulating Biomarkers
3.1.1. CgA
3.1.2. Circulating Tumor Cells
3.1.3. Other Biomarkers
| Mono-Analyte Biomarkers | Strengths | Flaws |
|---|---|---|
| CgA | Acceptable sensitivity only for well-differentiated NEN [20] | 30–50% of false negative in patients with NEN [19] |
| Associated to a longer PFS in GEP-NET (RADIANT-2 clinical trial) [26] | Non-standardized method of analysis [17,59] | |
| Marker of disease progression in advanced NENs and gastrinomas [33,34,35] | Poor specificity in NEN and poor sensitivity for non-functioning localized NET [20] | |
| Not effective in monitoring the disease in: GEP-NET treated with Everolimus (RADIANT-2 and 3 clinical trials) and GEP- and bp NENs treated with PRRT [17,25,110] | ||
| Not effective in monitoring tumor relapse in medullary thyroid NETs and lung NENs [46,47,111] | ||
| CTC | Correlation between amount and treatment response in midgut NET (CALM-NET trial) [61] | EpCAM expression required for isolation method FDA-approved [52] |
| Correlation between amount and disease progression in post-therapy metastatic nonfunctioning midgut and bp NET [62] | Detectable in less than 50% NENs [21,57] | |
| Analysis of CTC-derived CNAs identify chemo-refractory and chemo-sensitive SCL NECs [63] | Low levels detectable in low-grade NETs [54] | |
| Correlation between amount and PFS and OS in metastatic NENs [60] | ||
| Correlation between amount and metastasis formation in NENs [62,112] |
| Type of Functioning NEN | Secreted Hormones |
|---|---|
| Pancreatic NENs | Insulin |
| Glucagon | |
| Somatostatin | |
| Gastrin | |
| Vasoactive intestinal polypeptide (VIP) | |
| Adrenocorticotropic hormone (ACTH) | |
| Gastrointestinal NENs | Serotonin |
| Gastrin | |
| Glucagon | |
| Lung NENs | Serotonin |
| Adrenocorticotropic hormone (ACTH) | |
| Pheochromocytoma and paraganglioma | Catecholamines (CAs) and metabolites |
| Thyroid NENs | Calcitonin (CT) |
| Pituitary NENs | Growth hormone (GH) |
| Prolactin | |
| Insulin growth factor 1 (IGF1) | |
| Cortisol |
3.2. Potential Novel Multi-Analytes Biomarkers for NENs
3.2.1. NETest
3.2.2. MicroRNAs
3.2.3. Circulating Tumor DNA
| Multi-Analyte Biomarkers | Strengths | Flaws |
|---|---|---|
| NETest | High diagnostic accuracy in NENs (>90%) [115,116,168] | Not standardized cut-off values to distinguish stable from progressive disease [72] |
| Able to differentiate stable (score < 40%) from progressive disease in NENs [72,116,117] | Specificity influenced by the presence of gastrointestinal tract benign diseases in GEP-NET [117,118] | |
| Able to monitor response to therapy in GEP-, bp NET and of unknown origin [72,117,121] | ||
| Able to tumor recurrence after surgery (score < 33–40%) in NEN [123,124,126] | ||
| miRNA | Able to discriminate NET from carcinoma and benign disease in the pNET and siNET [134,138,139] | Different expression between tumor tissue and body fluids for the same miRNA [136] |
| Correlation between expression variation and tumor progression in different NENs [139,141,142,144] | Lack of standardization guidelines for analysis methods [136] | |
| Correlation between expression variation metastatization and worse prognosis [149,150,151] | Not evaluable in G1 and G2 NET [111,143] | |
| ctDNA | Able to discriminate pNET and siNET from healthy controls [157] | Limited diagnostic value in low tumor burden NENs [59,162] |
| Able to discriminate metastatic and localized pancreatic NETs [157] | ||
| Variations in the amount predicts PFS in lung and GEP-NET [157] | ||
| Mutations and CNAs detected are useful to predict response to treatment in GEP-NET, NEC, and Merkel cell carcinoma [157,158] | ||
| Methylome profile obtained is able to identify tumor progression and evaluates presence of metastasis in NENs [164] |
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms; Springer: Berlin/Heidelberg, Germany, 2022; Volume 33, ISBN 1-202-20220-9. [Google Scholar]
- Fraenkel, M.; Faggiano, A.; Valk, G.D. Epidemiology of Neuroendocrine Tumors. Front. Horm. Res. 2015, 44, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Raphael, M.J.; Chan, D.L.; Law, C.; Singh, S. Principles of Diagnosis and Management of Neuroendocrine Tumours. Cmaj 2017, 189, E398–E404. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Digestive System Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019. [Google Scholar]
- Mafficini, A.; Scarpa, A. Genetics and Epigenetics of Gastroenteropancreatic Neuroendocrine Neoplasms. Endocr. Rev. 2019, 40, 506–536. [Google Scholar] [CrossRef]
- Scarpa, A. The Landscape of Molecular Alterations in Pancreatic and Small Intestinal Neuroendocrine Tumours. Ann. D’endocrinologie 2019, 80, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Berner, A.M.; Pipinikas, C.; Ryan, A.; Dibra, H.; Moghul, I.; Webster, A.; Luong, T.V.; Thirlwell, C. Diagnostic Approaches to Neuroendocrine Neoplasms of Unknown Primary Site. Neuroendocrinology 2020, 110, 563–573. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic Neuroendocrine Neoplasms: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Uccella, S.; La Rosa, S.; Metovic, J.; Marchiori, D.; Scoazec, J.-Y.; Volante, M.; Mete, O.; Papotti, M. Genomics of High-Grade Neuroendocrine Neoplasms: Well-Differentiated Neuroendocrine Tumor with High-Grade Features (G3 NET) and Neuroendocrine Carcinomas (NEC) of Various Anatomic Sites. Endocr. Pathol. 2021, 32, 192–210. [Google Scholar] [CrossRef]
- Modlin, I.M.; Oberg, K.; Taylor, A.; Drozdov, I.; Bodei, L.; Kidd, M. Neuroendocrine Tumor Biomarkers: Current Status and Perspectives. Neuroendocrinology 2014, 100, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.M.; Bodei, L.; Kidd, M. Neuroendocrine Tumor Biomarkers: From Monoanalytes to Transcripts and Algorithms. Best. Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 59–77. [Google Scholar] [CrossRef]
- Smolkova, B.; Kataki, A.; Earl, J.; Ruz-Caracuel, I.; Cihova, M.; Urbanova, M.; Buocikova, V.; Tamargo, S.; Rovite, V.; Niedra, H.; et al. Liquid Biopsy and Preclinical Tools for Advancing Diagnosis and Treatment of Patients with Pancreatic Neuroendocrine Neoplasms. Crit. Rev. Oncol./Hematol. 2022, 180, 103865. [Google Scholar] [CrossRef]
- Rizzo, F.; Meyer, T. Liquid Biopsies for NETs—Circulating Tumour Cells. DNA MicroRNAs. 2018, 139, 1–21. [Google Scholar]
- Taupenot, L. The Chromogranin–Secretogranin Family. N. Engl. J. Med. 2003, 348, 1134–1149. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.M.; Gustafsson, B.I.; Moss, S.F.; Pavel, M.; Tsolakis, A.V.; Kidd, M. Chromogranin A—Biological Function and Clinical Utility in Neuro Endocrine Tumor Disease. Ann. Surg. Oncol. 2010, 17, 2427–2443. [Google Scholar] [CrossRef]
- Oberg, K.; Couvelard, A.; Delle Fave, G.; Gross, D.; Grossman, A.; Jensen, R.T.; Pape, U.-F.; Perren, A.; Rindi, G.; Ruszniewski, P.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Biochemical Markers. Neuroendocrinology 2017, 105, 201–211. [Google Scholar] [CrossRef]
- Marotta, V.; Zatelli, M.C.; Sciammarella, C.; Ambrosio, M.R.; Bondanelli, M.; Colao, A.; Faggiano, A. Chromogranin A as Circulating Marker for Diagnosis and Management of Neuroendocrine Neoplasms: More Flaws than Fame. Endocr. -Relat. Cancer 2018, 25, R11–R29. [Google Scholar] [CrossRef]
- Gkolfinopoulos, S.; Tsapakidis, K.; Papadimitriou, K.; Papamichael, D.; Kountourakis, P. Chromogranin A as a Valid Marker in Oncology: Clinical Application or False Hopes? WJM 2017, 7, 9. [Google Scholar] [CrossRef]
- Lindholm, D.P.; Oberg, K. Biomarkers and Molecular Imaging in Gastroenteropancreatic Neuroendocrine Tumors. Horm. Metab. Res. 2011, 43, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Korse, C.M.; Taal, B.G.; Vincent, A.; van Velthuysen, M.-L.F.; Baas, P.; Buning-Kager, J.C.G.M.; Linders, T.C.; Bonfrer, J.M.G. Choice of Tumour Markers in Patients with Neuroendocrine Tumours Is Dependent on the Histological Grade. A Marker Study of Chromogranin A, Neuron Specific Enolase, Progastrin-Releasing Peptide and Cytokeratin Fragments. Eur. J. Cancer 2012, 48, 662–671. [Google Scholar] [CrossRef]
- Oberg, K.; Modlin, I.M.; De Herder, W.; Pavel, M.; Klimstra, D.; Frilling, A.; Metz, D.C.; Heaney, A.; Kwekkeboom, D.; Strosberg, J.; et al. Consensus on Biomarkers for Neuroendocrine Tumour Disease. Lancet Oncol. 2015, 16, e435–e446. [Google Scholar] [CrossRef]
- Capdevila, J.; Meeker, A.; García-Carbonero, R.; Pietras, K.; Astudillo, A.; Casanovas, O.; Scarpa, A. Molecular Biology of Neuroendocrine Tumors: From Pathways to Biomarkers and Targets. Cancer Metastasis Rev. 2014, 33, 345–351. [Google Scholar] [CrossRef]
- Tsai, H.-J.; Hsiao, C.-F.; Chang, J.S.; Chen, L.-T.; Chao, Y.-J.; Yen, C.-J.; Shan, Y.-S. The Prognostic and Predictive Role of Chromogranin A in Gastroenteropancreatic Neuroendocrine Tumors—A Single-Center Experience. Front. Oncol. 2021, 11, 741096. [Google Scholar] [CrossRef]
- Pavel, M.E.; Baudin, E.; Öberg, K.E.; Hainsworth, J.D.; Voi, M.; Rouyrre, N.; Peeters, M.; Gross, D.J.; Yao, J.C. Efficacy of Everolimus plus Octreotide LAR in Patients with Advanced Neuroendocrine Tumor and Carcinoid Syndrome: Final Overall Survival from the Randomized, Placebo-Controlled Phase 3 RADIANT-2 Study. Ann. Oncol. 2017, 28, 1569–1575. [Google Scholar] [CrossRef]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the Treatment of Advanced, Non-Functional Neuroendocrine Tumours of the Lung or Gastrointestinal Tract (RADIANT-4): A Randomised, Placebo-Controlled, Phase 3 Study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef]
- Baudin, E.; Wolin, E.; Castellano, D.; Kaltsas, G.; Panneerselvam, A.; Tsuchihashi, Z.; Saletan, S.; Yao, J.C.; Gross, D. 6564 POSTER Correlation of PFS with Early Response of Chromogranin A and 5-Hydroxyindoleacetic Acid Levels in Pts with Advanced Neuroendocrine Tumours: Phase III RADIANT-2 Study Results. Eur. J. Cancer 2011, 47, S460. [Google Scholar] [CrossRef]
- Buil-Bruna, N.; Dehez, M.; Manon, A.; Nguyen, T.X.Q.; Trocóniz, I.F. Establishing the Quantitative Relationship Between Lanreotide Autogel®, Chromogranin A, and Progression-Free Survival in Patients with Nonfunctioning Gastroenteropancreatic Neuroendocrine Tumors. AAPS J. 2016, 18, 703–712. [Google Scholar] [CrossRef]
- Saif, M.W. Lanreotide for the Treatment of Gastroenteropancreatic Neuroendocrine Tumors. Expert. Opin. Pharmacother. 2016, 17, 443–456. [Google Scholar] [CrossRef]
- Klempner, S.J.; Gershenhorn, B.; Tran, P.; Lee, T.K.; Erlander, M.G.; Gowen, K.; Schrock, A.B.; Morosini, D.; Ross, J.S.; Miller, V.A.; et al. BRAFV600E Mutations in High-Grade Colorectal Neuroendocrine Tumors May Predict Responsiveness to BRAF-MEK Combination Therapy. Cancer Discov. 2016, 6, 594–600. [Google Scholar] [CrossRef]
- Stivanello, M.; Berruti, A.; Torta, M.; Termine, A.; Tampellini, M.; Gorzegno, G.; Angeli, A.; Dogliotti, L. Circulating Chromogranin A in the Assessment of Patients with Neuroendocrine Tumours. A Single Institution Experience. Ann. Oncol. 2001, 12 (Suppl. S2), S73–S77. [Google Scholar] [CrossRef]
- Rossi, R.E.; Garcia-Hernandez, J.; Meyer, T.; Thirlwell, C.; Watkins, J.; Martin, N.G.; Caplin, M.E.; Toumpanakis, C. Chromogranin A as a Predictor of Radiological Disease Progression in Neuroendocrine Tumours. Ann. Transl. Med. 2015, 3, 118. [Google Scholar] [CrossRef]
- Massironi, S.; Rossi, R.E.; Casazza, G.; Conte, D.; Ciafardini, C.; Galeazzi, M.; Peracchi, M. Chromogranin A in Diagnosing and Monitoring Patients with Gastroenteropancreatic Neuroendocrine Neoplasms: A Large Series from a Single Institution. Neuroendocrinology 2014, 100, 240–249. [Google Scholar] [CrossRef]
- Nobels, F.R.; Kwekkeboom, D.J.; Coopmans, W.; Schoenmakers, C.H.; Lindemans, J.; De Herder, W.W.; Krenning, E.P.; Bouillon, R.; Lamberts, S.W. Chromogranin A as Serum Marker for Neuroendocrine Neoplasia: Comparison with Neuron-Specific Enolase and the Alpha-Subunit of Glycoprotein Hormones. J. Clin. Endocrinol. Metab. 1997, 82, 2622–2628. [Google Scholar] [CrossRef]
- Arnold, R.; Wilke, A.; Rinke, A.; Mayer, C.; Kann, P.H.; Klose, K.-J.; Scherag, A.; Hahmann, M.; Müller, H.-H.; Barth, P. Plasma Chromogranin A as Marker for Survival in Patients with Metastatic Endocrine Gastroenteropancreatic Tumors. Clin. Gastroenterol. Hepatol. 2008, 6, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Gao, J.; Li, N.; Li, Y.; Lu, M.; Li, Z.; Lu, Z.; Li, J.; Shen, L. Circulating Chromogranin A as A Marker for Monitoring Clinical Response in Advanced Gastroenteropancreatic Neuroendocrine Tumors. PLoS ONE 2016, 11, e0154679. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.E.; Ciafardini, C.; Sciola, V.; Conte, D.; Massironi, S. Chromogranin A in the Follow-up of Gastroenteropancreatic Neuroendocrine Neoplasms: Is It Really Game Over? A Systematic Review and Meta-Analysis. Pancreas 2018, 47, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Li, M.; Travers, A.; Segelov, E. Role of Chromogranin A in the Diagnosis and Follow-up of Neuroendocrine Tumors: Real-World Experience. Pancreas 2022, 51, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Matar, S.; Malczewska, A.; Oberg, K.; Bodei, L.; Aslanian, H.; Lewczuk-Myślicka, A.; Filosso, P.L.; Suarez, A.L.; Kolasińska-Ćwikła, A.; Roffinella, M.; et al. Blood Chromogranin A Is Not Effective as a Biomarker for Diagnosis or Management of Bronchopulmonary Neuroendocrine Tumors/Neoplasms. Neuroendocrinology 2020, 110, 185–197. [Google Scholar] [CrossRef]
- Woltering, E.A.; Beyer, D.T.; Thiagarajan, R.; Ramirez, R.A.; Wang, Y.-Z.; Ricks, M.J.; Boudreaux, J.P. Elevated Plasma Pancreastatin, but Not Chromogranin A, Predicts Survival in Neuroendocrine Tumors of the Duodenum. J. Am. Coll. Surg. 2016, 222, 534–542. [Google Scholar] [CrossRef]
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021, 19, 839–868. [Google Scholar] [CrossRef]
- Shanahan, M.A.; Salem, A.; Fisher, A.; Cho, C.S.; Leverson, G.; Winslow, E.R.; Weber, S.M. Chromogranin A Predicts Survival for Resected Pancreatic Neuroendocrine Tumors. J. Surg. Res. 2016, 201, 38–43. [Google Scholar] [CrossRef]
- Nanno, Y.; Toyama, H.; Matsumoto, I.; Otani, K.; Asari, S.; Goto, T.; Ajiki, T.; Zen, Y.; Fukumoto, T.; Ku, Y. Baseline Plasma Chromogranin A Levels in Patients with Well-Differentiated Neuroendocrine Tumors of the Pancreas: A Potential Predictor of Postoperative Recurrence. Pancreatology 2017, 17, 291–294. [Google Scholar] [CrossRef]
- Fisher, A.V.; Lopez-Aguiar, A.G.; Rendell, V.R.; Pokrzywa, C.; Rocha, F.G.; Kanji, Z.S.; Poultsides, G.A.; Makris, E.A.; Dillhoff, M.E.; Beal, E.W.; et al. Predictive Value of Chromogranin A and a Pre-Operative Risk Score to Predict Recurrence after Resection of Pancreatic Neuroendocrine Tumors. J. Gastrointest. Surg. 2019, 23, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Raoof, M.; Jutric, Z.; Melstrom, L.G.; Lee, B.; Li, D.; Warner, S.G.; Fong, Y.; Singh, G. Prognostic Significance of Chromogranin A in Small Pancreatic Neuroendocrine Tumors. Surgery 2019, 165, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Jilesen, A.P.J.; Busch, O.R.C.; van Gulik, T.M.; Gouma, D.J.; Nieveen van Dijkum, E.J.M. Standard Pre- and Postoperative Determination of Chromogranin a in Resectable Non-Functioning Pancreatic Neuroendocrine Tumors--Diagnostic Accuracy: NF-PNET and Low Tumor Burden. Dig. Surg. 2014, 31, 407–414. [Google Scholar] [CrossRef]
- Malczewska, A.; Kidd, M.; Matar, S.; Kos-Kudła, B.; Bodei, L.; Oberg, K.; Modlin, I.M. An Assessment of Circulating Chromogranin A as a Biomarker of Bronchopulmonary Neuroendocrine Neoplasia: A Systematic Review and Meta-Analysis. Neuroendocrinology 2020, 110, 198–216. [Google Scholar] [CrossRef]
- Zarkesh, M.; Arab, N.; Tavangar, S.M.; Nozhat, Z.; Fanaei, S.M.; Hedayati, M. Utilizing the Circulating Tumor Markers in Diagnosis and Management of Medullary Thyroid Cancer. Pathol. Res. Pract. 2022, 229, 153694. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, A.; Kiavue, N.; Bidard, F.-C.; Pierga, J.-Y.; Cabel, L. Clinical Utility of Circulating Tumor Cells: An Update. Mol. Oncol. 2021, 15, 1647–1666. [Google Scholar] [CrossRef] [PubMed]
- Mandair, D.; Khan, M.S.; Lopes, A.; Furtado O’Mahony, L.; Ensell, L.; Lowe, H.; Hartley, J.A.; Toumpanakis, C.; Caplin, M.; Meyer, T. Prognostic Threshold for Circulating Tumor Cells in Patients with Pancreatic and Midgut Neuroendocrine Tumors. J. Clin. Endocrinol. Metab. 2021, 106, 872–882. [Google Scholar] [CrossRef]
- Grover, P.K.; Cummins, A.G.; Price, T.J.; Roberts-Thomson, I.C.; Hardingham, J.E. Circulating Tumour Cells: The Evolving Concept and the Inadequacy of Their Enrichment by EpCAM-Based Methodology for Basic and Clinical Cancer Research. Ann. Oncol. 2014, 25, 1506–1516. [Google Scholar] [CrossRef]
- Zatelli, M.C.; Grossrubatscher, E.M.; Guadagno, E.; Sciammarella, C.; Faggiano, A.; Colao, A. Circulating Tumor Cells and MiRNAs as Prognostic Markers in Neuroendocrine Neoplasms. Endocr. -Relat. Cancer 2017, 24, R223–R237. [Google Scholar] [CrossRef]
- Ju, S.; Chen, C.; Zhang, J.; Xu, L.; Zhang, X.; Li, Z.; Chen, Y.; Zhou, J.; Ji, F.; Wang, L. Detection of Circulating Tumor Cells: Opportunities and Challenges. Biomark. Res. 2022, 10, 58. [Google Scholar] [CrossRef]
- Jan, Y.J.; Chen, J.-F.; Zhu, Y.; Lu, Y.-T.; Chen, S.H.; Chung, H.; Smalley, M.; Huang, Y.-W.; Dong, J.; Yu, H.-H.; et al. NanoVelcro Rare-Cell Assays for Detection and Characterization of Circulating Tumor Cells. Adv. Drug Deliv. Rev. 2018, 125, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. The Epidemiology of Metastases in Neuroendocrine Tumors: Epidemiology of Metastases. Int. J. Cancer 2016, 139, 2679–2686. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Kirkwood, A.; Tsigani, T.; Garcia-Hernandez, J.; Hartley, J.A.; Caplin, M.E.; Meyer, T. Circulating Tumor Cells As Prognostic Markers in Neuroendocrine Tumors. JCO 2013, 31, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Blom, A.; Bhatia, S.; Pietromonaco, S.; Koehler, K.; Iyer, J.G.; Nagase, K.; Paulson, K.; Sabath, D.E.; Nghiem, P. Clinical Utility of a Circulating Tumor Cell Assay in Merkel Cell Carcinoma. J. Am. Acad. Dermatol. 2014, 70, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Öberg, K. Molecular Genomic Blood Biomarkers for Neuroendocrine Tumors: The Long and Winding Road from Berzelius and Bence Jones to a Neuroendocrine Destination. Neuroendocrinology 2021, 111, 297–303. [Google Scholar] [CrossRef]
- Childs, A.; Steele, C.D.; Vesely, C.; Rizzo, F.M.; Ensell, L.; Lowe, H.; Dhami, P.; Vaikkinen, H.; Luong, T.V.; Conde, L.; et al. Whole-Genome Sequencing of Single Circulating Tumor Cells from Neuroendocrine Neoplasms. Endocr. -Relat. Cancer 2021, 28, 631–644. [Google Scholar] [CrossRef]
- Komarnicki, P.; Musiałkiewicz, J.; Stańska, A.; Maciejewski, A.; Gut, P.; Mastorakos, G.; Ruchała, M. Circulating Neuroendocrine Tumor Biomarkers: Past, Present and Future. J. Clin. Med. 2022, 11, 5542. [Google Scholar] [CrossRef]
- Khan, M.S.; Kirkwood, A.A.; Tsigani, T.; Lowe, H.; Goldstein, R.; Hartley, J.A.; Caplin, M.E.; Meyer, T. Early Changes in Circulating Tumor Cells Are Associated with Response and Survival Following Treatment of Metastatic Neuroendocrine Neoplasms. Clin. Cancer Res. 2016, 22, 79–85. [Google Scholar] [CrossRef]
- Meyer, T.; Caplin, M.; Khan, M.S.; Toumpanakis, C.; Shetty, S.; Ramage, J.K.; Houchard, A.; Higgs, K.; Shah, T. Circulating Tumour Cells and Tumour Biomarkers in Functional Midgut Neuroendocrine Tumours. J. Neuroendocrinol. 2022, 34, e13096. [Google Scholar] [CrossRef]
- Hsieh, J.C.-H.; Chen, G.-Y.; Jhou, D.D.-W.; Chou, W.-C.; Yeh, C.-N.; Hwang, T.-L.; Lin, H.-C.; Chu, H.-C.; Wang, H.-M.; Yen, T.-C.; et al. The Prognostic Value of Circulating Tumor Cells in Asian Neuroendocrine Tumors. Sci. Rep. 2019, 9, 19917. [Google Scholar] [CrossRef]
- Carter, L.; Rothwell, D.G.; Mesquita, B.; Smowton, C.; Leong, H.S.; Fernandez-Gutierrez, F.; Li, Y.; Burt, D.J.; Antonello, J.; Morrow, C.J.; et al. Molecular Analysis of Circulating Tumor Cells Identifies Distinct Copy-Number Profiles in Patients with Chemosensitive and Chemorefractory Small-Cell Lung Cancer. Nat. Med. 2017, 23, 114–119. [Google Scholar] [CrossRef]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating Tumor Cell Clusters Are Oligoclonal Precursors of Breast Cancer Metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef]
- Gandini, S.; Zanna, I.; De Angelis, S.P.; Cocorocchio, E.; Queirolo, P.; Lee, J.H.; Carlino, M.S.; Mazzarella, L.; Achutti Duso, B.; Palli, D.; et al. Circulating Tumour DNA and Melanoma Survival: A Systematic Literature Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2021, 157, 103187. [Google Scholar] [CrossRef]
- Krebs, M.G.; Sloane, R.; Priest, L.; Lancashire, L.; Hou, J.-M.; Greystoke, A.; Ward, T.H.; Ferraldeschi, R.; Hughes, A.; Clack, G.; et al. Evaluation and Prognostic Significance of Circulating Tumor Cells in Patients with Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2011, 29, 1556–1563. [Google Scholar] [CrossRef]
- de Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.W.M.; Pienta, K.J.; Raghavan, D. Circulating Tumor Cells Predict Survival Benefit from Treatment in Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef]
- Sun, N.; Yang, Y.; Miao, H.; Redublo, P.; Liu, H.; Liu, W.; Huang, Y.-W.; Teng, P.-C.; Zhang, C.; Zhang, R.Y.; et al. Discovery and Characterization of Circulating Tumor Cell Clusters in Neuroendocrine Tumor Patients Using Nanosubstrate-Embedded Microchips. Biosens. Bioelectron. 2022, 199, 113854. [Google Scholar] [CrossRef]
- Sansone, A.; Lauretta, R.; Vottari, S.; Chiefari, A.; Barnabei, A.; Romanelli, F.; Appetecchia, M. Specific and Non-Specific Biomarkers in Neuroendocrine Gastroenteropancreatic Tumors. Cancers 2019, 11, 1113. [Google Scholar] [CrossRef]
- Isgrò, M.A.; Bottoni, P.; Scatena, R. Neuron-Specific Enolase as a Biomarker: Biochemical and Clinical Aspects. In Advances in Cancer Biomarkers: From Biochemistry to Clinic for a Critical Revision; Scatena, R., Ed.; Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherland, 2015; pp. 125–143. ISBN 978-94-017-7215-0. [Google Scholar]
- Xu, C.-M.; Luo, Y.-L.; Li, S.; Li, Z.-X.; Jiang, L.; Zhang, G.-X.; Owusu, L.; Chen, H.-L. Multifunctional Neuron-Specific Enolase: Its Role in Lung Diseases. Biosci. Rep. 2019, 39, BSR20192732. [Google Scholar] [CrossRef]
- Puliani, G.; Di Vito, V.; Feola, T.; Sesti, F.; Centello, R.; Pandozzi, C.; Tarsitano, M.G.; Verrico, M.; Lenzi, A.; Isidori, A.M.; et al. NETest: A Systematic Review Focusing on the Prognostic and Predictive Role. Neuroendocrinology 2022, 112, 523–536. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Zheng, G.; Yang, Y.; Du, L.; Dong, Z.; Zhang, X.; Wang, C. Clinical Evaluation and Therapeutic Monitoring Value of Serum Tumor Markers in Lung Cancer. Int. J. Biol. Markers 2016, 31, e80–e87. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, J.-G.; Yao, W.-X.; Tian, X.; Lv, S.-P.; Zhang, T.-Y.; Jin, S.-H.; Bai, Y.-J.; Ma, H. Systematic Review and Meta-Analysis of the Efficacy of Serum Neuron-Specific Enolase for Early Small Cell Lung Cancer Screening. Oncotarget 2017, 8, 64358–64372. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Reichmann, F.; Farzi, A. Neuropeptide Y, Peptide YY and Pancreatic Polypeptide in the Gut-Brain Axis. Neuropeptides 2012, 46, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; Chardon, L.; Chopin-laly, X.; Raverot, V.; Caffin, A.-G.; Chayvialle, J.-A.; Scoazec, J.-Y.; Lombard-Bohas, C. Is the Combination of Chromogranin A and Pancreatic Polypeptide Serum Determinations of Interest in the Diagnosis and Follow-up of Gastro-Entero-Pancreatic Neuroendocrine Tumours? Eur. J. Cancer 2012, 48, 1766–1773. [Google Scholar] [CrossRef]
- Panzuto, F.; Severi, C.; Cannizzaro, R.; Falconi, M.; Angeletti, S.; Pasquali, A.; Corleto, V.D.; Annibale, B.; Buonadonna, A.; Pederzoli, P.; et al. Utility of Combined Use of Plasma Levels of Chromogranin A and Pancreatic Polypeptide in the Diagnosis of Gastrointestinal and Pancreatic Endocrine Tumors. J. Endocrinol. Investig. 2004, 27, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.L.; Rodríguez, F.D.; Coveñas, R. Neuropeptide Y Peptide Family and Cancer: Antitumor Therapeutic Strategies. Int. J. Mol. Sci. 2023, 24, 9962. [Google Scholar] [CrossRef] [PubMed]
- Philippe, J.; Maurer, J.; Vocat, C.; Abid, K.; Matter, M.; Wuerzner, G.; Trepp, R.; Fischli, S.; Henzen, C.; Kolb, W.; et al. Proneuropeptide Y and Neuropeptide Y Metabolites in Healthy Volunteers and Patients with a Pheochromocytoma or Paraganglioma. Clin. Chim. Acta 2022, 534, 146–155. [Google Scholar] [CrossRef]
- Ciobanu, O.; Martin, S.; Fica, S. Perspectives on the Diagnostic, Predictive and Prognostic Markers of Neuroendocrine Neoplasms (Review). Exp. Ther. Med. 2021, 22, 1479. [Google Scholar] [CrossRef]
- Bevere, M.; Gkountakos, A.; Martelli, F.M.; Scarpa, A.; Luchini, C.; Simbolo, M. An Insight on Functioning Pancreatic Neuroendocrine Neoplasms. Biomedicines 2023, 11, 303. [Google Scholar] [CrossRef]
- Cryer, P.E.; Axelrod, L.; Grossman, A.B.; Heller, S.R.; Montori, V.M.; Seaquist, E.R.; Service, F.J. Endocrine Society Evaluation and Management of Adult Hypoglycemic Disorders: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2009, 94, 709–728. [Google Scholar] [CrossRef]
- Guettier, J.-M.; Lungu, A.; Goodling, A.; Cochran, C.; Gorden, P. The Role of Proinsulin and Insulin in the Diagnosis of Insulinoma: A Critical Evaluation of the Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4752–4758. [Google Scholar] [CrossRef]
- Hirshberg, B.; Livi, A.; Bartlett, D.L.; Libutti, S.K.; Alexander, H.R.; Doppman, J.L.; Skarulis, M.C.; Gorden, P. Forty-Eight-Hour Fast: The Diagnostic Test for Insulinoma. J. Clin. Endocrinol. Metab. 2000, 85, 3222–3226. [Google Scholar] [CrossRef]
- Ito, T.; Cadiot, G.; Jensen, R.T. Diagnosis of Zollinger-Ellison Syndrome: Increasingly Difficult. World J. Gastroenterol. 2012, 18, 5495–5503. [Google Scholar] [CrossRef]
- Varro, A.; Ardill, J.E.S. Gastrin: An Analytical Review. Ann. Clin. Biochem. 2003, 40, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Zandee, W.T.; Kamp, K.; van Adrichem, R.C.; Feelders, R.A.; de Herder, W.W. Effect of Hormone Secretory Syndromes on Neuroendocrine Tumor Prognosis. Endocr. -Relat. Cancer 2017, 24, R261–R274. [Google Scholar] [CrossRef]
- Bloom, S.R. Vasoactive Intestinal Peptide, the Major Mediator of the WDHA (Pancreatic Cholera) Syndrome: Value of Measurement in Diagnosis and Treatment. Dig. Dis. Sci. 1978, 23, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Una Cidon, E. Vasoactive Intestinal Peptide Secreting Tumour: An Overview. World J. Gastrointest. Oncol. 2022, 14, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Meijer, W.G.; Kema, I.P.; Volmer, M.; Willemse, P.H.; de Vries, E.G. Discriminating Capacity of Indole Markers in the Diagnosis of Carcinoid Tumors. Clin. Chem. 2000, 46, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Ram, P.; Penalver, J.L.; Lo, K.B.U.; Rangaswami, J.; Pressman, G.S. Carcinoid Heart Disease: Review of Current Knowledge. Tex. Heart Inst. J. 2019, 46, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Burgess, M.I.; Banks, M.; Pritchard, D.M.; Vora, J.; Valle, J.W.; Wong, C.; Chadwick, C.; George, K.; Keevil, B.; et al. The Association of a Panel of Biomarkers with the Presence and Severity of Carcinoid Heart Disease: A Cross-Sectional Study. PLoS ONE 2013, 8, e73679. [Google Scholar] [CrossRef]
- Davi, M.V.; Cosaro, E.; Piacentini, S.; Reimondo, G.; Albiger, N.; Arnaldi, G.; Faggiano, A.; Mantovani, G.; Fazio, N.; Piovesan, A.; et al. Prognostic Factors in Ectopic Cushing’s Syndrome Due to Neuroendocrine Tumors: A Multicenter Study. Eur. J. Endocrinol. 2017, 176, 453–461. [Google Scholar] [CrossRef]
- Paravati, S.; Rosani, A.; Warrington, S.J. Physiology, Catecholamines. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Jain, A.; Baracco, R.; Kapur, G. Pheochromocytoma and Paraganglioma—An Update on Diagnosis, Evaluation, and Management. Pediatr. Nephrol. 2020, 35, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Mete, O.; Asa, S.L.; Gill, A.J.; Kimura, N.; de Krijger, R.R.; Tischler, A. Overview of the 2022 WHO Classification of Paragangliomas and Pheochromocytomas. Endocr. Pathol. 2022, 33, 90–114. [Google Scholar] [CrossRef] [PubMed]
- Lenders, J.W.M.; Eisenhofer, G. Update on Modern Management of Pheochromocytoma and Paraganglioma. Endocrinol. Metab. 2017, 32, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.J.; Schaab, M.; Kratzsch, J. Calcitonin as Biomarker for the Medullary Thyroid Carcinoma. Recent. Results Cancer Res. 2015, 204, 117–137. [Google Scholar] [CrossRef]
- Master, S.R.; Burns, B. Medullary Thyroid Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Toledo, S.P.A.; Lourenço, D.M.; Santos, M.A.; Tavares, M.R.; Toledo, R.A.; Correia-Deur, J.E.d.M. Hypercalcitoninemia Is Not Pathognomonic of Medullary Thyroid Carcinoma. Clinics 2009, 64, 699–706. [Google Scholar] [CrossRef]
- Gambardella, C.; Offi, C.; Patrone, R.; Clarizia, G.; Mauriello, C.; Tartaglia, E.; Di Capua, F.; Di Martino, S.; Romano, R.M.; Fiore, L.; et al. Calcitonin Negative Medullary Thyroid Carcinoma: A Challenging Diagnosis or a Medical Dilemma? BMC Endocr. Disord. 2019, 19, 45. [Google Scholar] [CrossRef]
- Giannetta, E.; Guarnotta, V.; Altieri, B.; Sciammarella, C.; Guadagno, E.; Malandrino, P.; Puliani, G.; Feola, T.; Isidori, A.M.; Colao, A.A.L.; et al. ENDOCRINE TUMOURS: Calcitonin in Thyroid and Extra-Thyroid Neuroendocrine Neoplasms: The Two-Faced Janus(Details of the Nike Group Are Presented in the Acknowledgements Section). Eur. J. Endocrinol. 2020, 183, R197–R215. [Google Scholar] [CrossRef]
- Costante, G.; Meringolo, D. Calcitonin as a Biomarker of C Cell Disease: Recent Achievements and Current Challenges. Endocrine 2020, 67, 273–280. [Google Scholar] [CrossRef]
- Wells, S.A.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association Guidelines for the Management of Medullary Thyroid Carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Nigam, A.; Xu, B.; Spanheimer, P.M.; Ganly, I.; Tuttle, R.M.; Wong, R.J.; Shaha, A.R.; Ghossein, R.A.; Untch, B.R. Tumor Grade Predicts for Calcitonin Doubling Times and Disease-Specific Outcomes after Resection of Medullary Thyroid Carcinoma. Thyroid 2022, 32, 1193–1200. [Google Scholar] [CrossRef]
- Park, H.; Park, S.Y.; Park, J.; Choe, J.H.; Chung, M.K.; Woo, S.-Y.; Choi, J.Y.; Kim, S.W.; Chung, J.H.; Kim, T.H. Prognostic Value of Preoperative Serum Calcitonin Levels for Predicting the Recurrence of Medullary Thyroid Carcinoma. Front. Endocrinol. 2021, 12, 749973. [Google Scholar] [CrossRef]
- Kratzsch, J.; Willenberg, A.; Frank-Raue, K.; Kempin, U.; Rocktäschel, J.; Raue, F. Procalcitonin Measured by Three Different Assays Is an Excellent Tumor Marker for the Follow-up of Patients with Medullary Thyroid Carcinoma. Clin. Chem. Lab. Med. 2021, 59, 1861–1868. [Google Scholar] [CrossRef]
- Trouillas, J.; Jaffrain-Rea, M.-L.; Vasiljevic, A.; Raverot, G.; Roncaroli, F.; Villa, C. How to Classify Pituitary Neuroendocrine Tumors (PitNET)s in 2020. Cancers 2020, 12, 514. [Google Scholar] [CrossRef]
- Sandra, I.; Cazacu, I.M.; Croitoru, V.M.; Mihaila, M.; Herlea, V.; Diculescu, M.M.; Dima, S.O.; Croitoru, A.E. Circulating Angiogenic Markers in Gastroenteropancreatic Neuroendocrine Neoplasms: A Systematic Review. Curr. Issues Mol. Biol. 2022, 44, 4001–4014. [Google Scholar] [CrossRef]
- Pavel, M.E.; Hainsworth, J.D.; Baudin, E.; Peeters, M.; Hörsch, D.; Winkler, R.E.; Klimovsky, J.; Lebwohl, D.; Jehl, V.; Wolin, E.M.; et al. Everolimus plus Octreotide Long-Acting Repeatable for the Treatment of Advanced Neuroendocrine Tumours Associated with Carcinoid Syndrome (RADIANT-2): A Randomised, Placebo-Controlled, Phase 3 Study. Lancet 2011, 378, 2005–2012. [Google Scholar] [CrossRef]
- Nisman, B.; Oleinikov, K.; Nechushtan, H.; Maimon, O.; Atlan, K.; Peled, N.; Gross, D.; Peretz, T.; Meirovitz, A.; Grozinsky-Glasberg, S. Plasma Progastrin-Releasing Peptide and Chromogranin A Assays for Diagnosing and Monitoring Lung Well-Differentiated Neuroendocrine Tumors: A Brief Report. J. Thorac. Oncol. 2023, 18, 369–376. [Google Scholar] [CrossRef]
- Ko, J.M.Y.; Lam, K.O.; Kwong, D.L.W.; Wong, I.Y.-H.; Chan, F.S.-Y.; Wong, C.L.-Y.; Chan, K.K.; Law, T.T.; Chiu, K.W.H.; Lam, C.C.S.; et al. Circulating Tumor Cell Enumeration for Serial Monitoring of Treatment Outcomes for Locally Advanced Esophageal Squamous Cell Carcinoma. Cancers 2023, 15, 832. [Google Scholar] [CrossRef]
- Modlin, I.M.; Drozdov, I.; Kidd, M. The Identification of Gut Neuroendocrine Tumor Disease by Multiple Synchronous Transcript Analysis in Blood. PLoS ONE 2013, 8, e63364. [Google Scholar] [CrossRef]
- Modlin, I.M.; Kidd, M.; Falconi, M.; Filosso, P.L.; Frilling, A.; Malczewska, A.; Toumpanakis, C.; Valk, G.; Pacak, K.; Bodei, L.; et al. A Multigenomic Liquid Biopsy Biomarker for Neuroendocrine Tumor Disease Outperforms CgA and Has Surgical and Clinical Utility. Ann. Oncol. 2021, 32, 1425–1433. [Google Scholar] [CrossRef]
- Malczewska, A.; Kos-Kudła, B.; Kidd, M.; Drozdov, I.; Bodei, L.; Matar, S.; Oberg, K.; Modlin, I.M. The Clinical Applications of a Multigene Liquid Biopsy (NETest) in Neuroendocrine Tumors. Adv. Med. Sci. 2020, 65, 18–29. [Google Scholar] [CrossRef]
- Öberg, K.; Califano, A.; Strosberg, J.R.; Ma, S.; Pape, U.; Bodei, L.; Kaltsas, G.; Toumpanakis, C.; Goldenring, J.R.; Frilling, A.; et al. A Meta-Analysis of the Accuracy of a Neuroendocrine Tumor MRNA Genomic Biomarker (NETest) in Blood. Ann. Oncol. 2020, 31, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Van Treijen, M.J.C.; Korse, C.M.; Van Leeuwaarde, R.S.; Saveur, L.J.; Vriens, M.R.; Verbeek, W.H.M.; Tesselaar, M.E.T.; Valk, G.D. Blood Transcript Profiling for the Detection of Neuroendocrine Tumors: Results of a Large Independent Validation Study. Front. Endocrinol. 2018, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Al-Toubah, T.; Cives, M.; Valone, T.; Blue, K.; Strosberg, J. Sensitivity and Specificity of the NETest: A Validation Study. Neuroendocrinology 2021, 111, 580–585. [Google Scholar] [CrossRef]
- Liu, E.; Paulson, S.; Gulati, A.; Freudman, J.; Grosh, W.; Kafer, S.; Wickremesinghe, P.C.; Salem, R.R.; Bodei, L. Assessment of NETest Clinical Utility in a U.S. Registry-Based Study. Oncologist 2019, 24, 783–790. [Google Scholar] [CrossRef]
- van Treijen, M.J.C.; van der Zee, D.; Heeres, B.C.; Staal, F.C.R.; Vriens, M.R.; Saveur, L.J.; Verbeek, W.H.M.; Korse, C.M.; Maas, M.; Valk, G.D.; et al. Blood Molecular Genomic Analysis Predicts the Disease Course of Gastroenteropancreatic Neuroendocrine Tumor Patients: A Validation Study of the Predictive Value of the NETest®. Neuroendocrinology 2021, 111, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Kidd, M.S.; Singh, A.; Van Der Zwan, W.A.; Severi, S.; Drozdov, I.A.; Malczewska, A.; Baum, R.P.; Kwekkeboom, D.J.; Paganelli, G.; et al. PRRT Neuroendocrine Tumor Response Monitored Using Circulating Transcript Analysis: The NETest. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 895–906. [Google Scholar] [CrossRef]
- Modlin, I.M.; Kidd, M.; Frilling, A.; Falconi, M.; Filosso, P.L.; Malczewska, A.; Kitz, A. Molecular Genomic Assessment Using a Blood-Based MRNA Signature (NETest) Is Cost-Effective and Predicts Neuroendocrine Tumor Recurrence with 94% Accuracy. Ann. Surg. 2021, 274, 481–490. [Google Scholar] [CrossRef]
- Laskaratos, F.-M.; Liu, M.; Malczewska, A.; Ogunbiyi, O.; Watkins, J.; Luong, T.V.; Mandair, D.; Caplin, M.; Toumpanakis, C. Evaluation of Circulating Transcript Analysis (NETest) in Small Intestinal Neuroendocrine Neoplasms after Surgical Resection. Endocrine 2020, 69, 430–440. [Google Scholar] [CrossRef]
- Modlin, I.M.; Kidd, M.; Oberg, K.; Falconi, M.; Filosso, P.L.; Frilling, A.; Malczewska, A.; Salem, R.; Toumpanakis, C.; Laskaratos, F.-M.; et al. Early Identification of Residual Disease after Neuroendocrine Tumor Resection Using a Liquid Biopsy Multigenomic MRNA Signature (NETest). Ann. Surg. Oncol. 2021, 28, 7506–7517. [Google Scholar] [CrossRef]
- NET Blood Transcript Analysis Defines the Crossing of the Clinical Rubicon: When Stable Disease Becomes Progressive|Neuroendocrinology|Karger Publishers. Available online: https://karger.com/nen/article/104/2/170/220131/NET-Blood-Transcript-Analysis-Defines-the-Crossing (accessed on 15 May 2023).
- Ćwikła, J.B.; Bodei, L.; Kolasinska-Ćwikła, A.; Sankowski, A.; Modlin, I.M.; Kidd, M. Circulating Transcript Analysis (NETest) in GEP-NETs Treated with Somatostatin Analogs Defines Therapy. J. Clin. Endocrinol. Metab. 2015, 100, E1437–E1445. [Google Scholar] [CrossRef]
- Frilling, A.; Clift, A.K.; Frampton, A.E.; Bomanji, J.; Kaemmerer, D.; Al-Nahhas, A.; Alsafi, A.; Kidd, M.; Modlin, I.M.; Hoersch, D.; et al. A Combination of Surgery, Theranostics, and Liquid Biopsy—A Personalised Oncologic Approach to Treatment of Patients with Advanced Metastatic Neuroendocrine Neoplasms. Int. J. Med. Sci. 2021, 18, 2166–2175. [Google Scholar] [CrossRef]
- Malczewska, A.; Bodei, L.; Kidd, M.; Modlin, I.M. Blood MRNA Measurement (NETest) for Neuroendocrine Tumor Diagnosis of Image-Negative Liver Metastatic Disease. J. Clin. Endocrinol. Metab. 2019, 104, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Ratnayake, G.M. Diagnostic and Therapeutic Advances in Neuroendocrine Tumours. Nat. Rev. Endocrinol. 2021, 17, 81–82. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a Big Role in Gene Regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, L. Expanding on Exosomes and Ectosomes in Cancer. N. Engl. J. Med. 2015, 372, 2359–2362. [Google Scholar] [CrossRef]
- Nanayakkara, J.; Tyryshkin, K.; Yang, X.; Wong, J.J.M.; Vanderbeck, K.; Ginter, P.S.; Scognamiglio, T.; Chen, Y.-T.; Panarelli, N.; Cheung, N.-K.; et al. Characterizing and Classifying Neuroendocrine Neoplasms through MicroRNA Sequencing and Data Mining. NAR Cancer 2020, 2, zcaa009. [Google Scholar] [CrossRef]
- Korotaeva, A.; Mansorunov, D.; Apanovich, N.; Kuzevanova, A.; Karpukhin, A. MiRNA Expression in Neuroendocrine Neoplasms of Frequent Localizations. ncRNA 2021, 7, 38. [Google Scholar] [CrossRef]
- Malczewska, A.; Frampton, A.E.; Mato Prado, M.; Ameri, S.; Dabrowska, A.F.; Zagorac, S.; Clift, A.K.; Kos-Kudła, B.; Faiz, O.; Stebbing, J.; et al. Circulating MicroRNAs in Small-Bowel Neuroendocrine Tumors: A Potential Tool for Diagnosis and Assessment of Effectiveness of Surgical Resection. Ann. Surg. 2021, 274, e1–e9. [Google Scholar] [CrossRef]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. MiRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef]
- Malczewska, A.; Kidd, M.; Matar, S.; Kos-Kudla, B.; Modlin, I.M. A Comprehensive Assessment of the Role of MiRNAs as Biomarkers in Gastroenteropancreatic Neuroendocrine Tumors. Neuroendocrinology 2018, 107, 73–90. [Google Scholar] [CrossRef]
- Thorns, C.; Schurmann, C.; Gebauer, N.; Wallaschofski, H.; Kümpers, C.; Bernard, V.; Feller, A.C.; Keck, T.; Habermann, J.K.; Begum, N.; et al. Global MicroRNA Profiling of Pancreatic Neuroendocrine Neoplasias. Anticancer. Res. 2014, 34, 2249–2254. [Google Scholar]
- Vicentini, C.; Fassan, M.; D’Angelo, E.; Corbo, V.; Silvestris, N.; Nuovo, G.; Scarpa, A. Clinical Application of MicroRNA Testing in Neuroendocrine Tumors of the Gastrointestinal Tract. Molecules 2014, 19, 2458–2468. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yu, J.; Kim, H.; Wolfgang, C.L.; Canto, M.I.; Hruban, R.H.; Goggins, M. MicroRNA Array Analysis Finds Elevated Serum MiR-1290 Accurately Distinguishes Patients with Low-Stage Pancreatic Cancer from Healthy and Disease Controls. Clin. Cancer Res. 2013, 19, 3600–3610. [Google Scholar] [CrossRef] [PubMed]
- Kooblall, K.G.; Stokes, V.J.; Shariq, O.A.; English, K.A.; Stevenson, M.; Broxholme, J.; Wright, B.; Lockstone, H.E.; Buck, D.; Grozinsky-Glasberg, S.; et al. MiR-3156-5p Is Downregulated in Serum of MEN1 Patients and Regulates Expression of MORF4L2. Endocr. -Relat. Cancer 2022, 29, 557–568. [Google Scholar] [CrossRef]
- Lloyd, K.A.; Moore, A.R.; Parsons, B.N.; O’Hara, A.; Boyce, M.; Dockray, G.J.; Varro, A.; Pritchard, D.M. Gastrin-Induced MiR-222 Promotes Gastric Tumor Development by Suppressing P27kip1. Oncotarget 2016, 7, 45462–45478. [Google Scholar] [CrossRef]
- Fan, K.; Ritter, C.; Nghiem, P.; Blom, A.; Verhaegen, M.E.; Dlugosz, A.A.; Ødum, N.; Woetmann, A.; Tothill, R.; Hicks, R.J.; et al. Circulating Cell-Free MiR-375 as Surrogate Marker of Tumor Burden in Merkel Cell Carcinoma. Clin. Cancer Res. 2018, 24, 5873–5882. [Google Scholar] [CrossRef]
- Bhagirath, D.; Liston, M.; Patel, N.; Akoto, T.; Lui, B.; Yang, T.L.; To, D.M.; Majid, S.; Dahiya, R.; Tabatabai, Z.L.; et al. MicroRNA Determinants of Neuroendocrine Differentiation in Metastatic Castration-Resistant Prostate Cancer. Oncogene 2020, 39, 7209–7223. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yuan, T.; Liang, M.; Du, M.; Xia, S.; Dittmar, R.; Wang, D.; See, W.; Costello, B.A.; Quevedo, F.; et al. Exosomal MiR-1290 and MiR-375 as Prognostic Markers in Castration-Resistant Prostate Cancer. Eur. Urol. 2015, 67, 33–41. [Google Scholar] [CrossRef]
- Niedra, H.; Peculis, R.; Litvina, H.D.; Megnis, K.; Mandrika, I.; Balcere, I.; Romanovs, M.; Steina, L.; Stukens, J.; Breiksa, A.; et al. Genome Wide Analysis of Circulating MiRNAs in Growth Hormone Secreting Pituitary Neuroendocrine Tumor Patients’ Plasma. Front. Oncol. 2022, 12, 894317. [Google Scholar] [CrossRef]
- Sato, J.; Shimomura, A.; Kawauchi, J.; Matsuzaki, J.; Yamamoto, Y.; Takizawa, S.; Sakamoto, H.; Ohno, M.; Narita, Y.; Ochiya, T.; et al. Brain Metastasis-Related MicroRNAs in Patients with Advanced Breast Cancer. PLoS ONE 2019, 14, e0221538. [Google Scholar] [CrossRef]
- Bowden, M.; Zhou, C.W.; Zhang, S.; Brais, L.; Rossi, A.; Naudin, L.; Thiagalingam, A.; Sicinska, E.; Kulke, M.H. Profiling of Metastatic Small Intestine Neuroendocrine Tumors Reveals Characteristic MiRNAs Detectable in Plasma. Oncotarget 2017, 8, 54331–54344. [Google Scholar] [CrossRef]
- Powrózek, T.; Porgador, A.; Małecka-Massalska, T. Detection, Prediction, and Prognosis: Blood Circulating MicroRNA as Novel Molecular Markers of Head and Neck Cancer Patients. Expert. Rev. Mol. Diagn. 2020, 20, 31–39. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, H.; Kim, H.W.; Lee, J.-C.; Paik, K.-H.; Kang, J.; Kim, J.; Yoon, Y.-S.; Han, H.-S.; Sohn, I.; et al. High Expression of MicroRNA-196a Indicates Poor Prognosis in Resected Pancreatic Neuroendocrine Tumor. Medicine 2015, 94, e2224. [Google Scholar] [CrossRef]
- Havasi, A.; Sur, D.; Cainap, S.S.; Lungulescu, C.-V.; Gavrilas, L.-I.; Cainap, C.; Vlad, C.; Balacescu, O. Current and New Challenges in the Management of Pancreatic Neuroendocrine Tumors: The Role of MiRNA-Based Approaches as New Reliable Biomarkers. Int. J. Mol. Sci. 2022, 23, 1109. [Google Scholar] [CrossRef]
- Roldo, C.; Missiaglia, E.; Hagan, J.P.; Falconi, M.; Capelli, P.; Bersani, S.; Calin, G.A.; Volinia, S.; Liu, C.-G.; Scarpa, A.; et al. MicroRNA Expression Abnormalities in Pancreatic Endocrine and Acinar Tumors Are Associated with Distinctive Pathologic Features and Clinical Behavior. J. Clin. Oncol. 2006, 24, 4677–4684. [Google Scholar] [CrossRef]
- Szilágyi, M.; Pös, O.; Márton, É.; Buglyó, G.; Soltész, B.; Keserű, J.; Penyige, A.; Szemes, T.; Nagy, B. Circulating Cell-Free Nucleic Acids: Main Characteristics and Clinical Application. Int. J. Mol. Sci. 2020, 21, 6827. [Google Scholar] [CrossRef]
- Cao, D.; Di, M.; Liang, J.; Shi, S.; Tan, Q.; Wang, Z. MicroRNA-183 in Cancer Progression. J. Cancer 2020, 11, 1315–1324. [Google Scholar] [CrossRef]
- Sharabi, A.; Kim, S.S.; Kato, S.; Sanders, P.D.; Patel, S.P.; Sanghvi, P.; Weihe, E.; Kurzrock, R. Exceptional Response to Nivolumab and Stereotactic Body Radiation Therapy (SBRT) in Neuroendocrine Cervical Carcinoma with High Tumor Mutational Burden: Management Considerations from the Center For Personalized Cancer Therapy at UC San Diego Moores Cancer Center. Oncologist 2017, 22, 631–637. [Google Scholar] [CrossRef]
- Raman, L.; Van Der Linden, M.; Van Der Eecken, K.; Vermaelen, K.; Demedts, I.; Surmont, V.; Himpe, U.; Dedeurwaerdere, F.; Ferdinande, L.; Lievens, Y.; et al. Shallow Whole-Genome Sequencing of Plasma Cell-Free DNA Accurately Differentiates Small from Non-Small Cell Lung Carcinoma. Genome Med. 2020, 12, 35. [Google Scholar] [CrossRef]
- Poulet, G.; Massias, J.; Taly, V. Liquid Biopsy: General Concepts. Acta Cytol. 2019, 63, 449–455. [Google Scholar] [CrossRef]
- Boons, G.; Vandamme, T.; Mariën, L.; Lybaert, W.; Roeyen, G.; Rondou, T.; Papadimitriou, K.; Janssens, K.; Op de Beeck, B.; Simoens, M.; et al. Longitudinal Copy-Number Alteration Analysis in Plasma Cell-Free DNA of Neuroendocrine Neoplasms Is a Novel Specific Biomarker for Diagnosis, Prognosis, and Follow-Up. Clin. Cancer Res. 2022, 28, 338–349. [Google Scholar] [CrossRef]
- Gerard, L.; Garcia, J.; Gauthier, A.; Lopez, J.; Durand, A.; Hervieu, V.; Lemelin, A.; Chardon, L.; Landel, V.; Gibert, B.; et al. CtDNA in Neuroendocrine Carcinoma of Gastroenteropancreatic Origin or of Unknown Primary: The CIRCAN-NEC Pilot Study. Neuroendocrinology 2021, 111, 951–964. [Google Scholar] [CrossRef]
- Beltran, H.; Romanel, A.; Conteduca, V.; Casiraghi, N.; Sigouros, M.; Franceschini, G.M.; Orlando, F.; Fedrizzi, T.; Ku, S.Y.; Dann, E.; et al. Circulating Tumor DNA Profile Recognizes Transformation to Castration-Resistant Neuroendocrine Prostate Cancer. J. Clin. Investig. 2020, 130, 1653–1668. [Google Scholar] [CrossRef]
- Riviere, P.; Fanta, P.T.; Ikeda, S.; Baumgartner, J.; Heestand, G.M.; Kurzrock, R. The Mutational Landscape of Gastrointestinal Malignancies as Reflected by Circulating Tumor DNA. Mol. Cancer Ther. 2018, 17, 297–305. [Google Scholar] [CrossRef]
- Schrock, A.B.; Pavlick, D.; Klempner, S.J.; Chung, J.H.; Forcier, B.; Welsh, A.; Young, L.; Leyland-Jones, B.; Bordoni, R.; Carvajal, R.D.; et al. Hybrid Capture–Based Genomic Profiling of Circulating Tumor DNA from Patients with Advanced Cancers of the Gastrointestinal Tract or Anus. Clin. Cancer Res. 2018, 24, 1881–1890. [Google Scholar] [CrossRef]
- Padda, S.K.; Aggarwal, R.R.; Ashok, A.; Mauer, E.; Shirazi, M.; Bergsland, E.K. Prevalence of High Tumor Mutational Burden (TMB-H) and Microsatellite Instability-High (MSI-H) Status in Neuroendocrine Neoplasms. JCO 2022, 40, 2625. [Google Scholar] [CrossRef]
- Malla, M.; Loree, J.M.; Kasi, P.M.; Parikh, A.R. Using Circulating Tumor DNA in Colorectal Cancer: Current and Evolving Practices. JCO 2022, 40, 2846–2857. [Google Scholar] [CrossRef]
- Prakash, V.; Gao, L.; Park, S.J. Evolving Applications of Circulating Tumor DNA in Merkel Cell Carcinoma. Cancers 2023, 15, 609. [Google Scholar] [CrossRef]
- Yeakel, J.; Kannan, A.; Rattigan, N.H.; Yamamoto, M.; Aleshin, A.; Harris, J.P.; Gao, L. Bespoke Circulating Tumor DNA as a Biomarker for Treatment Response in a Refractory Merkel Cell Carcinoma Patient. JAAD Case Rep. 2021, 18, 94–98. [Google Scholar] [CrossRef]
- Herrgott, G.A.; Asmaro, K.P.; Wells, M.; Sabedot, T.S.; Malta, T.M.; Mosella, M.S.; Nelson, K.; Scarpace, L.; Barnholtz-Sloan, J.S.; Sloan, A.E.; et al. Detection of Tumor-Specific DNA Methylation Markers in the Blood of Patients with Pituitary Neuroendocrine Tumors. Neuro-Oncol. 2022, 24, 1126–1139. [Google Scholar] [CrossRef]
- Mettler, E.; Fottner, C.; Bakhshandeh, N.; Trenkler, A.; Kuchen, R.; Weber, M.M. Quantitative Analysis of Plasma Cell-Free DNA and Its DNA Integrity and Hypomethylation Status as Biomarkers for Tumor Burden and Disease Progression in Patients with Metastatic Neuroendocrine Neoplasias. Cancers 2022, 14, 1025. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.M.; Kidd, M.; Malczewska, A.; Drozdov, I.; Bodei, L.; Matar, S.; Chung, K.M. The NETest: The Clinical Utility of Multigene Blood Analysis in the Diagnosis and Management of Neuroendocrine Tumors. Endocrinol. Metab. Clin. North. Am. 2018, 47, 485–504. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bevere, M.; Masetto, F.; Carazzolo, M.E.; Bettega, A.; Gkountakos, A.; Scarpa, A.; Simbolo, M. An Overview of Circulating Biomarkers in Neuroendocrine Neoplasms: A Clinical Guide. Diagnostics 2023, 13, 2820. https://doi.org/10.3390/diagnostics13172820
Bevere M, Masetto F, Carazzolo ME, Bettega A, Gkountakos A, Scarpa A, Simbolo M. An Overview of Circulating Biomarkers in Neuroendocrine Neoplasms: A Clinical Guide. Diagnostics. 2023; 13(17):2820. https://doi.org/10.3390/diagnostics13172820
Chicago/Turabian StyleBevere, Michele, Francesca Masetto, Maria Elena Carazzolo, Alice Bettega, Anastasios Gkountakos, Aldo Scarpa, and Michele Simbolo. 2023. "An Overview of Circulating Biomarkers in Neuroendocrine Neoplasms: A Clinical Guide" Diagnostics 13, no. 17: 2820. https://doi.org/10.3390/diagnostics13172820
APA StyleBevere, M., Masetto, F., Carazzolo, M. E., Bettega, A., Gkountakos, A., Scarpa, A., & Simbolo, M. (2023). An Overview of Circulating Biomarkers in Neuroendocrine Neoplasms: A Clinical Guide. Diagnostics, 13(17), 2820. https://doi.org/10.3390/diagnostics13172820






