Abstract
When hypostatic pneumonia is present at the same time as COVID-19 pneumonia, the clinical course is almost always prolonged (prolonged-COVID-19) due to persistent inflammation, long-term anti-inflammatory syndrome, followed by immune exhaustion, i.e., by immunosuppression and catabolic syndrome. In the immunosuppression phase, viral reactivation can be accompanied by a secondary infection, which, in this case, is pulmonary tuberculosis. Pulmonary tuberculosis in post-COVID-19 patients and in patients with spastic quadriplegic cerebral palsy does not have a typical clinical course nor laboratory, radiological, immunological, microbiological, or fiberbronchoscopic pathohistological confirmation. Due to this, the treatment of pulmonary tuberculosis was not carried out on time, postponed after the unsuccessful treatment of sepsis, post-COVID-19, and other accompanying viral (adenovirus, RSV) and bacterial (streptococcus viridans) infections. The treatment of pulmonary tuberculosis was possible only “ex juvantibus” (trial) post-COVID-19. It becomes imperative to search for a new, more precise and reliable diagnostic test for the detection of tuberculosis bacillus.
A boy with a body weigh of 15 kg, who has spastic quadriplegic cerebral palsy (SQCP), was down clinically inapparent COVID-19 which we confirmed due to an elevated titer of IgG antibodies for SARS-CoV-2 (40.6 BAU/mL) on admission to the Clinic of Paediatrics (PC-Kg) (Table 1). The child was referred to PC-Kg due to experiencing intermittent fever during the previous 3 months, but with a continuous fever 2 weeks before admission to PC-Kg. During the last 2 weeks, he was treated with antibiotics (semi-synthetic penicillin, then third-generation cephalosporin, and the day before admission, azithromycin) via bronchodilator inhalation, so that C-reactive protein (CRP) fell to 114 mg/L, compared to the previous level (305 mg/L). According to the anamnestic data of the last 3 months, the patient had no weight loss, no sweating, no cough “with a full mouth”, no history of tuberculosis in the family and environment, and showed no evidence of suffering from COVID-19. The Quantiferon TB gold immunoassay (interferon-γ release assay, IGRA) result was negative, and there was no increase in total serum immunoglobulin M nor decrease in total serum immunoglobulin A during the 34 days of hospitalization, due to the patient’s suppressed immune response during the period of post-COVID-19 (Table 1) [1]. A negative tuberculin skin test (TST) is a consequence of suppression by systemic corticosteroids (Table 1). The direct microscopy of all three samples of tracheal aspirate and gastric washings, after staining with Ziehl-Neelsen, on the 28th, 29th and 30th day of hospitalization did not find tuberculosis bacilli, nor did the analysis of the fiber-aspirate sample on the 36th day of hospitalization (Institute for Mother and Child New Belgrade (IMC) (Table 1). Only 45 days after the start of “ex juvantibus” treatment with antituberculotics (H,R,Z,E), was pulmonary tuberculosis confirmed by microbiological examination of the tracheal aspirate and gastric washings in a Loweinsten medium, in a patient with post-COVID-19 and SQCP [2,3,4,5]. Other results were within the reference values: urine, blood gas analysis, erythrocyte count in blood test, prothrombin time, activated partial thromboplastin time, transaminases (AST, ALT), gamma-GT, glycemia, urea, creatinine, alkaline phosphatase, creatinine kinase (total, muscle), urine culture, virological serology for cytomegalovirus, toxoplasma, rubella, herpes simplex 1 + 2, parvo-B19, Epstein-Barr virus (Table 1). Streptococcus viridans was isolated in 1 blood culture, but 11 blood cultures with two samples were left without pathogenic germs (Table 1). Adenovirus and respiratory syncytial virus (RSV) have been proven via polymerase chain reaction (PCR) diagnostics for the 17 most common respiratory pathogens (Table 1) [6,7,8].

Table 1.
Relevant hematological, biochemical, immunological, and microbiological results in an 11-year-old boy on admission and during 36 days of hospitalization.
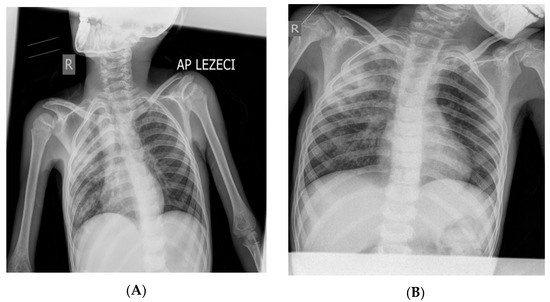
Figure 1.
X-ray of the boy’s lungs on admission (A) and after 28 days of hospitalization (B). (A) X-rays of the lungs (in the supine position) were performed on admission: right (“R”) diffusely spotted shadows confluent in the upper and middle lung field, left hiloapically spotted shadows. Cardiophrenic sinuses free, both hemidiaphragm clearly contoured (Figure 1). (B) X-rays of the lungs (in the supine position) were performed on the 28th day of hospitalization: diffusely accentuated interstitium with a zone of consolidation in the upper and middle pulmonary field on the right (“R”). Hemidiaphragms are clearly contoured (Figure 1). The pediatric scoring system A for pulmonary tuberculosis was not sufficient to confirm pulmonary tuberculosis (mark 3) [9,10,11,12,13]. During the 34 days of hospitalization, the boy was febrile all the time with occasional temperature jumps up to 39.5 °C (septic type); he was breathing stably throughout, without the need for oxygen therapy. Only the patient’s fever, which manifested itself continuously for 4 weeks, raised the suspicion of pulmonary tuberculosis. The cough was intermittent and discreet during the 34 days of hospitalization in PC-Kg.
Figure 1.
X-ray of the boy’s lungs on admission (A) and after 28 days of hospitalization (B). (A) X-rays of the lungs (in the supine position) were performed on admission: right (“R”) diffusely spotted shadows confluent in the upper and middle lung field, left hiloapically spotted shadows. Cardiophrenic sinuses free, both hemidiaphragm clearly contoured (Figure 1). (B) X-rays of the lungs (in the supine position) were performed on the 28th day of hospitalization: diffusely accentuated interstitium with a zone of consolidation in the upper and middle pulmonary field on the right (“R”). Hemidiaphragms are clearly contoured (Figure 1). The pediatric scoring system A for pulmonary tuberculosis was not sufficient to confirm pulmonary tuberculosis (mark 3) [9,10,11,12,13]. During the 34 days of hospitalization, the boy was febrile all the time with occasional temperature jumps up to 39.5 °C (septic type); he was breathing stably throughout, without the need for oxygen therapy. Only the patient’s fever, which manifested itself continuously for 4 weeks, raised the suspicion of pulmonary tuberculosis. The cough was intermittent and discreet during the 34 days of hospitalization in PC-Kg.
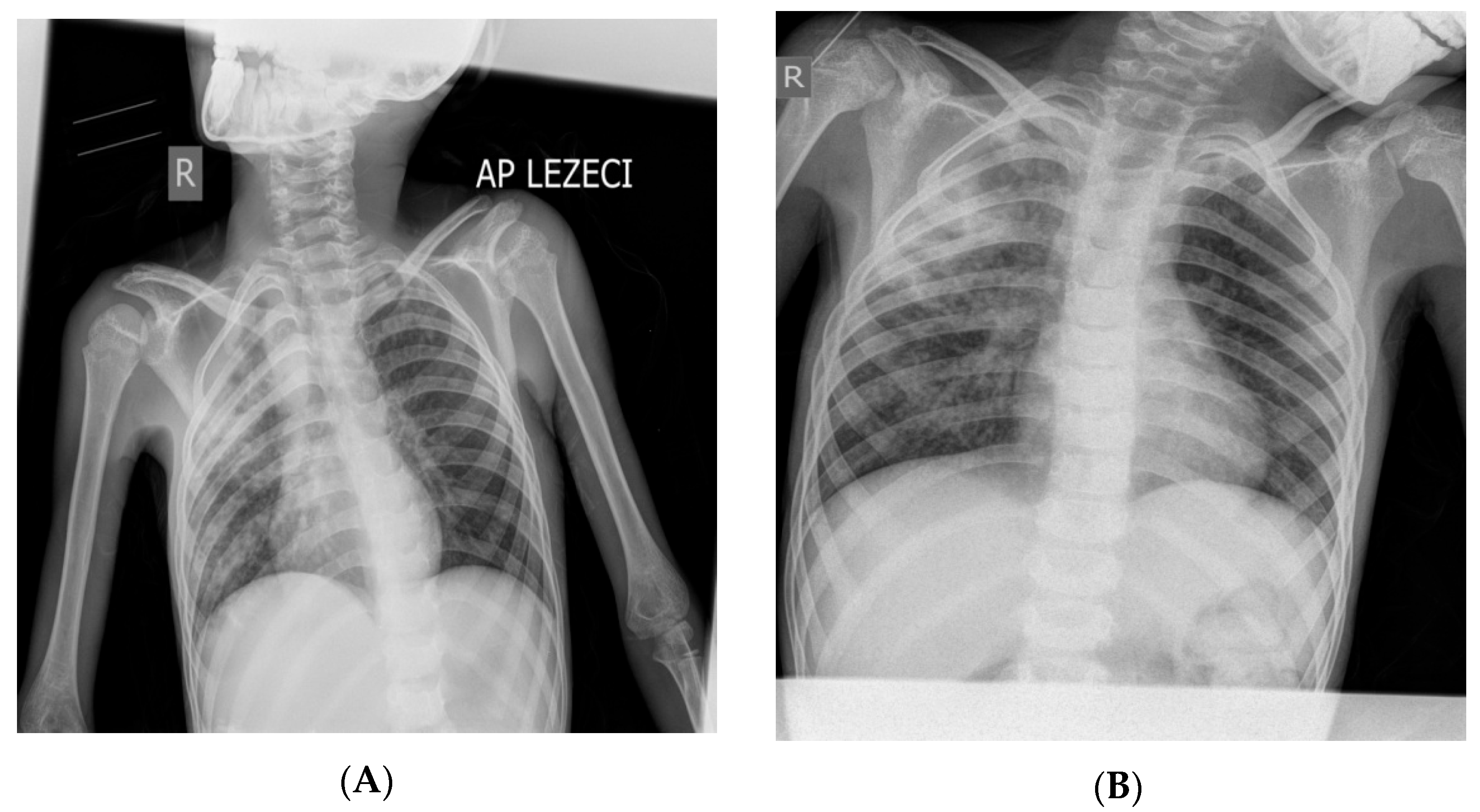
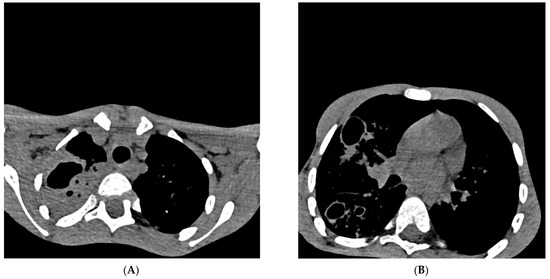
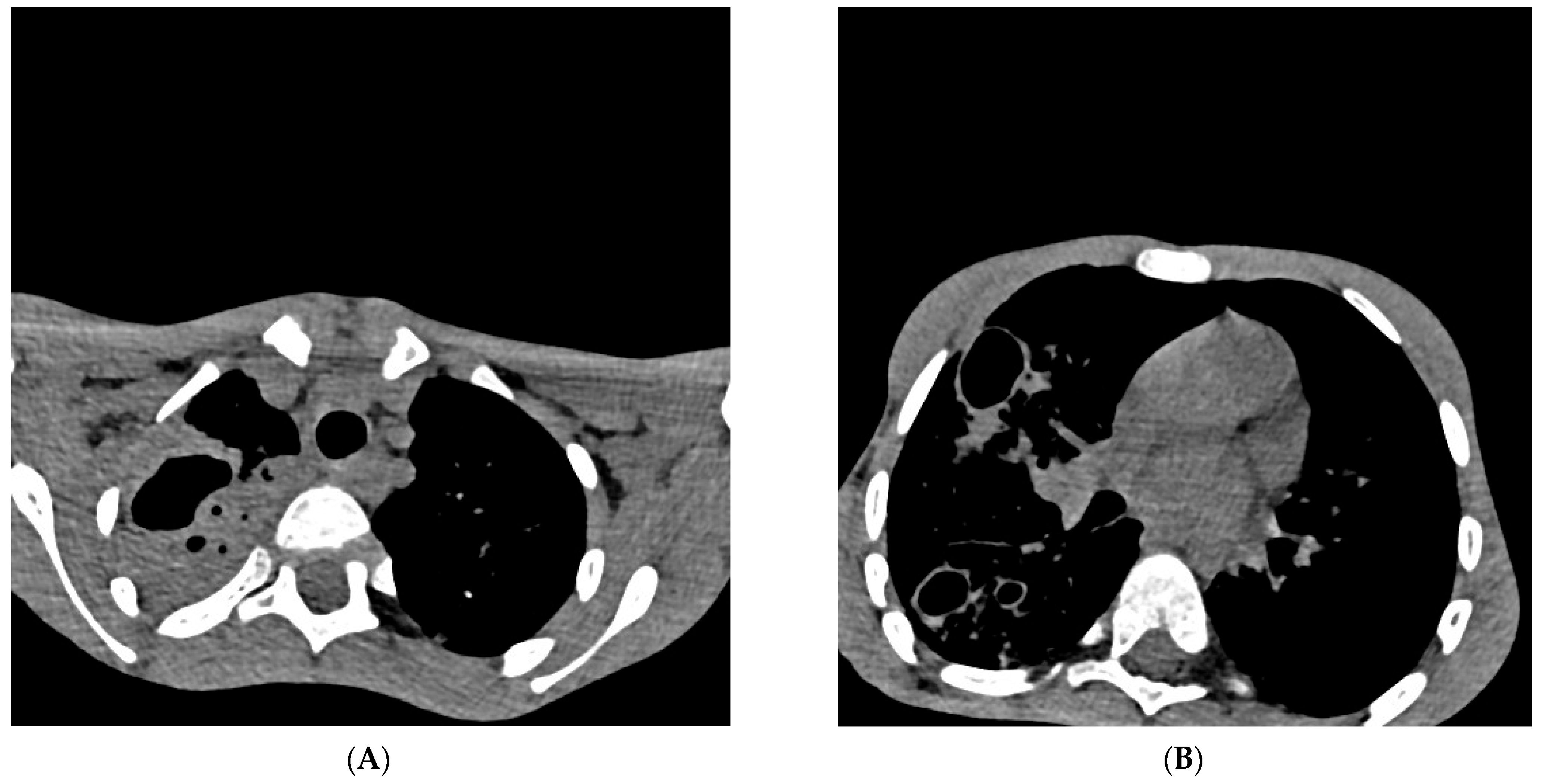
Figure 2.
Cross-section of a multislice chest scanner (MSCT). Chest MSCT native and with iv contrast with MPR (28th day of hospitalization), Section 54/209 ((A) in Figure 2) and Section 100/209 ((B) in Figure 2): on the right, there is a consolidation of the entire upper lobe with signs of excavation (lobar pneumonia) which aroused suspicion, differentially diagnostic of pulmonary tuberculosis. Cystic and traction bronchiectasis are observed in the upper lobe. On both sides of the lung parenchyma, multiple centrilobular nodules (“tree in the bud”) with cystic changes of the thick walls, excavated (obs. Histiocytosis), are more pronounced on the right. Smaller zones of a grouping of spotted consolidation zones are present on both sides in the lower lobes. The trachea and both principal bronchi are passable. Lymphadenopathy of 6 mm paratracheal and established bronchiectasis seen on MSCT of the chest are not relevant exclusively for pulmonary tuberculosis and can already be considered differentially diagnostically in many other respiratory and lung diseases [14]. Pleural spaces are free. The hematological examination of blood smears and myelogram confirmed the infection and rejected the hypothesis of malignant blood disease and solid tumor on the 29th day of hospitalization. Cardiac and ultrasound examination of the heart raised suspicion of infectious endocarditis (mild dysplasia of mitral vela prolapse and a small thickening of 1 × 2 mm in the upper third). Ultrasound examination of the abdomen revealed normal findings, as well as a gentle digital rectal examination. The boy was treated according to protocols for post-COVID-19, sepsis, and pneumonia with antibiotics from the group of reserve antibiotics, mainly parenteral: meropenem + vancomycin 7 days, ciprofloxacin 9 days with concomitant inhalation of colomycin (total 18 days), piperacillin/tazobactam 4 days, vancomycin 8 days, azithromycin 3 days, then, simultaneously for 3 days fluconazole, amoxicillin, and metronidazole, and then, simultaneously for 6 days trimethoprim/sulfamethoxazole and caspofungin. In addition to antibiotics, an antipyretic (ibuprofen, paracetamol) was applied all the time. The systemic corticosteroid methylprednisolone was administered for 20 days, and one dose of normal human immunoglobulins for intravenous use (IgM 6 mg, IgA 6 mg, IgG 38 mg) was administered to treat “post-covid19 inflammation” [15,16]. The patient was referred to IMC, where four antituberculotics (isoniazid, rifampicin, pyrazinamide, ethambutol—H,R,Z,E) were introduced “ex juvantibus” (trial), after which clinical improvement occurred. During the prolonged/post-COVID-19 period, pulmonary tuberculosis can progress “quietly” and “clinically unconvincingly”, which delays diagnosis and delays initiation of adequate treatment. Parents of a sick child may ask you “Why did you introduce four antituberculosis drugs for my child, and you have no evidence that it is tuberculosis?” In the same way, parents can come after a month of treatment with antituberculosis drugs and ask “Why are you excluding antituberculosis drugs now?” “So it’s some other disease after all?”. A new diagnostic test for pulmonary tuberculosis needs to be considered, especially since the eradication of tuberculosis in the world has failed for many years. It is necessary to conduct research on better and more reliable diagnostic tests (microbiological, immunological, or any new type of analysis).
Figure 2.
Cross-section of a multislice chest scanner (MSCT). Chest MSCT native and with iv contrast with MPR (28th day of hospitalization), Section 54/209 ((A) in Figure 2) and Section 100/209 ((B) in Figure 2): on the right, there is a consolidation of the entire upper lobe with signs of excavation (lobar pneumonia) which aroused suspicion, differentially diagnostic of pulmonary tuberculosis. Cystic and traction bronchiectasis are observed in the upper lobe. On both sides of the lung parenchyma, multiple centrilobular nodules (“tree in the bud”) with cystic changes of the thick walls, excavated (obs. Histiocytosis), are more pronounced on the right. Smaller zones of a grouping of spotted consolidation zones are present on both sides in the lower lobes. The trachea and both principal bronchi are passable. Lymphadenopathy of 6 mm paratracheal and established bronchiectasis seen on MSCT of the chest are not relevant exclusively for pulmonary tuberculosis and can already be considered differentially diagnostically in many other respiratory and lung diseases [14]. Pleural spaces are free. The hematological examination of blood smears and myelogram confirmed the infection and rejected the hypothesis of malignant blood disease and solid tumor on the 29th day of hospitalization. Cardiac and ultrasound examination of the heart raised suspicion of infectious endocarditis (mild dysplasia of mitral vela prolapse and a small thickening of 1 × 2 mm in the upper third). Ultrasound examination of the abdomen revealed normal findings, as well as a gentle digital rectal examination. The boy was treated according to protocols for post-COVID-19, sepsis, and pneumonia with antibiotics from the group of reserve antibiotics, mainly parenteral: meropenem + vancomycin 7 days, ciprofloxacin 9 days with concomitant inhalation of colomycin (total 18 days), piperacillin/tazobactam 4 days, vancomycin 8 days, azithromycin 3 days, then, simultaneously for 3 days fluconazole, amoxicillin, and metronidazole, and then, simultaneously for 6 days trimethoprim/sulfamethoxazole and caspofungin. In addition to antibiotics, an antipyretic (ibuprofen, paracetamol) was applied all the time. The systemic corticosteroid methylprednisolone was administered for 20 days, and one dose of normal human immunoglobulins for intravenous use (IgM 6 mg, IgA 6 mg, IgG 38 mg) was administered to treat “post-covid19 inflammation” [15,16]. The patient was referred to IMC, where four antituberculotics (isoniazid, rifampicin, pyrazinamide, ethambutol—H,R,Z,E) were introduced “ex juvantibus” (trial), after which clinical improvement occurred. During the prolonged/post-COVID-19 period, pulmonary tuberculosis can progress “quietly” and “clinically unconvincingly”, which delays diagnosis and delays initiation of adequate treatment. Parents of a sick child may ask you “Why did you introduce four antituberculosis drugs for my child, and you have no evidence that it is tuberculosis?” In the same way, parents can come after a month of treatment with antituberculosis drugs and ask “Why are you excluding antituberculosis drugs now?” “So it’s some other disease after all?”. A new diagnostic test for pulmonary tuberculosis needs to be considered, especially since the eradication of tuberculosis in the world has failed for many years. It is necessary to conduct research on better and more reliable diagnostic tests (microbiological, immunological, or any new type of analysis).
Author Contributions
Conceptualization, A.S.; methodology, M.I.; software, I.I.; validation, A.S. and M.I.; formal analysis, K.D.; investigation, Z.R. and J.N.; resources, A.K.; data curation, K.D. and A.K.; writing—original draft preparation, A.S. and K.D.; writing—review and editing, A.S., I.I., A.K., K.D., Z.R., J.N. and M.I.; visualization, I.I.; supervision, A.S. and M.I.; project administration, I.I.; funding acquisition, A.S. and M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to it being a retrospective case report which did not impact the management of the patient.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data associated with this study has been deposited at the electronic archive of the University Clinical Centre Pediatric Clinic in Kragujevac, Serbia.
Acknowledgments
We would like to thank Predrag Minic from the Institute for Mother and Child, New Belgrade, Serbia, for performing the fiber-bronchoscopy and for supplying results of tracheal aspirate in Lowenstein medium.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Channappanavar, R. Interferons in coronavirus pathogenesis: The good, the bad, and the ugly. Cell Host Microbe 2022, 30, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Larson, C.; Hammond, T.C.; Oronsky, A.; Kesari, S.; Lybeck, M.; Reid, T.R. A Review of Persistent Post-COVID Syndrome (PPCS). Clin. Rev. Allergy Immunol. 2021, 64, 66–74. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Kılınç, E.; Baranoğlu, Y. Mast cell stabilizers as a supportive therapy can contribute to alleviate fatal inflammatory responses and severity of pulmonary complications in COVID-19 infection. Anadolu Klin. 2020, 25, 111–118. [Google Scholar] [CrossRef][Green Version]
- Sun, B.; Tang, N.; Peluso, M.J.; Iyer, N.S.; Torres, L.; Donatelli, J.L.; Munter, S.E.; Nixon, C.C.; Rutishauser, R.L.; Rodriguez-Barraquer, I.; et al. Characterization and Biomarker Analyses of Post-COVID-19 Complications and Neurological Manifestations. Cells 2021, 10, 386. [Google Scholar] [CrossRef]
- Lewis, D. Long COVID and kids: Scientists race to find answers. Nature 2021, 595, 482–483. [Google Scholar] [CrossRef]
- Buonsenso, D.; Munblit, D.; De Rose, C.; Sinatti, D.; Ricchiuto, A.; Carfi, A.; Valentini, P. Preliminary evidence on long COVID in children. Acta Paediatr. 2021, 110, 2208–2211. [Google Scholar] [CrossRef]
- Osmanov, I.M.; Spiridonova, E.; Bobkova, P.; Gamirova, A.; Shikhaleva, A.; Andreeva, M.; Blyuss, O.; El-Taravi, Y.; DunnGalvin, A.; Comberiati, P. Risk factors for long covid in previously hospitalized children using the ISARIC Global follow-up protocol: A prospective cohort study. medRxiv 2021. [Google Scholar] [CrossRef]
- Diagnosis of TB in Children. In Guidance for National Tuberculosis Programs on the Management of Tuberculosis in Children, 2nd ed.; World Health Organization: Geneva, Switzerland, 2014.
- Crofton, J.; Horne, N.; Miller, F. Klinička Tuberkuloza, 2nd ed.; Ministarstvo Zdravlja Republike Srbije: Belgrade, Serbia; Stojkov: Novi Sad, Serbia, 2005; pp. 29–90. [Google Scholar]
- Global Tuberculosis Report 2021. World Health Organization. 2021. Available online: https://www.who.int/publications/i/item/9789240037021 (accessed on 10 March 2023).
- Hypostatic Pneumonia. Merriam-Webster.com Medical Dictionary, Merriam-Webster. Available online: https://www.merriam-webster.com/medical/hypostatic%20pneumonia (accessed on 17 April 2022).
- Cellina, M.; Orsi, M.A.; Oliva, G. Peribronchial Consolidation with Surrounding Ground-Glass Opacity in COVID-19 Pneumonia: 3D Reconstruction of a Chest Computed Tomography. Am. J. Trop. Med. Hyg. 2020, 103, 7. [Google Scholar] [CrossRef] [PubMed]
- Sinigaglia, A.; Peta, E.; Riccetti, S.; Venkateswaran, S.; Manganelli, R.; Barzon, L. Tuberculosis-Associated MicroRNAs: From Pathogenesis to Disease Biomarkers. Cells 2020, 9, 2160. [Google Scholar] [CrossRef]
- Visca, D.; Ong, C.; Tiberi, S.; Centis, R.; D’ambrosio, L.; Chen, B.; Mueller, J.; Mueller, P.; Duarte, R.; Dalcolmo, M.; et al. Tuberculosis and COVID-19 interaction: A review of biological, clinical and public health effects. Pulmonology 2021, 27, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Gopalaswamy, R.; Subbian, S. Corticosteroids for COVID-19 Therapy: Potential Implications on Tuberculosis. Int. J. Mol. Sci. 2021, 22, 3773. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
