Acute Effects of Running on Shear Wave Elastography Measures of the Achilles Tendon and Calf Muscles in Professional Female Handball and Volleyball Players
Abstract
:1. Introduction
1.1. Development of Ultrasound as Multiparametric Technique
1.2. Shear Wave Elastography Application
1.3. Shear Wave Elastography in Musculoskeletal Tissue
1.4. Shear Wave Elastography in Professional Sports
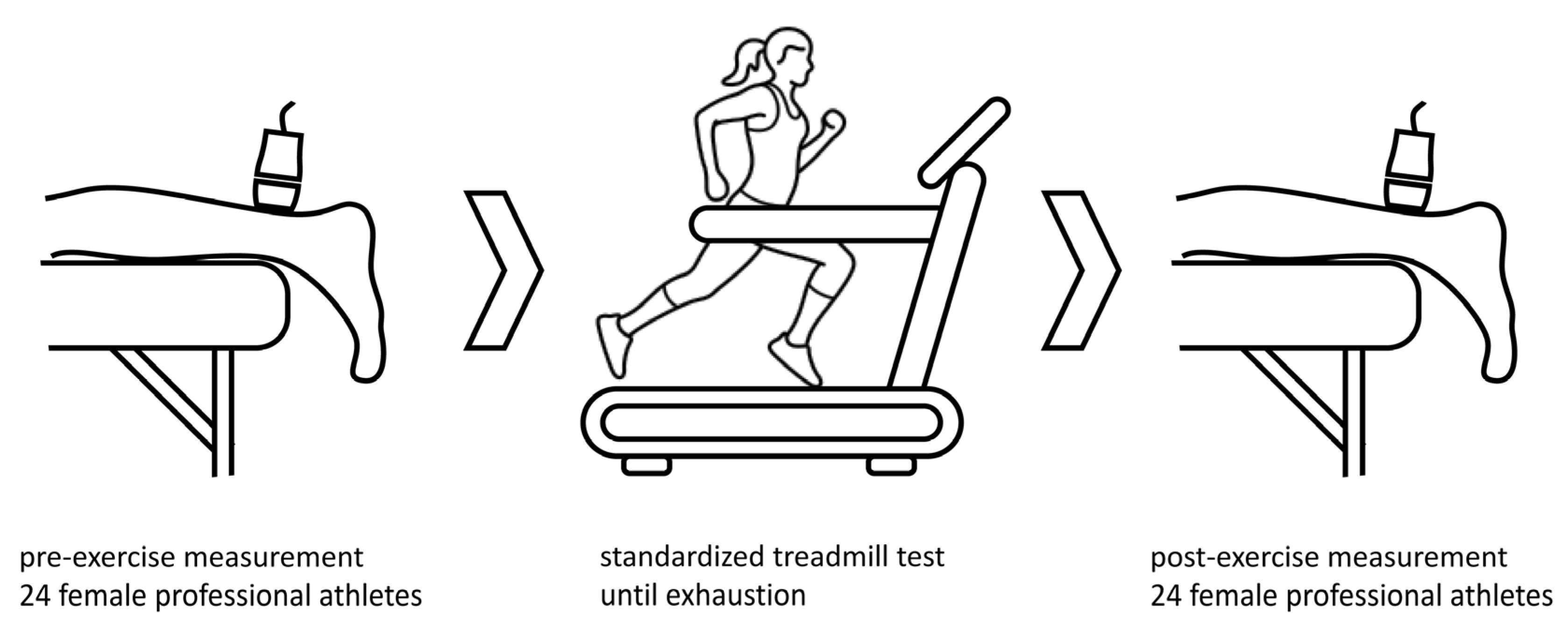
2. Methods
2.1. Participants
2.2. Shear Wave Elastography Protocol
2.3. Statistical Analysis
3. Results
Results of SWE Examination
4. Discussion
4.1. Shear Wave Elastography of Achilles Tendon
4.2. Shear Wave Elastography of Soleus und Gastrocnemius Muscle
4.3. The Potential of Shear Wave Elastography to Detect Inflammatory Tissue
Perspective Section
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AT | Achilles tendon |
| MRI | magnetic resonance imaging |
| MS | musculus soleus |
| MG | musculus gastrocnemius |
| SWE | shear wave elastography |
| SWS | shear wave speed |
| US | ultrasound |
References
- Aubry, S.; Nueffer, J.P.; Tanter, M.; Becce, F.; Vidal, C.; Michel, F. Viscoelasticity in Achilles tendonopathy: Quantitative assessment by using real-time shear-wave elastography. Radiology 2015, 274, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Akkoc, O.; Caliskan, E.; Bayramoglu, Z. Effects of passive muscle stiffness measured by Shear Wave Elastography, muscle thickness, and body mass index on athletic performance in adolescent female basketball players. Med. Ultrason. 2018, 20, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.S.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Radiographics 2017, 37, 855–870. [Google Scholar] [CrossRef] [PubMed]
- Grazynska, A.; Kufel, J.; Dudek, A.; Cebula, M. Shear Wave and Strain Elastography in Crohn’s Disease-A Systematic Review. Diagnostics 2021, 11, 1609. [Google Scholar] [CrossRef]
- Costello, J.T.; Bieuzen, F.; Bleakley, C.M. Where are all the female participants in Sports and Exercise Medicine research? Eur. J. Sports Sci. 2014, 14, 847–851. [Google Scholar] [CrossRef]
- Tas, S.; Onur, M.R.; Yilmaz, S.; Soylu, A.R.; Korkusuz, F. Shear Wave Elastography Is a Reliable and Repeatable Method for Measuring the Elastic Modulus of the Rectus Femoris Muscle and Patellar Tendon. J. Ultrasound Med. 2017, 36, 565–570. [Google Scholar] [CrossRef]
- Dirrichs, T.; Quack, V.; Gatz, M.; Tingart, M.; Kuhl, C.K.; Schrading, S. Shear Wave Elastography (SWE) for the Evaluation of Patients with Tendinopathies. Acad. Radiol. 2016, 23, 1204–1213. [Google Scholar] [CrossRef]
- Corrigan, P.; Zellers, J.A.; Balascio, P.; Silbernagel, K.G.; Cortes, D.H. Quantification of Mechanical Properties in Healthy Achilles Tendon Using Continuous Shear Wave Elastography: A Reliability and Validation Study. Ultrasound Med. Biol. 2019, 45, 1574–1585. [Google Scholar] [CrossRef]
- Haen, T.X.; Roux, A.; Soubeyrand, M.; Laporte, S. Shear waves elastography for assessment of human Achilles tendon’s biomechanical properties: An experimental study. J. Mech. Behav. Biomed. Mater. 2017, 69, 178–184. [Google Scholar] [CrossRef]
- Bernabei, M.; Lee, S.S.M.; Perreault, E.J.; Sandercock, T.G. Shear wave velocity is sensitive to changes in muscle stiffness that occur independently from changes in force. J. Appl. Physiol. (1985) 2020, 128, 8–16. [Google Scholar] [CrossRef]
- Chen, X.M.; Cui, L.G.; He, P.; Shen, W.W.; Qian, Y.J.; Wang, J.R. Shear wave elastographic characterization of normal and torn achilles tendons: A pilot study. J. Ultrasound Med. 2013, 32, 449–455. [Google Scholar] [CrossRef]
- Ivanac, G.; Lemac, D.; Kosovic, V.; Bojanic, K.; Cengic, T.; Dumic-Cule, I.; Pecina, M.; Brkljacic, B. Importance of shear-wave elastography in prediction of Achilles tendon rupture. Int. Orthop. 2021, 45, 1043–1047. [Google Scholar] [CrossRef]
- Dirrichs, T.; Quack, V.; Gatz, M.; Tingart, M.; Rath, B.; Betsch, M.; Kuhl, C.K.; Schrading, S. Shear Wave Elastography (SWE) for Monitoring of Treatment of Tendinopathies: A Double-blinded, Longitudinal Clinical Study. Acad. Radiol. 2018, 25, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Docking, S.I.; Rio, E.; Cook, J.; Orchard, J.W.; Fortington, L.V. The prevalence of Achilles and patellar tendon injuries in Australian football players beyond a time-loss definition. Scand. J. Med. Sci. Sports 2018, 28, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Gisslen, K.; Gyulai, C.; Soderman, K.; Alfredson, H. High prevalence of jumper’s knee and sonographic changes in Swedish elite junior volleyball players compared to matched controls. Br. J. Sports Med. 2005, 39, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Berko, N.S.; Hanstein, R.; Burton, D.A.; Fornari, E.D.; Schulz, J.F.; Levin, T.L. Ultrasound elastography of the patellar tendon in young, asymptomatic sedentary and moderately active individuals. Clin. Imaging 2019, 54, 172–177. [Google Scholar] [CrossRef]
- Dirrichs, T.; Schrading, S.; Gatz, M.; Tingart, M.; Kuhl, C.K.; Quack, V. Shear Wave Elastography (SWE) of Asymptomatic Achilles Tendons: A Comparison Between Semiprofessional Athletes and the Nonathletic General Population. Acad. Radiol. 2019, 26, 1345–1351. [Google Scholar] [CrossRef]
- Mannarino, P.; Lima, K.M.M.; Fontenelle, C.R.C.; Matta, T.T.; de Salles, B.F.; Simao, R.; Oliveira, L.F. Analysis of the correlation between knee extension torque and patellar tendon elastic property. Clin. Physiol. Funct. Imaging 2018, 38, 378–383. [Google Scholar] [CrossRef]
- Mannarino, P.; Matta, T.T.D.; Oliveira, L.F. An 8-week resistance training protocol is effective in adapting quadriceps but not patellar tendon shear modulus measured by Shear Wave Elastography. PLoS ONE 2019, 14, e0205782. [Google Scholar] [CrossRef]
- Andonian, P.; Viallon, M.; Le Goff, C.; de Bourguignon, C.; Tourel, C.; Morel, J.; Giardini, G.; Gergele, L.; Millet, G.P.; Croisille, P. Shear-Wave Elastography Assessments of Quadriceps Stiffness Changes prior to, during and after Prolonged Exercise: A Longitudinal Study during an Extreme Mountain Ultra-Marathon. PLoS ONE 2016, 11, e0161855. [Google Scholar] [CrossRef]
- Siu, W.L.; Chan, C.H.; Lam, C.H.; Lee, C.M.; Ying, M. Sonographic evaluation of the effect of long-term exercise on Achilles tendon stiffness using shear wave elastography. J. Sci. Med. Sport 2016, 19, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Romer, C.; Czupajllo, J.; Zessin, E.; Fischer, T.; Wolfarth, B.; Lerchbaumer, M.H. Stiffness of Muscles and Tendons of the Lower Limb of Professional and Semiprofessional Athletes Using Shear Wave Elastography. J. Ultrasound Med. 2022, 41, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.E.; Vosseller, J.T. Maximizing Return to Sports After Achilles Tendon Rupture in Athletes. Foot Ankle Clin. 2019, 24, 439–445. [Google Scholar] [CrossRef]
- Leung, W.K.C.; Chu, K.L.; Lai, C. Sonographic evaluation of the immediate effects of eccentric heel drop exercise on Achilles tendon and gastrocnemius muscle stiffness using shear wave elastography. PeerJ 2017, 5, e3592. [Google Scholar] [CrossRef]
- Ooi, C.C.; Schneider, M.E.; Malliaras, P.; Counsel, P.; Connell, D.A. Prevalence of morphological and mechanical stiffness alterations of mid Achilles tendons in asymptomatic marathon runners before and after a competition. Skeletal Radiol. 2015, 44, 1119–1127. [Google Scholar] [CrossRef]
- Risch, L.; Mayer, F.; Cassel, M. Doppler Flow Response Following Running Exercise Differs Between Healthy and Tendinopathic Achilles Tendons. Front. Physiol. 2021, 12, 650507. [Google Scholar] [CrossRef]
- Feng, Y.N.; Li, Y.P.; Liu, C.L.; Zhang, Z.J. Assessing the elastic properties of skeletal muscle and tendon using shearwave ultrasound elastography and MyotonPRO. Sci. Rep. 2018, 8, 17064. [Google Scholar] [CrossRef]
- Coombes, B.K.; Tucker, K.; Vicenzino, B.; Vuvan, V.; Mellor, R.; Heales, L.; Nordez, A.; Hug, F. Achilles and patellar tendinopathy display opposite changes in elastic properties: A shear wave elastography study. Scand. J. Med. Sci. Sports 2018, 28, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Kot, B.C.; Zhang, Z.J.; Lee, A.W.; Leung, V.Y.; Fu, S.N. Elastic modulus of muscle and tendon with shear wave ultrasound elastography: Variations with different technical settings. PLoS ONE 2012, 7, e44348. [Google Scholar] [CrossRef] [PubMed]
- Payne, C.; Watt, P.; Cercignani, M.; Webborn, N. Reproducibility of shear wave elastography measuresof the Achilles tendon. Skeletal Radiol. 2018, 47, 779–784. [Google Scholar] [CrossRef]
- Mazza, S.; Conforti, F.S.; Forzenigo, L.V.; Piazza, N.; Berte, R.; Costantino, A.; Fraquelli, M.; Coletta, M.; Rimola, J.; Vecchi, M.; et al. Agreement between real-time elastography and delayed enhancement magnetic resonance enterography on quantifying bowel wall fibrosis in Crohn’s disease. Dig. Liver Dis. 2022, 54, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Salomone Megna, A.; Ragucci, M.; De Luca, M.; Marino Marsilia, G.; Nardone, G.; Coccoli, P.; Prinster, A.; Mannelli, L.; Vergara, E.; et al. Reproducibility of shear wave elastography (SWE) in patients with chronic liver disease. PLoS ONE 2017, 12, e0185391. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.P.; Chen, I.J.; Chang, K.V.; Wu, W.T.; Ozcakar, L. Utility of Ultrasound Elastography in Evaluation of Carpal Tunnel Syndrome: A Systematic Review and Meta-analysis. Ultrasound Med. Biol. 2019, 45, 2855–2865. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qian, Z.; Liang, W.; Liu, J.; Ren, L.; Ren, L. In vivo assessment of material properties of muscles and connective tissues around the knee joint based on shear wave elastography. J. Mech. Behav. Biomed. Mater. 2020, 109, 103829. [Google Scholar] [CrossRef]
- Ooi, C.C.; Schneider, M.E.; Malliaras, P.; Jones, D.; Saunders, S.; McMahon, A.; Connell, D. Sonoelastography of the Achilles Tendon: Prevalence and Prognostic Value Among Asymptomatic Elite Australian Rules Football Players. Clin. J. Sports Med. 2016, 26, 299–306. [Google Scholar] [CrossRef]
- Fusini, F.; Langella, F.; Busilacchi, A.; Tudisco, C.; Gigante, A.; Masse, A.; Bisicchia, S. Real-time sonoelastography: Principles and clinical applications in tendon disorders. A systematic review. Muscles Ligaments Tendons J. 2017, 7, 467–477. [Google Scholar] [CrossRef]
- Washburn, N.; Onishi, K.; Wang, J.H. Ultrasound elastography and ultrasound tissue characterisation for tendon evaluation. J. Orthop. Translat. 2018, 15, 9–20. [Google Scholar] [CrossRef]
- Gatz, M.; Betsch, M.; Bode, D.; Schweda, S.; Dirrichs, T.; Migliorini, F.; Tingart, M.; Quack, V. Intra individual comparison of unilateral Achilles tendinopathy using B-mode, power Doppler, ultrasound tissue characterization and shear wave elastography. J. Sports Med. Phys. Fit. 2020, 60, 1462–1469. [Google Scholar] [CrossRef]
- Capalbo, E.; Peli, M.; Stradiotti, P. Sonoelastography of the distal third of the Achilles tendon in asymptomatic volunteers: Correlation with anthropometric data, ultrasound findings and reproducibility of the method. Radiol. Med. 2016, 121, 667–674. [Google Scholar] [CrossRef]
- Stiver, M.L.; Mirjalili, S.A.; Agur, A.M.R. Measuring Shear Wave Velocity in Adult Skeletal Muscle with Ultrasound 2-D Shear Wave Elastography: A Scoping Review. Ultrasound Med. Biol. 2023, 49, 1353–1362. [Google Scholar] [CrossRef]
- Zardi, E.M.; Franceschetti, E.; Giorgi, C.; Palumbo, A.; Franceschi, F. Reliability of quantitative point shear-wave ultrasound elastography on vastus medialis muscle and quadriceps and patellar tendons. Med. Ultrason. 2019, 21, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cai, Y.; Hua, Y.; Shi, J.; Wang, Y.; Wang, Y. Sonoelastography shows that Achilles tendons with insertional tendinopathy are harder than asymptomatic tendons. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Frankewycz, B.; Henssler, L.; Weber, J.; Silva, N.; Koch, M.; Jung, E.M.; Docheva, D.; Alt, V.; Pfeifer, C.G. Changes of Material Elastic Properties during Healing of Ruptured Achilles Tendons Measured with Shear Wave Elastography: A Pilot Study. Int. J. Mol. Sci. 2020, 21, 3427. [Google Scholar] [CrossRef] [PubMed]
- Römer, C.; Legerlotz, K.; Czupajllo, J.; Fischer, T.; Wolfarth, B.; Lerchbaumer, M.H. Training-induced change of tendon and muscle stiffness of the lower limb in professional female athletes: Assessment with shear wave elastography. Res. Sq. 2022, preprint. [Google Scholar] [CrossRef]
- Ham, S.; Kim, S.; Choi, H.; Lee, Y.; Lee, H. Greater Muscle Stiffness during Contraction at Menstruation as Measured by Shear-Wave Elastography. Tohoku J. Exp. Med. 2020, 250, 207–213. [Google Scholar] [CrossRef]
- Mackay, K.; González, C.; Zbinden-Foncea, H.; Peñailillo, L. Effects of oral contraceptive use on female sexual salivary hormones and indirect markers of muscle damage following eccentric cycling in women. Eur. J. Appl. Physiol. 2019, 119, 2733–2744. [Google Scholar] [CrossRef]
- Huston, L.J.; Greenfield, M.L.; Wojtys, E.M. Anterior cruciate ligament injuries in the female athlete. Potential risk factors. Clin. Orthop. Relat. Res. 2000, 372, 50–63. [Google Scholar] [CrossRef]
- Romer, C.; Czupajllo, J.; Zessin, E.; Fischer, T.; Wolfarth, B.; Lerchbaumer, M.H. Muscle and Tendon Stiffness of the Lower Limb of Professional Adolescent Soccer Athletes Measured Using Shear Wave Elastography. Diagnostics 2022, 12, 2453. [Google Scholar] [CrossRef]





| Female Athletes (n = 24) | ||
|---|---|---|
| Age | 21 | 18–26 |
| BMI | 22.47 | 19.60–27.82 |
| VB | 13 | |
| HB | 11 | |
| Estimation | MS | p-Value | MG | p-Value | AT | p-Value |
|---|---|---|---|---|---|---|
| Intercept | 1.5540 | <0.001 | 1.0682 | <0.001 | 6.8831 | <0.001 |
| SWS pre-exercise (m/s) | −0.9045 | <0.001 | −0.6644 | <0.001 | −0.6149 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Römer, C.; Legerlotz, K.; Czupajllo, J.; Fischer, T.; Wolfarth, B.; Lerchbaumer, M.H. Acute Effects of Running on Shear Wave Elastography Measures of the Achilles Tendon and Calf Muscles in Professional Female Handball and Volleyball Players. Diagnostics 2023, 13, 2957. https://doi.org/10.3390/diagnostics13182957
Römer C, Legerlotz K, Czupajllo J, Fischer T, Wolfarth B, Lerchbaumer MH. Acute Effects of Running on Shear Wave Elastography Measures of the Achilles Tendon and Calf Muscles in Professional Female Handball and Volleyball Players. Diagnostics. 2023; 13(18):2957. https://doi.org/10.3390/diagnostics13182957
Chicago/Turabian StyleRömer, Claudia, Kirsten Legerlotz, Julia Czupajllo, Thomas Fischer, Bernd Wolfarth, and Markus Herbert Lerchbaumer. 2023. "Acute Effects of Running on Shear Wave Elastography Measures of the Achilles Tendon and Calf Muscles in Professional Female Handball and Volleyball Players" Diagnostics 13, no. 18: 2957. https://doi.org/10.3390/diagnostics13182957
APA StyleRömer, C., Legerlotz, K., Czupajllo, J., Fischer, T., Wolfarth, B., & Lerchbaumer, M. H. (2023). Acute Effects of Running on Shear Wave Elastography Measures of the Achilles Tendon and Calf Muscles in Professional Female Handball and Volleyball Players. Diagnostics, 13(18), 2957. https://doi.org/10.3390/diagnostics13182957








