A Key Role of CD8+ T Cells in Controlling of Tuberculosis Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Sample Collection
2.3. Immunophenotyping of Peripheral Blood CD8+ T Cell Subset Maturation Stages and CD57 Expression
2.4. Immunophenotyping of Peripheral Blood ‘Polarized’ CD8+ T Cell Subsets
2.5. Statistical Analysis
3. Results
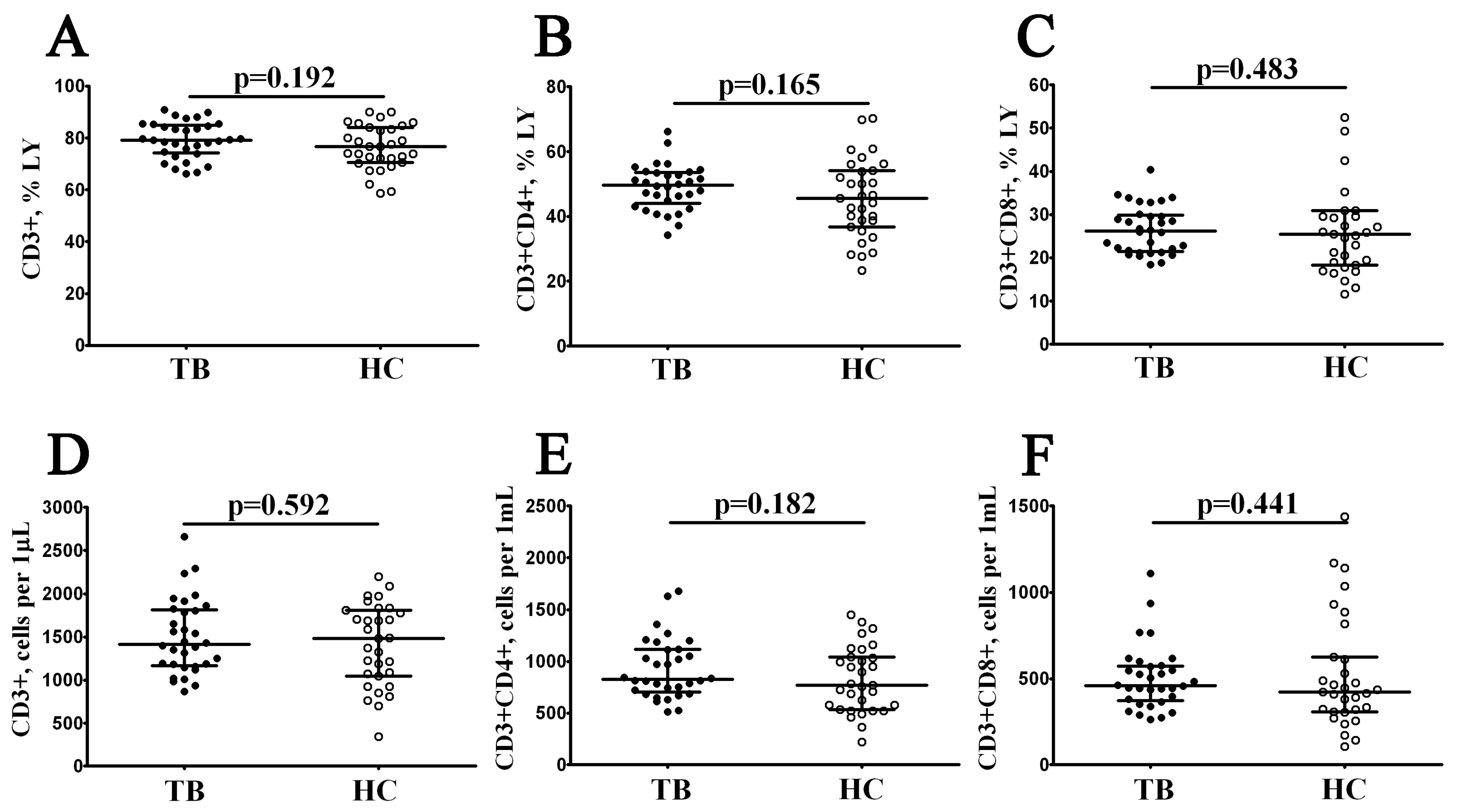
3.1. Main Peripheral Blood T Cell Subsets in Patients with Pulmonary Tuberculosis
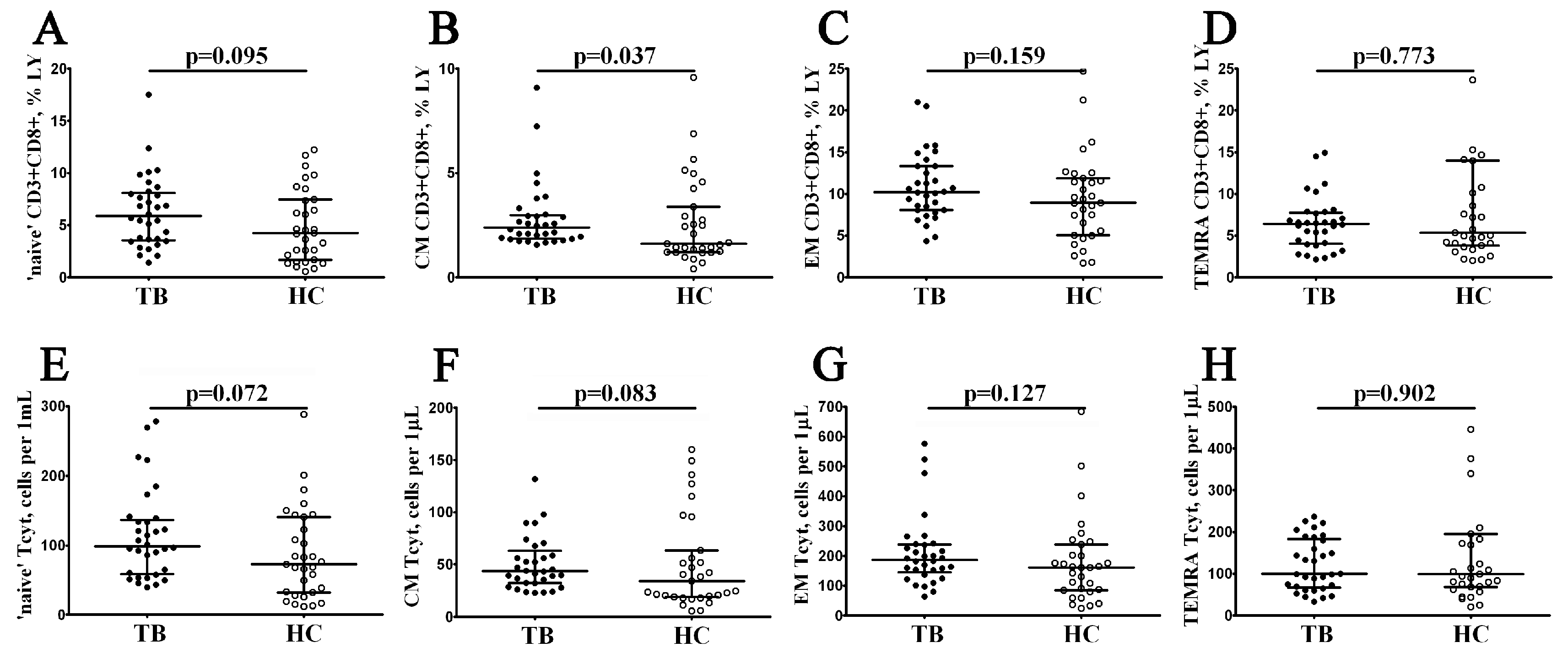
3.2. Alterations in Peripheral Blood CD8+ T Cell Maturation Subsets in Patients with Pulmonary Tuberculosis
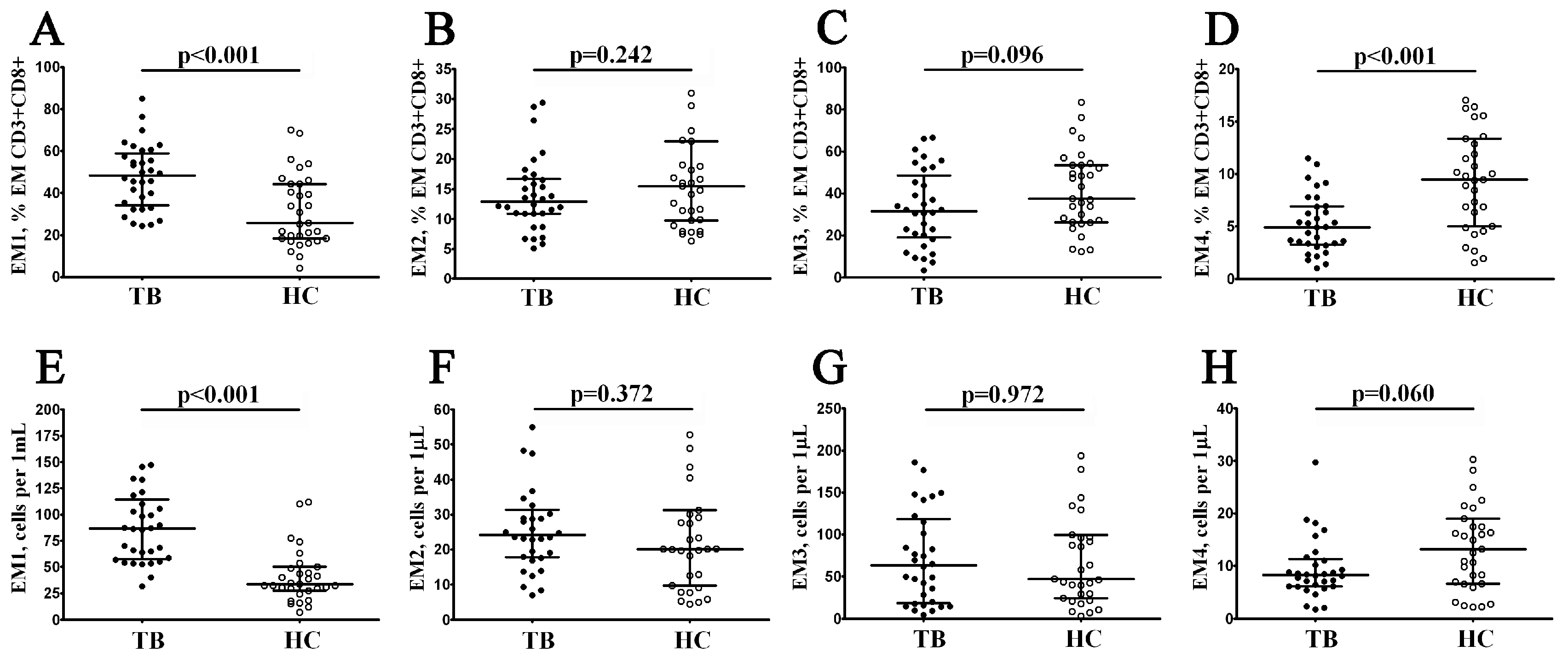
3.3. Alterations in EM and TEMRA CD8+ T Cell Subsets in Patients with Pulmonary Tuberculosis
3.4. Alterations in CD57 Expression on CD8+ T Cell Subsets from Patients with Pulmonary Tuberculosis
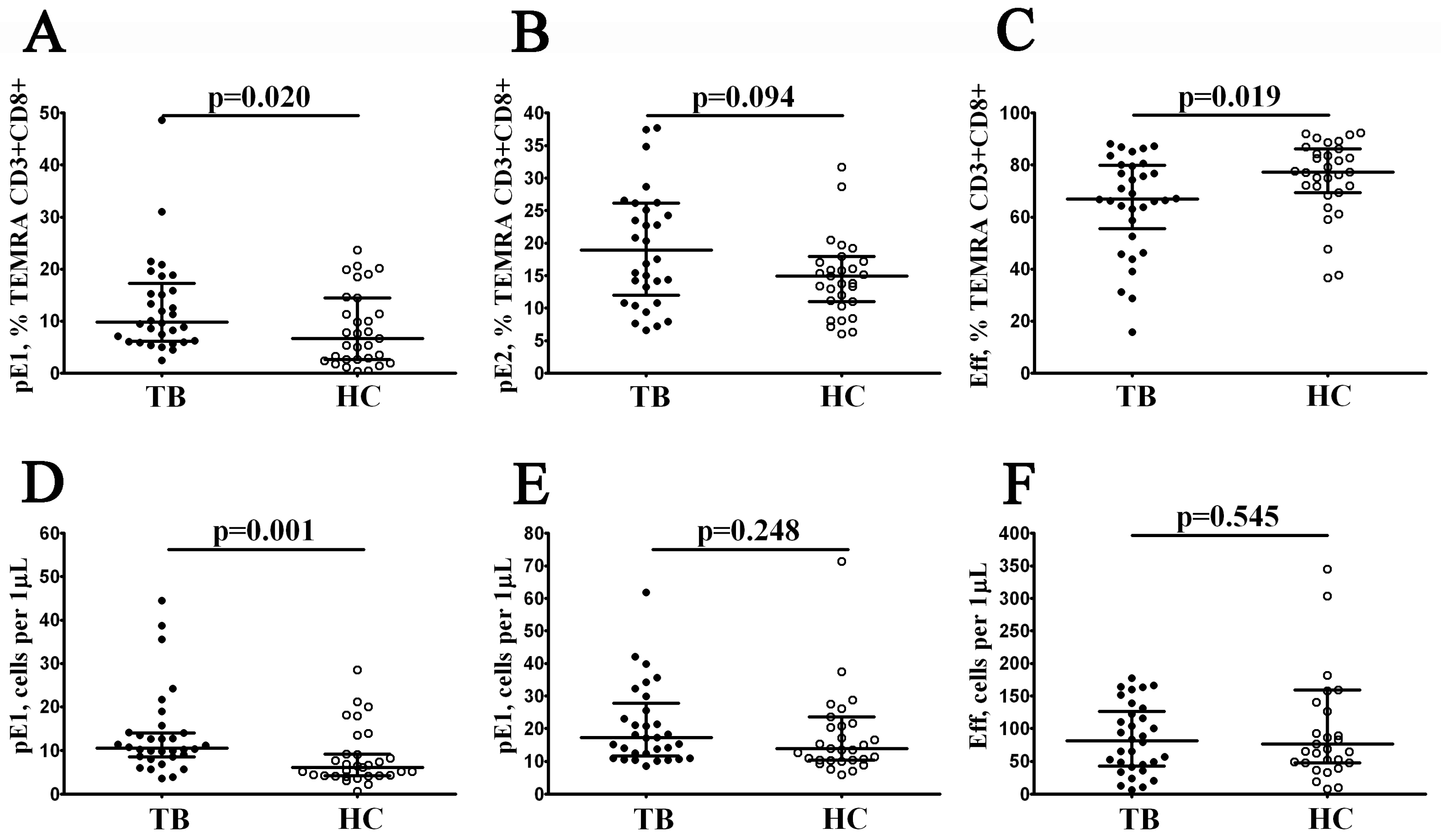
3.5. Imbalance in Peripheral Blood Main ‘Polarized’ CD8+ T Cell Subsets in Patients with Pulmonary Tuberculosis
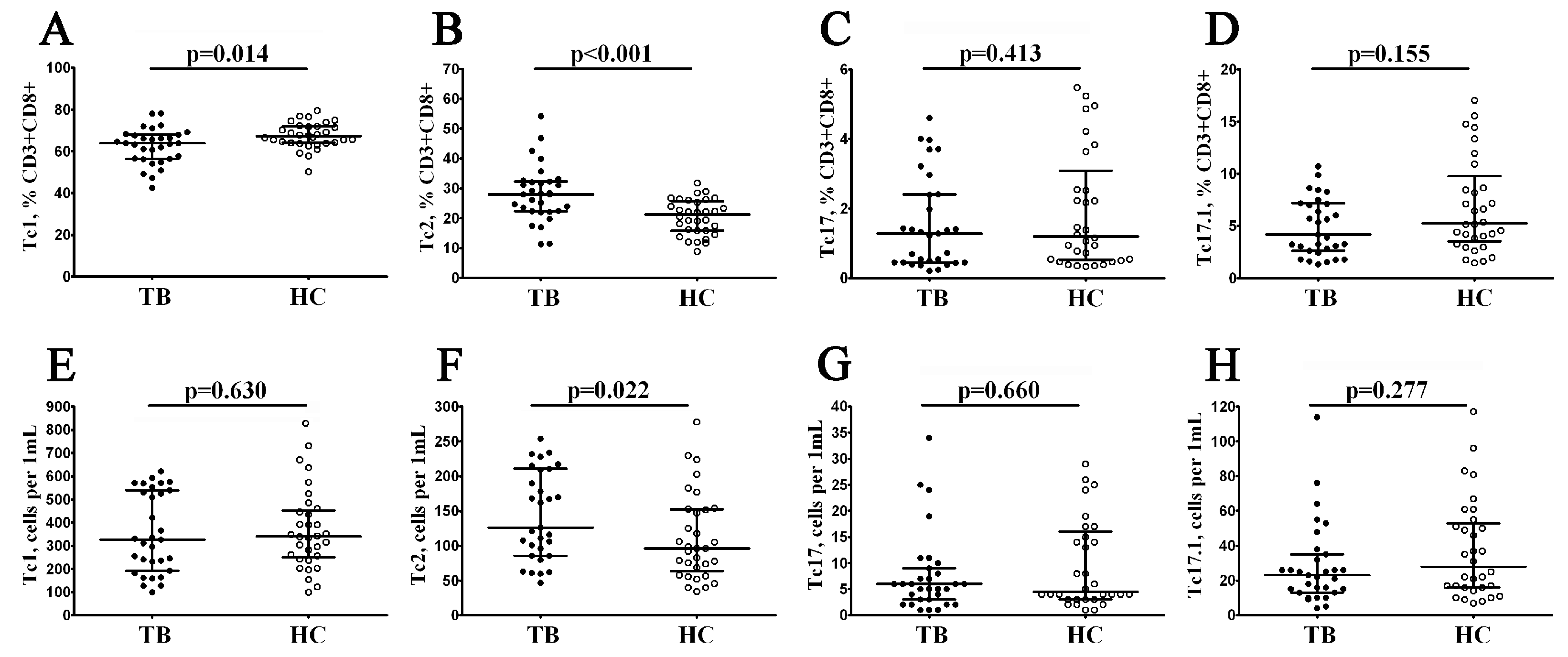
3.6. Imbalance in Tc1, Tc2, and Tc17 Cells in Peripheral Blood CD8+ T Cell Maturation Subsets in Patients with Pulmonary Tuberculosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement:
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020; Volume 4, p. 250. [Google Scholar]
- World Health Organization. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- McQuaid, C.F.; Henrion, M.Y.R.; Burke, R.M.; MacPherson, P.; Nzawa-Soko, R.; Horton, K.C. Inequalities in the impact of COVID-19-associated disruptions on tuberculosis diagnosis by age and sex in 45 high TB burden countries. BMC Med. 2022, 20, 432. [Google Scholar] [CrossRef]
- Kessel, B.; Heinsohn, T.; Ott, J.J.; Wolff, J.; Hassenstein, M.J.; Lange, B. Impact of COVID-19 pandemic and anti-pandemic measures on tuberculosis, viral hepatitis, HIV/AIDS and malaria-A systematic review. PLoS Glob. Public Health 2023, 3, e0001018. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report 2021; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2021; p. 57. [Google Scholar]
- World Health Organization. Consolidated Guidelines on Tuberculosis. In Module 3: Diagnosis. Tests for Tuberculosis Infection; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Zellweger, J.P.; Sotgiu, G.; Corradi, M.; Durando, P. The diagnosis of latent tuberculosis infection (LTBI): Currently 622 available tests, future developments, and perspectives to eliminate tuberculosis (TB). Med. Lav. 2020, 111, 170–183. [Google Scholar] [CrossRef]
- Starshinova, A.A.; Nazarenko, M.M.; Belyaeva, E.N.; Kudlay, D.A.; Pavlova, M.V.; Yablonskiy, P.K. Effectiveness of bedaquiline use in patients with multidrug and extensive drug TB. sustainability. Tuberc. Lung Dis. 2022, 100, 56–63. [Google Scholar] [CrossRef]
- Guglielmetti, L.; Veziris, N.; Aubry, A.; Brossier, F.; Bernard, C.; Sougakoff, W.; Jarlier, V.; Robert, J.; Surveillan, F.T.L. Risk factors for extensive drug resistance in multidrug-resistant tuberculosis cases: A case-case study. Int. J. Tuberc. Lung Dis. 2018, 22, 54–59. [Google Scholar] [CrossRef]
- Starshinova, A.; Kudryavtsev, I.; Rubinstein, A.; Malkova, A.; Dovgaluk, I.; Kudlay, D. Tuberculosis and COVID-19 Dually Affect Human Th17 Cell Immune Response. Biomedicines 2023, 11, 2123. [Google Scholar] [CrossRef]
- Ribeiro, F.M.; Goldenberg, T. Mycobacteria and autoimmunity. Lupus 2015, 24, 374–381. [Google Scholar] [CrossRef]
- Elkington, P.; Tebruegge, M.; Mansour, S. Tuberculosis: An Infection-Initiated Autoimmune Disease? Trends Immunol. 2016, 37, 815–818. [Google Scholar] [CrossRef]
- Kudryavtsev, I.V.; Serebriakova, M.K.; Starshinova, A.A.; Zinchenko, Y.S.; Basantsova, N.Y.; Belyaeva, E.N.; Pavlova, M.V.; Yablonskiy, P.K. Altered peripheral blood Th17 and follicular T-helper subsets in patients with pulmonary tuberculosis. Russ. J. Infect. Immun. 2019, 9, 304–314. [Google Scholar] [CrossRef]
- Monin, L.; Griffiths, K.L.; Slight, S.; Lin, Y.; Rangel-Moreno, J.; Khader, S.A. Immune requirements for protective Th17 recall responses to Mycobacterium tuberculosis challenge. Mucosal Immunol. 2015, 8, 1099–1109. [Google Scholar] [CrossRef]
- Maglione, P.J.; Xu, J.; Chan, J. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. J. Immunol. 2007, 178, 7222–7234. [Google Scholar] [CrossRef]
- Kozlov, V.A.; Tikhonova, E.P.; Savchenko, A.A.; Kudryavtsev, I.V.; Andronova, N.V.; Anisimova, E.N.; Golovkin, A.S.; Demina, D.V.; Zdzitovetsky, D.E.; Kalinina, Y.S.; et al. Clinical immunology. In A Practical Guide for Infectious Disease Specialists; Polikor: Krasnoyarsk, Russia, 2021; 563p. (In Russian) [Google Scholar] [CrossRef]
- Boom, W.H.; Canaday, D.H.; Fulton, S.A.; Gehring, A.J.; Rojas, R.E.; Torres, M. Human immunity to M. tuberculosis: T cell subsets and antigen processing. Tuberculosis 2003, 83, 98–106. [Google Scholar] [CrossRef]
- Kudryavtsev, I.V.; Arsentieva, N.A.; Korobova, Z.R.; Isakov, D.V.; Rubinstein, A.A.; Batsunov, O.K.; Khamitova, I.V.; Kuznetsova, R.N.; Savin, T.V.; Akisheva, T.V.; et al. Heterogenous CD8+ T Cell Maturation and ‘Polarization’ in Acute and Convalescent COVID-19 Patients. Viruses 2022, 14, 1906. [Google Scholar] [CrossRef]
- Dolfi, D.V.; Katsikis, P.D. CD28 and CD27 costimulation of CD8+ T cells: A story of survival. Adv. Exp. Med. Biol. 2007, 590, 149–170. [Google Scholar] [CrossRef]
- Romero, P.; Zippelius, A.; Kurth, I.; Pittet, M.J.; Touvrey, C.; Iancu, E.M.; Corthesy, P.; Devevre, E.; Speiser, D.E.; Rufer, N. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J. Immunol. 2007, 178, 4112–4119. [Google Scholar] [CrossRef]
- Rufer, N.; Zippelius, A.; Batard, P.; Pittet, M.J.; Kurth, I.; Corthesy, P.; Cerottini, J.C.; Leyvraz, S.; Roosnek, E.; Nabholz, M.; et al. Ex vivo characterization of human CD8+ T subsets with distinct replicative history and partial effector functions. Blood 2003, 102, 1779–1787. [Google Scholar] [CrossRef]
- Hoji, A.; Connolly, N.C.; Buchanan, W.G.; Rinaldo, C.R., Jr. CD27 and CD57 expression reveals atypical differentiation of human immunodeficiency virus type 1-specific memory CD8+ T cells. Clin. Vaccine Immunol. 2007, 14, 74–80. [Google Scholar] [CrossRef]
- Strioga, M.; Pasukoniene, V.; Characiejus, D. CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology 2011, 134, 17–32. [Google Scholar] [CrossRef]
- Chattopadhyay, P.K.; Betts, M.R.; Price, D.A.; Gostick, E.; Horton, H.; Roederer, M.; De Rosa, S.C. The cytolytic enzymes granyzme A, granzyme B, and perforin: Expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J. Leukoc. Biol. 2009, 85, 88–97. [Google Scholar] [CrossRef]
- Loyal, L.; Warth, S.; Jürchott, K.; Mölder, F.; Nikolaou, C.; Babel, N.; Nienen, M.; Durlanik, S.; Stark, R.; Kruse, B.; et al. SLAMF7 and IL-6R define distinct cytotoxic versus helper memory CD8+ T cells. Nat. Commun. 2020, 11, 6357. [Google Scholar] [CrossRef]
- Trombke, J.; Loyal, L.; Braun, J.; Pleyer, U.; Thiel, A.; Pohlmann, D. Analysis of peripheral inflammatory T cell subsets and their effector function in patients with Birdshot Retinochoroiditis. Sci. Rep. 2021, 11, 8604. [Google Scholar] [CrossRef]
- Brighenti, S.; Andersson, J. Local immune responses in human tuberculosis: Learning from the site of infection. J. Infect. Dis. 2012, 205 (Suppl. S2), S316–S324. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kaneda, K.; Kasama, T. Immunopathogenesis of delayed-type hypersensitivity. Microsc. Res. Tech. 2001, 53, 241–245. [Google Scholar] [CrossRef]
- Mazzarella, G.; Bianco, A.; Perna, F.; D’Auria, D.; Grella, E.; Moscariello, E.; Sanduzzi, A. T lymphocyte phenotypic profile in lung segments affected by cavitary and non-cavitary tuberculosis. Clin. Exp. Immunol. 2003, 132, 283–288. [Google Scholar] [CrossRef]
- Shepherd, F.R.; McLaren, J.E. T Cell Immunity to Bacterial Pathogens: Mechanisms of Immune Control and Bacterial Evasion. Int. J. Mol. Sci. 2020, 21, 6144. [Google Scholar] [CrossRef]
- Raghuvanshi, S.; Sharma, P.; Singh, S.; Van Kaer, L.; Das, G. Mycobacterium tuberculosis evades host immunity by recruiting mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 21653–21658. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, B.; Li, M.; Deng, Q.; Wu, X.; Le, X.; Wu, C.; Larmonier, N.; Zhang, W.; Zhang, H.; et al. CD4(+)CD25(+)FoxP3(+) regulatory T cells suppress Mycobacterium tuberculosis immunity in patients with active disease. Clin. Immunol. 2007, 123, 50–59. [Google Scholar] [CrossRef]
- Moguche, A.O.; Musvosvi, M.; Penn-Nicholson, A.; Plumlee, C.R.; Mearns, H.; Geldenhuys, H.; Smit, E.; Abrahams, D.; Rozot, V.; Dintwe, O.; et al. Antigen Availability Shapes T Cell Differentiation and Function during Tuberculosis. Cell Host Microbe 2017, 21, 695–706.e5. [Google Scholar] [CrossRef]
- Ocaña-Guzmán, R.; Téllez-Navarrete, N.A.; Ramón-Luing, L.A.; Herrera, I.; De Ita, M.; Carrillo-Alduenda, J.L.; Choreño-Parra, J.A.; Medina-Quero, K.; Zúñiga, J.; Chávez-Galán, L. Leukocytes from Patients with Drug-Sensitive and Multidrug-Resistant Tuberculosis Exhibit Distinctive Profiles of Chemokine Receptor Expression and Migration Capacity. J. Immunol. Res. 2021, 2021, 6654220. [Google Scholar] [CrossRef]
- Chávez-Galán, L.; Illescas-Eugenio, J.; Alvarez-Sekely, M.; Baez-Saldaña, R.; Chávez, R.; Lascurain, R. Tuberculosis patients display a high proportion of CD8+ T cells with a high cytotoxic potential. Microbiol. Immunol. 2019, 63, 316–327. [Google Scholar] [CrossRef]
- An, H.R.; Bai, X.J.; Liang, J.Q.; Wang, T.; Wang, Z.Y.; Xue, Y.; Liu, Y.P.; Wang, L.; Wu, X.Q. The relationship between absolute counts of lymphocyte subsets and clinical features in patients with pulmonary tuberculosis. Clin. Respir. J. 2022, 16, 369–379. [Google Scholar] [CrossRef]
- Qin, S.; Chen, R.; Jiang, Y.; Zhu, H.; Chen, L.; Chen, Y.; Shen, M.; Lin, X. Multifunctional T cell response in active pulmonary tuberculosis patients. Int. Immunopharmacol. 2021, 99, 107898. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ran, R.; Jiang, Z.; Fan, C.; Li, T.; Yin, Z. Changes in T-lymphocyte subsets and risk factors in human immunodeficiency virus-negative patients with active tuberculosis. Infection 2020, 48, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.N.; Henson, S.M. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat. Rev. Immunol. 2011, 11, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, J.; Niu, Y.; Yao, L.; Liu, X.; Chen, H.; Chen, S.; Wu, M.; Yu, X.; Xu, P. The elevated expression of LAG-3 on CD8+T cells correlates with disease severity of pulmonary TB. Microb. Pathog. 2023, 179, 106089. [Google Scholar] [CrossRef]
- Yu, X.; Lin, Y.; Chen, H.; Wu, M.J.; Huang, L.N.; Song, Y.Y.; Gu, B.B.; Ye, Z.J.; Xu, P.; Zhang, J.P.; et al. Distribution and clinical significance of circulating CD8+CD28− regulatory T cells in the peripheral blood of patients with pulmonary tuberculosis. BMC Pulm. Med. 2022, 22, 291. [Google Scholar] [CrossRef]
- St Paul, M.; Ohashi, P.S. The Roles of CD8+ T Cell Subsets in Antitumor Immunity. Trends Cell Biol. 2020, 30, 695–704. [Google Scholar] [CrossRef]
- Doan Ngoc, T.M.; Tilly, G.; Danger, R.; Bonizec, O.; Masset, C.; Guérif, P.; Bruneau, S.; Glemain, A.; Harb, J.; Cadoux, M.; et al. Effector Memory-Expressing CD45RA (TEMRA) CD8+ T Cells from Kidney Transplant Recipients Exhibit Enhanced Purinergic P2X4 Receptor-Dependent Proinflammatory and Migratory Responses. J. Am. Soc. Nephrol. 2022, 33, 2211–2231. [Google Scholar] [CrossRef]
- Andersson, J.; Samarina, A.; Fink, J.; Rahman, S.; Grundström, S. Impaired expression of perforin and granulysin in CD8+ T cells at the site of infection in human chronic pulmonary tuberculosis. Infect. Immun. 2007, 75, 5210–5222. [Google Scholar] [CrossRef]
- Taqueti, V.R.; Grabie, N.; Colvin, R.; Pang, H.; Jarolim, P.; Luster, A.D.; Glimcher, L.H.; Lichtman, A.H. T-bet controls pathogenicity of CTLs in the heart by separable effects on migration and effector activity. J. Immunol. 2006, 177, 5890–5901. [Google Scholar] [CrossRef] [PubMed]
- Hickman, H.D.; Reynoso, G.V.; Ngudiankama, B.F.; Cush, S.S.; Gibbs, J.; Bennink, J.R.; Yewdell, J.W. CXCR3 chemokine receptor enables local CD8(+) T cell migration for the destruction of virus-infected cells. Immunity 2015, 42, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Mittrücker, H.W.; Visekruna, A.; Huber, M. Heterogeneity in the differentiation and function of CD8⁺ T cells. Arch. Immunol. Ther. Exp. 2014, 62, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Guenova, E.; Watanabe, R.; Teague, J.E.; Desimone, J.A.; Jiang, Y.; Dowlatshahi, M.; Schlapbach, C.; Schaekel, K.; Rook, A.H.; Tawa, M.; et al. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clin. Cancer Res. 2013, 19, 3755–3763. [Google Scholar] [CrossRef] [PubMed]
- Ashenafi, S.; Aderaye, G.; Bekele, A.; Zewdie, M.; Aseffa, G.; Hoang, A.T.; Carow, B.; Habtamu, M.; Wijkander, M.; Rottenberg, M.; et al. Progression of clinical tuberculosis is associated with a Th2 immune response signature in combination with elevated levels of SOCS3. Clin. Immunol. 2014, 151, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Morais-Papini, T.F.; Coelho-Dos-Reis, J.G.A.; Wendling, A.P.B.; do Vale Antonelli, L.R.; Wowk, P.F.; Bonato, V.L.D.; Augusto, V.M.; Elói-Santos, S.; Martins-Filho, O.A.; Carneiro, C.M.; et al. Systemic Immunological changes in patients with distinct clinical outcomes during Mycobacterium tuberculosis infection. Immunobiology 2017, 222, 1014–1024. [Google Scholar] [CrossRef]
- Malkova, A.; Kudryavtsev, I.; Starshinova, A.; Kudlay, D.; Zinchenko, Y.; Glushkova, A.; Yablonskiy, P.; Shoenfeld, Y. Post COVID-19 Syndrome in Patients with Asymptomatic/Mild Form. Pathogens 2021, 10, 1408. [Google Scholar] [CrossRef]
- Savchenko, A.A.; Tikhonova, E.; Kudryavtsev, I.; Kudlay, D.; Korsunsky, I.; Beleniuk, V.; Borisov, A. TREC/KREC Levels and T and B Lymphocyte Subpopulations in COVID-19 Patients at Different Stages of the Disease. Viruses 2022, 14, 646. [Google Scholar] [CrossRef]

| Characteristics | Tbc Patients n (%) (n = 32) |
|---|---|
| Men | 22 (68.7) |
| Women | 10 (31.3) |
| Age | 36.5 (±10.6) years |
| Clinical symptoms | 29 (90.6) |
| Fever | 20/32 (62.5) |
| General weakness | 21/32 (65.6) |
| Sweating | 18/32 (56.2) |
| Weight loss | 21/32 (65.6) |
| Respiratory symptoms | |
| Cough | 22/32 (68.7) |
| Shortness of breath | 11/32 (34.3) |
| Chest pain | 5/32 (15.6) |
| X-Ray and CT changes | |
| Infiltrates in the lungs | 15 (46.8) |
| Focus on the lungs | 11 (34.3) |
| Focal infiltrates and focus in the lungs | 6 (18.7) |
| Bacteriologic data | |
| Sputum positive for MBT | 32 (100.0) |
| MDR | 10 (31.2) |
| CD8+ T Cell Subset | Phenotype | TB Group (n = 32) (%, Med Q25; Q75) | Healthy Control (n = 31) (%, Med Q25; Q75) | Significant Differences (p < 0.05) |
|---|---|---|---|---|
| Naïve | CD45RA+CD62L+ | 0.96 (0.48; 3.21) | 1.62 (0.63; 3.49) | 0.417 |
| CM | CD45RA–CD62L+ | 3.56 (1.75; 7.86) | 3.01 (1.65; 5.28) | 0.343 |
| EM: | CD45RA–CD62L– | 36.97 (29.85; 44.67) | 39.87 (30.94; 59.8) | 0.211 |
| EM1 | CD27+CD28+ | 5.63 (3.49; 8.46) | 10.60 (6.57; 12.82) | 0.001 |
| EM2 | CD27+CD28− | 37.59 (27.69; 49.63) | 46.43 (34.60; 62.03) | 0.035 |
| EM3 | CD27–CD28− | 81.96 (76.81; 90.52) | 76.75 (66.55; 83.60) | 0.037 |
| EM4 | CD27–CD28+ | 10.25 (4.98;16.80) | 12.51 (8.40; 20.78) | 0.114 |
| TEMRA: | CD45RA+CD62L– | 59.83 (51.15; 70.09) | 64.42 (53.26; 76.4) | 0.157 |
| pE1 | CD27+CD28+ | 3.53 (2.34; 6.37) | 6.55 (2.87; 10.00) | 0.029 |
| pE2 | CD27+CD28− | 34.19 (27.25; 42.69) | 44.31 (32.08; 52.00) | 0.243 |
| effectors | CD27–CD28− | 74.44 (68.59; 84.89) | 80.08 (65.07; 86.90) | 0.433 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudryavtsev, I.; Zinchenko, Y.; Serebriakova, M.; Akisheva, T.; Rubinstein, A.; Savchenko, A.; Borisov, A.; Belenjuk, V.; Malkova, A.; Yablonskiy, P.; et al. A Key Role of CD8+ T Cells in Controlling of Tuberculosis Infection. Diagnostics 2023, 13, 2961. https://doi.org/10.3390/diagnostics13182961
Kudryavtsev I, Zinchenko Y, Serebriakova M, Akisheva T, Rubinstein A, Savchenko A, Borisov A, Belenjuk V, Malkova A, Yablonskiy P, et al. A Key Role of CD8+ T Cells in Controlling of Tuberculosis Infection. Diagnostics. 2023; 13(18):2961. https://doi.org/10.3390/diagnostics13182961
Chicago/Turabian StyleKudryavtsev, Igor, Yulia Zinchenko, Maria Serebriakova, Tatiana Akisheva, Artem Rubinstein, Andrei Savchenko, Alexandr Borisov, Vasilij Belenjuk, Anna Malkova, Piotr Yablonskiy, and et al. 2023. "A Key Role of CD8+ T Cells in Controlling of Tuberculosis Infection" Diagnostics 13, no. 18: 2961. https://doi.org/10.3390/diagnostics13182961
APA StyleKudryavtsev, I., Zinchenko, Y., Serebriakova, M., Akisheva, T., Rubinstein, A., Savchenko, A., Borisov, A., Belenjuk, V., Malkova, A., Yablonskiy, P., Kudlay, D., & Starshinova, A. (2023). A Key Role of CD8+ T Cells in Controlling of Tuberculosis Infection. Diagnostics, 13(18), 2961. https://doi.org/10.3390/diagnostics13182961









