Patellar Tendon Elasticity and Temperature Following after a 448 Kilohertz Radiofrequency Intervention on Active Healthy Subjects: An Open Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Setting
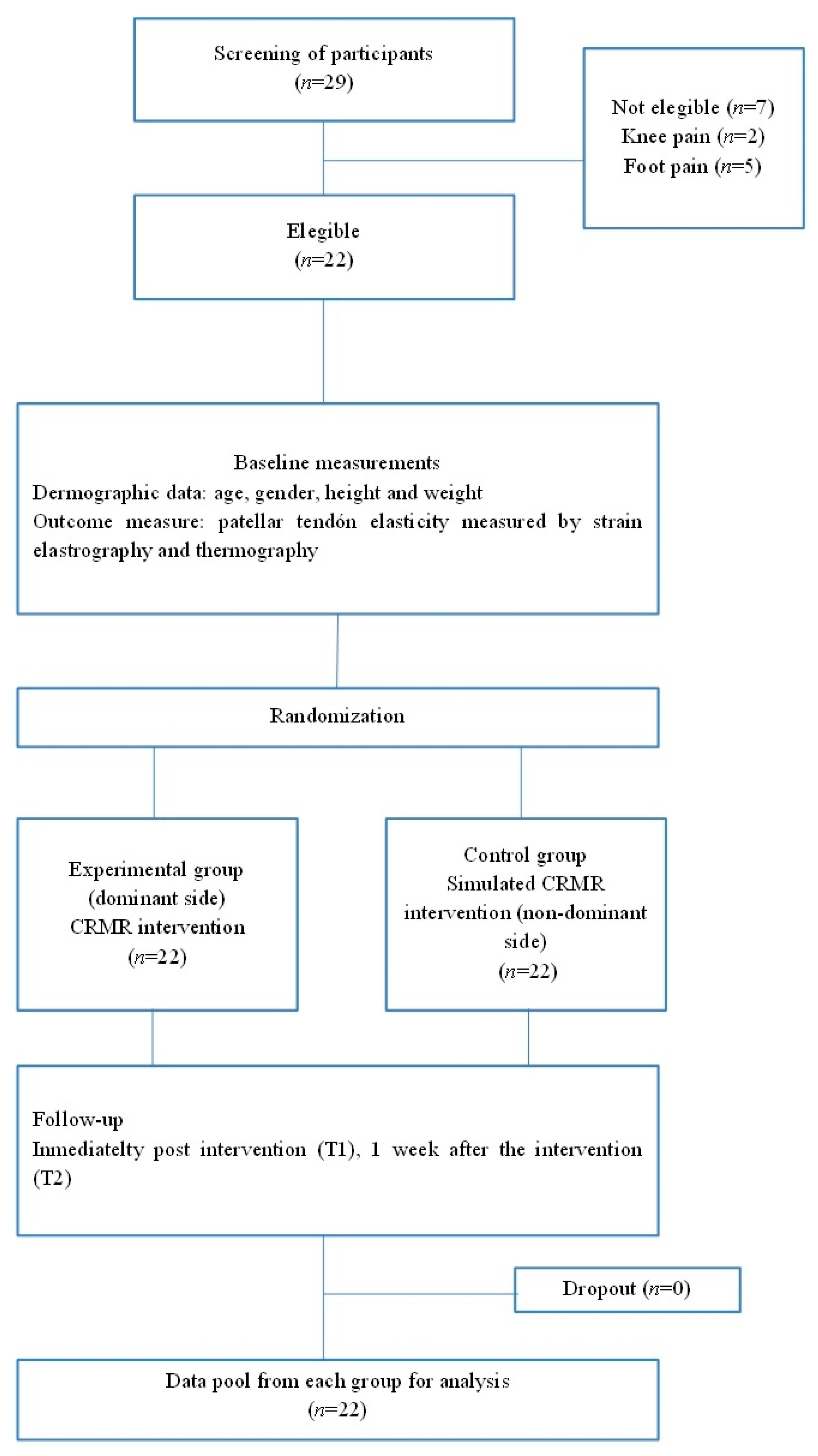
2.3. Participants
2.4. Inclusion Criteria
2.5. Exclusion Criteria
2.5.1. Allocation
2.5.2. Sample Size Calculation
2.6. Intervention Description
2.6.1. Experimental Group
2.6.2. Control Group
2.7. Outcome Measures
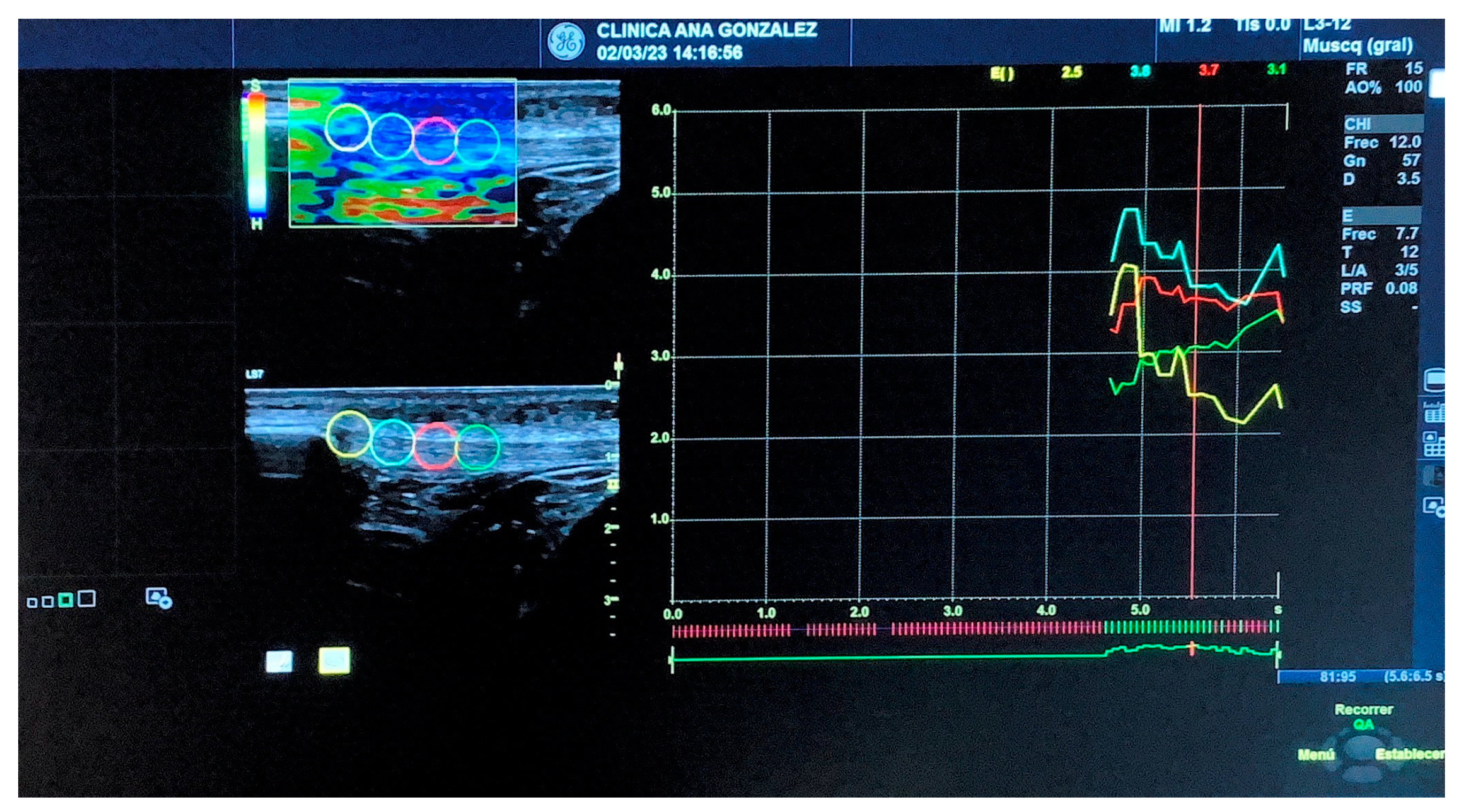
2.7.1. Strain Elastography
2.7.2. Thermography
2.8. Statistical Analysis
3. Results
3.1. Differences in Patellar Tendon Elasticity and Temperature at Baseline after the Intervention and One-Week Follow-up
3.2. Within-Group Differences in Patellar Tendon Elasticity and Temperature
3.3. Association between Patellar Tendon Strain Elastography and Thermography
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sprague, A.; Epsley, S.; Silbernagel, K.G. Distinguishing Quadriceps Tendinopathy and Patellar Tendinopathy: Semantics or Significant? J. Orthop. Sports Phys. Ther. 2019, 49, 627–630. [Google Scholar] [CrossRef]
- Götschi, T.; Hanimann, J.; Schulz, N.; Huser, S.; Held, V.; Frey, W.O.; Snedeker, J.G.; Spörri, J. Patellar Tendon Shear Wave Velocity Is Higher and has Different Regional Patterns in Elite Competitive Alpine Skiers than in Healthy Controls. Front. Bioeng. Biotechnol. 2022, 10, 858610. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Long, Y.; Yu, M.; Guo, J.; Tang, Y.; Li, F.; Li, Q.; Zhang, Y.; Zheng, Z.; Hou, J.; et al. Allogeneic Tendon Transplantation for the Treatment of Pathological Patellar Ligament Defect in Children: Technical Note and 4-Year Follow-Up. Orthop. Surg. 2022, 14, 3431–3440. [Google Scholar] [CrossRef]
- Aicale, R.; Oliviero, A.; Maffulli, N. Management of Achilles and patellar tendinopathy: What we know, what we can do. J. Foot Ankle Res. 2020, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, S.R.; Ramakanth, R.; Rajasekaran, S. Concomitant Patellar Tendon Tear (PTT) with Cruciate and/Collateral ligament injury (Multi-Ligamentous Knee Injury—MLKI) and new pathoanatomical-Ganga PTT classification aids to strategize treatment options. Injury 2023, 54, 712–721. [Google Scholar] [CrossRef]
- Steele, R.; Hayden, S.R.; Ward, N.; Macias, M. Patellar Tendon Rupture Bedside Diagnosis. J. Emerg. Med. 2021, 60, 384–386. [Google Scholar] [CrossRef]
- Dilip, D.; Khaladkar, S.M.; Chanabasanavar, V.; Parripati, S.S.V.K. REAL-TIME strain elastography: Applications in musculoskeletal system. J. Clin. Orthop. Trauma 2022, 26, 101784. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.C.; Richards, P.J.; Maffulli, N.; Ede, D.; Schneider, M.E.; Connell, D.; Morrissey, D.; Malliaras, P. A soft patellar tendon on ultrasound elastography is associated with pain and functional deficit in volleyball players. J. Sci. Med. Sport 2016, 19, 373–378. [Google Scholar] [CrossRef]
- Dirrichs, T.; Quack, V.; Gatz, M.; Tingart, M.; Kuhl, C.K.; Schrading, S. Shear Wave Elastography (SWE) for the Evaluation of Patients with Tendinopathies. Acad. Radiol. 2016, 23, 1204–1213. [Google Scholar] [CrossRef]
- Breda, S.J.; van der Vlist, A.; de Vos, R.J.; Krestin, G.P.; Oei, E.H.G. The association between patellar tendon stiffness measured with shear-wave elastography and patellar tendinopathy—A case-control study. Eur. Radiol. 2020, 30, 5942–5951. [Google Scholar] [CrossRef]
- Zhang, C.; Duan, L.; Liu, Q.; Zhang, W. Application of shear wave elastography and B-mode ultrasound in patellar tendinopathy after extracorporeal shockwave therapy. J. Med. Ultrason. 2020, 47, 469–476. [Google Scholar] [CrossRef]
- Majano, C.; García-Unanue, J.; Hernandez-Martin, A.; Sánchez-Sánchez, J.; Gallardo, L.; Felipe, J.L. Relationship between Repeated Sprint Ability, Countermovement Jump and Thermography in Elite Football Players. Sensors 2023, 23, 631. [Google Scholar] [CrossRef] [PubMed]
- Destrempes, F.; Gesnik, M.; Chayer, B.; Roy-Cardinal, M.H.; Olivié, D.; Giard, J.M.; Sebastiani, G.; Nguyen, B.N.; Cloutier, G.; Tang, A. Quantitative ultrasound, elastography, and machine learning for assessment of steatosis, inflammation, and fibrosis in chronic liver disease. PLoS ONE 2022, 17, e0262291. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, B.; Watson, T. Thermal build-up, decay and retention responses to local therapeutic application of 448 kHz capacitive resistive monopolar radiofrequency: A prospective randomised crossover study in healthy adults. Int. J. Hyperth. 2015, 31, 883–895. [Google Scholar] [CrossRef]
- Fousekis, K.; Chrysanthopoulos, G.; Tsekoura, M.; Mandalidis, D.; Mylonas, K.; Angelopoulos, P.; Koumoundourou, D.; Billis, V.; Tsepis, E. Posterior thigh thermal skin adaptations to radiofrequency treatment at 448 kHz applied with or without Indiba® fascia treatment tools. J. Phys. Ther. Sci. 2020, 32, 292–296. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Puig, J.; Martí, R.; Lladó, X.; Corral-Baqués, M.I.; Sendrós-Tolsau, S. Structural changes in subcutaneous and visceral abdominal fatty tissue induced by local application of 448 kHz capacitive resistive monopolar radiofrequency: A magnetic resonance imaging case study. Lasers Med. Sci. 2022, 37, 3739–3748. [Google Scholar] [CrossRef]
- Beltrame, R.; Ronconi, G.; Ferrara, P.E.; Salgovic, L.; Vercelli, S.; Solaro, C.; Ferriero, G. Capacitive and resistive electric transfer therapy in rehabilitation: A systematic review. Int. J. Rehabil. Res. 2020, 43, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Avendaño-Coy, J.; Aceituno-Gómez, J.; García-Durán, S.; Arroyo-Fernández, R.; Blázquez-Gamallo, R.; García-Madero, V.M.; Escribá-de-la-Fuente, S.M.; Fernández-Pérez, C. Capacitive resistive monopolar radiofrequency at 448 kHz plus exercising versus exercising alone for subacromial pain: A sham-controlled randomized clinical trial. Clin. Rehabil. 2022, 36, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Ledesma, S.; Gonzalez-Muñoz, A. Mid- and Long-Term Results Using 448 kHz Stimulation on the Elasticity of the Supraspinatus Tendon Measured by Quantitative Ultrasound Elastographyin Badminton Professionals: Prospective Randomized Double-Blinded Clinical Trial with Nine Months of Follow-up. J. Clin. Med. 2022, 11, 1664. [Google Scholar]
- Navarro-Ledesma, S.; Gonzalez-Muñoz, A. Short-term effects of 448 kilohertz radiofrequency stimulation on supraspinatus tendon elasticity measured by quantitative ultrasound elastography in professional badminton players: A double-blinded randomized clinical trial. Int. J. Hyperth. 2021, 38, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ 2016, 2, 355. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. J. Clin. Epidemiol. 2010, 63, e1–e37. [Google Scholar] [CrossRef] [PubMed]
- Damsted, C.; Parner, E.T.; Sørensen, H.; Malisoux, L.; Nielsen, R.O. Design of ProjectRun21: A 14-week prospective cohort study of the influence of running experience and running pace on running-related injury in half-marathoners. Inj. Epidemiol. 2017, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.M.; Smith, S.L.; Hendry, G.J. Strain sonoelastography in asymptomatic individuals and individuals with knee osteoarthritis: An evaluation of quadriceps and patellar tendon. Rheumatol. Int. 2022, 42, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Reina-Martin, I.; Navarro-Ledesma, S.; Ortega-Avila, A.B.; Deschamps, K.; Martinez-Franco, A.; Luque-Suarez, A.; Gijon-Nogueron, G. Morphological Characteristics of Passive and Active Structures of the Foot Across Populations with Different Levels of Physical Activity. J. Sport Rehabil. 2021, 30, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Villafañe, J.H.; De-las-Peñas, C.F.; Silva, G.B.; Negrini, S. Contralateral Sensory and Motor Effects of Unilateral Kaltenborn Mobilization in Patients with Thumb Carpometacarpal Osteoarthritis: A Secondary Analysis. J. Phys. Ther. Sci. 2014, 26, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Baar, K. Stress Relaxation and Targeted Nutrition to Treat Patellar Tendinopathy. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhang, S.; Yang, C.; Wang, Y.; Shi, B.; Zheng, Q.; Zeng, N.; Huang, H. Mechanotransduction in skin wound healing and scar formation: Potential therapeutic targets for controlling hypertrophic scarring. Front. Immunol. 2022, 13, 1028410. [Google Scholar] [CrossRef] [PubMed]
- Kuehlmann, B.; Bonham, C.A.; Zucal, I.; Prantl, L.; Gurtner, G.C. Mechanotransduction in Wound Healing and Fibrosis. J. Clin. Med. 2020, 9, 1423. [Google Scholar] [CrossRef] [PubMed]
- Benage, L.G.; Sweeney, J.D.; Giers, M.B.; Balasubramanian, R. Dynamic Load Model Systems of Tendon Inflammation and Mechanobiology. Front. Bioeng. Biotechnol. 2022, 10, 896336. [Google Scholar] [CrossRef]
- Logerstedt, D.S.; Ebert, J.R.; MacLeod, T.D.; Heiderscheit, B.C.; Gabbett, T.J.; Eckenrode, B.J. Effects of and Response to Mechanical Loading on the Knee. Sports Med. 2022, 52, 201–235. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, C.; Netterström-Wedin, F. Does mechanical loading restore ligament biomechanics after injury? A systematic review of studies using animal models. BMC Musculoskelet. Disord. 2023, 24, 511. [Google Scholar] [CrossRef] [PubMed]
- Zeynep Bekin Sarikaya, P.; Sarikaya, B.; Bozkurt, C.; Dere, O.; Balevi Batur, E.; Duşak, A. Evaluation of the anterior shoulder instability using ultrasound shear wave elastography. Jt. Dis. Relat. Surg. 2023, 34, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Chen, W.S.; Wang, T.G. Elasticity of the Coracohumeral Ligament in Patients with Adhesive Capsulitis of the Shoulder. Radiology 2016, 278, 458–464. [Google Scholar] [CrossRef]
- Aguilar-Nuñez, D.; Cervera-Garvi, P.; Aguilar-Garcia, M.; Cuevas-Cervera, M.; Gonzalez-Muñoz, A.; Navarro-Ledesma, S. Ultrasound Strain Elastography Reliability in the Assessment of the Plantar Fascia and Its Relationship with the Plantar Thickness in Healthy Adults: An Intra and Interobserver Reliability Study in Novice Evaluators. Biomedicines 2023, 11, 2040. [Google Scholar] [CrossRef] [PubMed]

| Intervention Group (CRMR) (n = 22) | Control Group (Placebo CRMR) (n = 22) | |
|---|---|---|
| Age (years), mean (SD) | 33.6 (10.6) | 33.6 (10.6) |
| Height (cm), mean (SD) | 175 (8.51) | 175 (8.51) |
| Weight (kg), mean (SD) | 73 (10.4) | 73 (10.4) |
| Patellar tendon elasticity point 1, mean (SD) | 1.77 (1.09) | 1.84 (0.695) |
| Patellar tendon elasticity point 2, mean (SD) | 2.16 (1.14) | 2.08 (0.695) |
| Patellar tendon elasticity point 3, mean (SD) | 2.55 (1.10) | 2.39 (0.907) |
| Patellar tendon elasticity point 4, mean (SD) | 2.55 (0.995) | 2.60 (1.23) |
| Patellar tendon thermography (SD) | 32.1 (1.47) | 32.2 (1.70) |
| T0 (Baseline) | T1 (Immediately after the CRMR Intervention) | T1–T2 (Changes from Immediately after the CRMR Intervention until T2) | T2 (One-Week Follow-Up from T0) | |
|---|---|---|---|---|
| Patellar tendon elasticity point 1 (mean) | 0.07273 p = 1.000 | 0.16818 p = 0.982 | 0.01818 p = 1.000 | −0.18636 p = 0.991 |
| Patellar tendon elasticity point 2 (mean) | −0.0818 p = 1.000 | 0.2227 p = 0.954 | −0.0364 p = 1.000 | 0.0909 p = 1.000 |
| Patellar tendon elasticity point 3 (mean) | −0.1636 p = 0.994 | 0.0727 p = 1.000 | −0.4500 p = 0.524 | 0.0364 p = 1.000 |
| Patellar tendon elasticity point 4 (mean) | 0.0500 p = 1.000 | 0.1909 p = 0.990 | −0.4636 p = 0.622 | 0.0727 p = 1.000 |
| Thermography (mean) | 0.0227 p = 1.000 | 0.2273 p = 0.996 | 3.1591 p < 0.001 | 0.4091 p = 0.970 |
| Intervention Group (CRMR) | Control Group (Placebo CRMR) | |||||||
|---|---|---|---|---|---|---|---|---|
| T0 (Baseline) | T1 (Immediately after the CRMR Intervention) | T1–T2 (Changes from Immediately after the CRMR Intervention until T2) | T2 (One-Week Follow-Up) | T0 (Baseline) | T1 (Immediately after the CRMR Intervention) | T1–T2 (Changes from Immediately after the CRMR Intervention until T2) | T2 (One-Week Follow-Up) | |
| Patellar tendon elasticity point 1 (mean) | - | 0.15000 p = 0.989 | −0.33182 p = 0.838 | −0.18182 p = 0.973 | - | 0.09545 p = 0.999 | −0.35455 p = 0.797 | −0.25909 p = 0.886 |
| Patellar tendon elasticity point 2 (mean) | - | 0.2591 p = 0.919 | −0.0818 p = 0.999 | 0.1773 p = 0.966 | - | 0.3045 p = 0.852 | −0.1318 p = 0.993 | 0.1727 p = 0.970 |
| Patellar tendon elasticity point 3 (mean) | - | 0.5227 p = 0.331 | −0.1364 p = 0.989 | 0.3864 p = 0.521 | - | 0.2364 p = 0.937 | −0.0364 p = 1.000 | 0.2000 p = 0.946 |
| Patellar tendon elasticity point 4 (mean) | - | 0.6545 p = 0.166 | −0.2091 p = 0.923 | 0.4455 p = 0.583 | - | 0.1409 p = 0.995 | −0.1182 p = 0.994 | 0.0227 p = 1.000 |
| Thermography (mean) | - | −2.9318 p < 0.001 | 3.2727 p < 0.001 | 0.3409 p = 0.851 | - | 0.2045 p = 0.995 | 0.1818 p = 0.998 | 0.3864 p = 0.772 |
| Intervention Group (CRMR) | Control Group (Placebo CRMR) | |||||
|---|---|---|---|---|---|---|
| T0 (Baseline) | T1 (Immediately after the CRMR Intervention) | T2 (One-Week Follow-Up) | T0 (Baseline) | T1 (Immediately after the CRMR Intervention) | T2 (One-Week Follow-Up) | |
| Patellar tendon elasticity point 1 | −0.242 p = 0.278 | 0.434 p = 0.044 | 0.031 p = 0.892 | −0.047 p = 0.835 | −0.346 p = 0.114 | 0.151 p = 0.503 |
| Patellar tendon elasticity point 2 | −0.352 p = 0.108 | 0.366 p = 0.094 | 0.005 p = 0.983 | −0.009 p = 0.968 | −0.405 p = 0.062 | −0.189 p = 0.399 |
| Patellar tendon elasticity point 3 | −0.357 p = 0.103 | 0.053 p = 0.816 | −0.247 p = 0.267 | 0.134 p = 0.553 | −0.245 p = 0.272 | −0.313 p = 0.156 |
| Patellar tendon elasticity point 4 | −0.329 p = 0.135 | −0.081 p = 0.721 | −0.365 p = 0.095 | 0.101 p = 0.654 | −0.285 p = 0.198 | −0.276 p = 0.213 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas-Cervera, M.; Aguilar-Nuñez, D.; Aguilar-García, M.; García-Ríos, M.C.; González-Muñoz, A.; Navarro-Ledesma, S. Patellar Tendon Elasticity and Temperature Following after a 448 Kilohertz Radiofrequency Intervention on Active Healthy Subjects: An Open Controlled Clinical Trial. Diagnostics 2023, 13, 2976. https://doi.org/10.3390/diagnostics13182976
Cuevas-Cervera M, Aguilar-Nuñez D, Aguilar-García M, García-Ríos MC, González-Muñoz A, Navarro-Ledesma S. Patellar Tendon Elasticity and Temperature Following after a 448 Kilohertz Radiofrequency Intervention on Active Healthy Subjects: An Open Controlled Clinical Trial. Diagnostics. 2023; 13(18):2976. https://doi.org/10.3390/diagnostics13182976
Chicago/Turabian StyleCuevas-Cervera, Maria, Daniel Aguilar-Nuñez, María Aguilar-García, María Carmen García-Ríos, Ana González-Muñoz, and Santiago Navarro-Ledesma. 2023. "Patellar Tendon Elasticity and Temperature Following after a 448 Kilohertz Radiofrequency Intervention on Active Healthy Subjects: An Open Controlled Clinical Trial" Diagnostics 13, no. 18: 2976. https://doi.org/10.3390/diagnostics13182976
APA StyleCuevas-Cervera, M., Aguilar-Nuñez, D., Aguilar-García, M., García-Ríos, M. C., González-Muñoz, A., & Navarro-Ledesma, S. (2023). Patellar Tendon Elasticity and Temperature Following after a 448 Kilohertz Radiofrequency Intervention on Active Healthy Subjects: An Open Controlled Clinical Trial. Diagnostics, 13(18), 2976. https://doi.org/10.3390/diagnostics13182976







