Molecular Mechanisms of Resistance to Direct-Acting Antiviral (DAA) Drugs for the Treatment of Hepatitis C Virus Infections
Abstract
1. Introduction
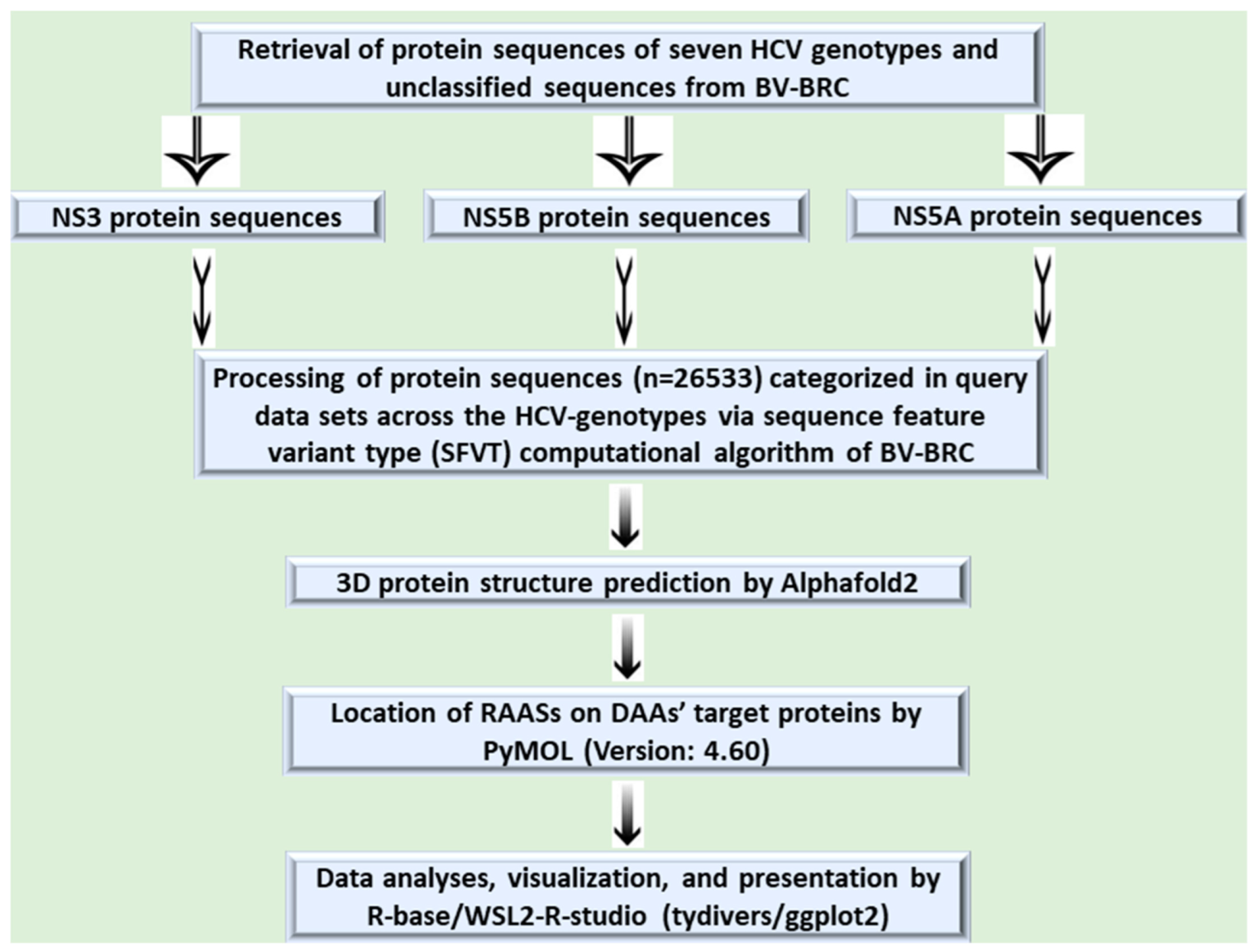
2. Materials and Methods
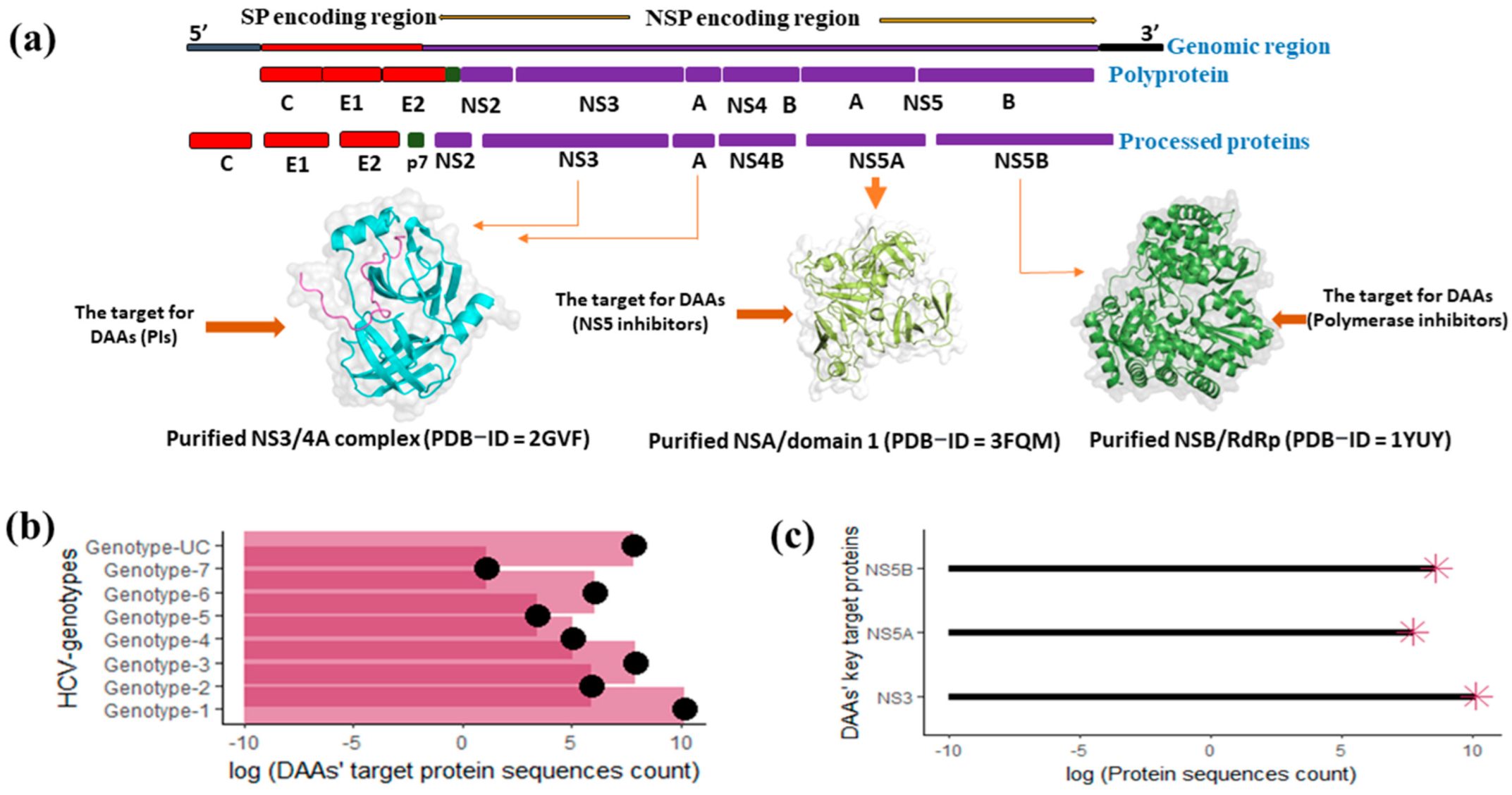
2.1. Retrieval of Target Nonstructural Protein Sequences
2.2. Operational Definition and Query Dataset Generation
2.3. Evaluation of RAASs in DAAs’ Target Proteins and Their Phenotypic (Resistance) Effects
3. Results
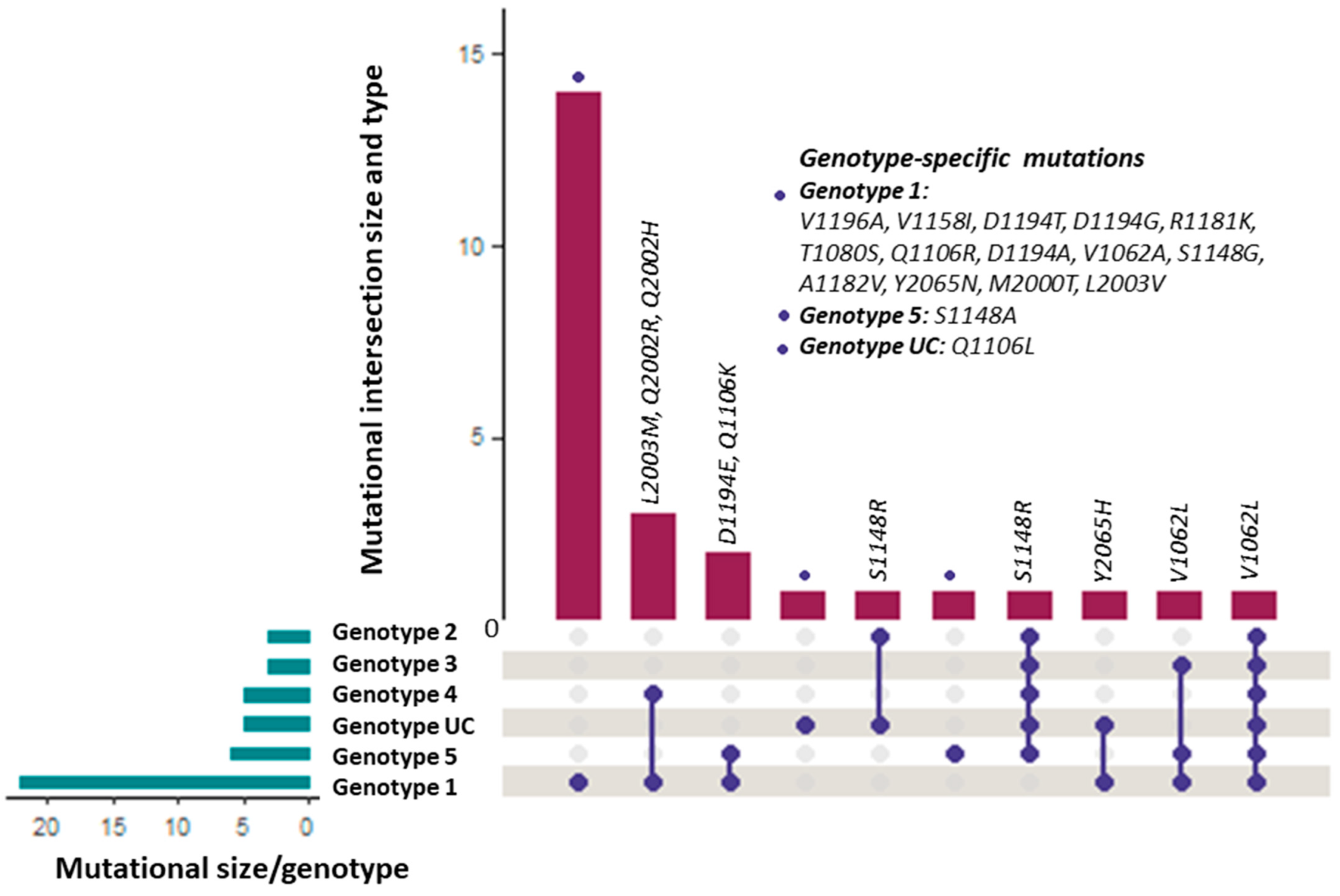
3.1. Distribution of the RAASs among HCV Genotypes
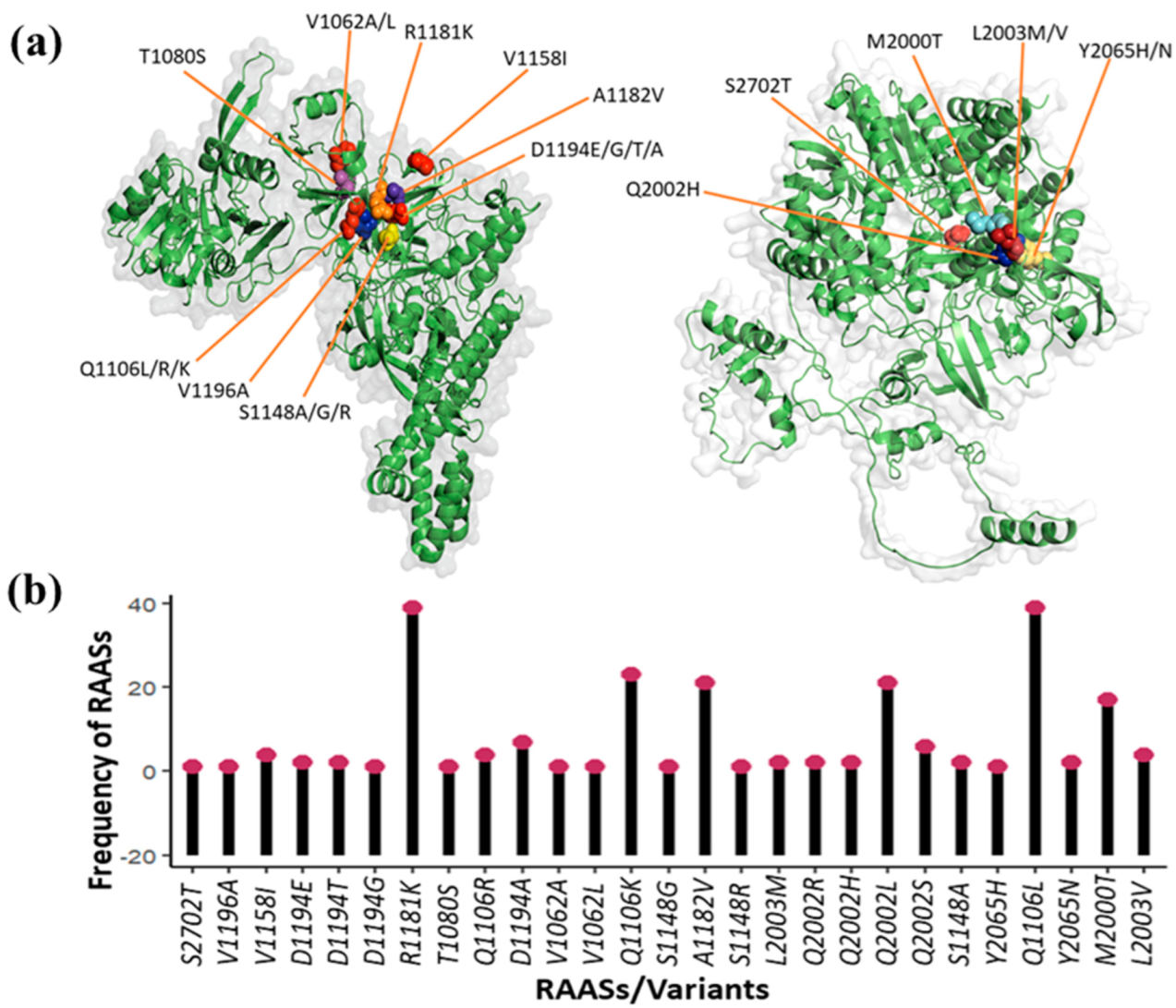
3.2. Number, Types, and Frequency of DAA Resistance-Associated Amino Acid Substitutions
3.3. DAA Resistance-Associated Substitutions and Their Phenotypic Effects (Enhanced Resistance)
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiyasu, P.K.; Caldwell, S.H. Diagnosis and treatment of the major hepatotropic viruses. Am. J. Med. Sci. 1993, 306, 248–261. [Google Scholar] [CrossRef]
- Craig, A.J.; Von Felden, J.; Garcia-Lezana, T.; Sarcognato, S.; Villanueva, A. Tumour evolution in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Cougot, D.; Neuveut, C.; Buendia, M.A. HBV induced carcinogenesis. J. Clin. Virol. 2005, 34, S75–S78. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Petruzziello, A.; Marigliano, S.; Loquercio, G.; Cozzolino, A.; Cacciapuoti, C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J. Gastroenterol. 2016, 22, 7824. [Google Scholar] [CrossRef]
- Ali, A.; Zaman, B.; Shoaib, M.A.; Dhanani, R.; Khan, R. CAUSES OF PREVALENCE OF HEPATITIS–C IN VILLAGE MALKANI SHARIF, DISTRICT BADIN, SINDH, PAKISTAN. FUUAST J. Biol. 2013, 3, 165–169. [Google Scholar]
- Gower, E.; Estes, C.; Blach, S.; Razavi-Shearer, K.; Razavi, H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 2014, 61, S45–S57. [Google Scholar] [CrossRef]
- Goto, K.; Roca Suarez, A.A.; Wrensch, F.; Baumert, T.F.; Lupberger, J. Hepatitis C Virus and Hepatocellular Carcinoma: When the Host Loses Its Grip. Int. J. Mol. Sci. 2020, 21, 3057. [Google Scholar] [CrossRef]
- Bailey, J.R.; Barnes, E.; Cox, A.L. Approaches, Progress, and Challenges to Hepatitis C Vaccine Development. Gastroenterology 2019, 156, 418–430. [Google Scholar] [CrossRef]
- Domingo, E.; Perales, C. Viral quasispecies. PLoS Genet. 2019, 15, e1008271. [Google Scholar] [CrossRef]
- Murphy, D.G.; Sablon, E.; Chamberland, J.; Fournier, E.; Dandavino, R.; Tremblay, C.L. Hepatitis C virus genotype 7, a new genotype originating from central Africa. J. Clin. Microbiol. 2015, 53, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.P.; Humphreys, I.; Flaxman, A.; Brown, A.; Cooke, G.S.; Pybus, O.G.; Barnes, E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2015, 61, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Crosignani, A.; Maisonneuve, P.; Rossi, S.; Silini, E.; Mondelli, M.U. Hepatitis C virus genotype 1b as a major risk factor associated with hepatocellular carcinoma in patients with cirrhosis: A seventeen-year prospective cohort study. Hepatology 2007, 46, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Vescovo, T.; Refolo, G.; Vitagliano, G.; Fimia, G.M.; Piacentini, M. Molecular mechanisms of hepatitis C virus–induced hepatocellular carcinoma. Clin. Microbiol. Infect. 2016, 22, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Bukh, J. The history of hepatitis C virus (HCV): Basic research reveals unique features in phylogeny, evolution and the viral life cycle with new perspectives for epidemic control. J. Hepatol. 2016, 65, S2–S21. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.; Lauer, G.; Isba, R.; Walker, B.; Klenerman, P. Cellular immune responses against hepatitis C virus: The evidence base 2002. Clin. Exp. Immunol. 2002, 128, 195–203. [Google Scholar] [CrossRef]
- Manns, M.P.; McHutchison, J.G.; Gordon, S.C.; Rustgi, V.K.; Shiffman, M.; Reindollar, R.; Goodman, Z.D.; Koury, K.; Ling, M.-H.; Albrecht, J.K. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet 2001, 358, 958–965. [Google Scholar] [CrossRef]
- Strader, D.B.; Wright, T.; Thomas, D.L.; Seeff, L.B. Diagnosis, management, and treatment of hepatitis C. Hepatology 2004, 39, 1147–1171. [Google Scholar] [CrossRef]
- Lebray, P.; Nalpas, B.; Vallet-Pichard, A.; Broissand, C.; Sobesky, R.; Serpaggi, J.; Fontaine, H.; Pol, S. The impact of haematopoietic growth factors on the management and efficacy of antiviral treatment in patients with hepatitis C virus. Antivir. Ther. 2005, 10, 769–776. [Google Scholar] [CrossRef]
- Schaefer, M.; Schmidt, F.; Folwaczny, C.; Lorenz, R.; Martin, G.; Schindlbeck, N.; Heldwein, W.; Soyka, M.; Grunze, H.; Koenig, A. Adherence and mental side effects during hepatitis C treatment with interferon alfa and ribavirin in psychiatric risk groups. Hepatology 2003, 37, 443–451. [Google Scholar] [CrossRef]
- Toniutto, P.; Fabris, C.; Fumo, E.; Apollonio, L.; Caldato, M.; Avellini, C.; Minisini, R.; Pirisi, M. Pegylated versus standard interferon-α in antiviral regimens for post-transplant recurrent hepatitis C: Comparison of tolerability and efficacy. J. Gastroenterol. Hepatol. 2005, 20, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Marciniewicz, E.; Podgórski, P.; Pawłowski, T.; Małyszczak, K.; Fleischer-Stępniewska, K.; Knysz, B.; Waliszewska-Prosół, M.; Żelwetro, A.; Rymer, W.; Inglot, M.; et al. Evaluation of brain volume alterations in HCV-infected patients after interferon-free therapy: A pilot study. J. Neurol. Sci. 2019, 399, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.R.; Peruhype-Magalhães, V.; Coelho-dos-Reis, J.G.A.; Chaves, L.P.V.; de Lima, T.A.; Pimentel, J.P.D.; de Paula, L.; de Almeida, C.M.; Tarragô, A.M.; Tateno, A. Dual role of IL-12 in the therapeutic efficacy or failure during combined PEG-Interferon-α2A and ribavirin therapy in patients with chronic hepatitis C. Immunol. Lett. 2013, 154, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hadziyannis, S.J.; Sette, H., Jr.; Morgan, T.R.; Balan, V.; Diago, M.; Marcellin, P.; Ramadori, G.; Bodenheimer Jr, H.; Bernstein, D.; Rizzetto, M. Peginterferon-α2a and ribavirin combination therapy in chronic hepatitis C: A randomized study of treatment duration and ribavirin dose. Ann. Intern. Med. 2004, 140, 346–355. [Google Scholar] [CrossRef]
- Asselah, T.; Boyer, N.; Saadoun, D.; Martinot-Peignoux, M.; Marcellin, P. Direct-acting antivirals for the treatment of hepatitis C virus infection: Optimizing current IFN-free treatment and future perspectives. Liver Int. 2016, 36, 47–57. [Google Scholar] [CrossRef]
- Kish, T.; Aziz, A.; Sorio, M. Hepatitis C in a New Era: A Review of Current Therapies. Pharm. Ther. 2017, 42, 316–329. [Google Scholar]
- Courcambeck, J.; Bouzidi, M.; Perbost, R.; Jouirou, B.; Amrani, N.; Cacoub, P.; Pèpe, G.; Sabatier, J.-M.; Halfon, P. Resistance of hepatitis C virus to NS3–4A protease inhibitors: Mechanisms of drug resistance induced by R155Q, A156T, D168A and D168V mutations. Antivir. Ther. 2006, 11, 847–856. [Google Scholar] [CrossRef]
- Lok, A.S.-F. HCV NS5A inhibitors in development. Clin. Liver Dis. 2013, 17, 111–121. [Google Scholar]
- Herbst, D.A.; Reddy, K.R. NS5A inhibitor, daclatasvir, for the treatment of chronic hepatitis C virus infection. Expert. Opin. Investig. Drugs 2013, 22, 1337–1346. [Google Scholar] [CrossRef]
- Ng, T.I.; Tripathi, R.; Reisch, T.; Lu, L.; Middleton, T.; Hopkins, T.A.; Pithawalla, R.; Irvin, M.; Dekhtyar, T.; Krishnan, P. In vitro antiviral activity and resistance profile of the next-generation hepatitis C virus NS3/4A protease inhibitor glecaprevir. Antimicrob. Agents Chemother. 2018, 62, 10-1128. [Google Scholar] [CrossRef]
- Pawlotsky, J.-M. Hepatitis C virus resistance to direct-acting antiviral drugs in interferon-free regimens. Gastroenterology 2016, 151, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Harrington, P.R.; Komatsu, T.E.; Deming, D.J.; Donaldson, E.F.; O’Rear, J.J.; Naeger, L.K. Impact of hepatitis C virus polymorphisms on direct-acting antiviral treatment efficacy: Regulatory analyses and perspectives. Hepatology 2018, 67, 2430–2448. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, C. The importance of resistance to direct antiviral drugs in HCV infection in clinical practice. J. Hepatol. 2016, 64, 486–504. [Google Scholar] [CrossRef] [PubMed]
- Shahid, I.; Ibrahim, M.M.; Nawaz, M.U.; Imam, M.T.; AlMalki, W.H. Resistance-Associated Substitutions/Variants Correlate to Therapeutic Outcomes of Novel Direct-Acting Antivirals in Different HCV Genotype Treated Individuals; IntechOpen: London, UK, 2018. [Google Scholar]
- Bull, R.A.; Luciani, F.; McElroy, K.; Gaudieri, S.; Pham, S.T.; Chopra, A.; Cameron, B.; Maher, L.; Dore, G.J.; White, P.A. Sequential bottlenecks drive viral evolution in early acute hepatitis C virus infection. PLoS Pathog. 2011, 7, e1002243. [Google Scholar] [CrossRef]
- Lu, J.; Feng, Y.; Chen, L.; Zeng, Z.; Liu, X.; Cai, W.; Wang, H.; Guo, X.; Zhou, H.; Tao, W. Subtype-specific prevalence of hepatitis C virus NS5A resistance associated substitutions in Mainland China. Front. Microbiol. 2019, 10, 535. [Google Scholar] [CrossRef]
- Gritsenko, D.; Hughes, G. Ledipasvir/Sofosbuvir (harvoni): Improving options for hepatitis C virus infection. Pharm. Ther. 2015, 40, 256. [Google Scholar]
- Geddawy, A.; Ibrahim, Y.F.; Elbahie, N.M.; Ibrahim, M.A. Direct Acting Anti-hepatitis C Virus Drugs: Clinical Pharmacology and Future Direction. J. Transl. Int. Med. 2017, 5, 8–17. [Google Scholar] [CrossRef]
- Ghany, M.G.; Nelson, D.R.; Strader, D.B.; Thomas, D.L.; Seeff, L.B. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011, 54, 1433. [Google Scholar] [CrossRef]
- Martínez, A.P.; Culasso, A.C.; Pérez, P.S.; Romano, V.; Campos, R.H.; Ridruejo, E.; García, G.; Di Lello, F.A. Polymorphisms associated with resistance to protease inhibitors in naïve patients infected with hepatitis C virus genotype 1 in Argentina: Low prevalence of Q80K. Virus Res. 2017, 240, 140–146. [Google Scholar] [CrossRef]
- Sarrazin, C.; Dvory-Sobol, H.; Svarovskaia, E.S.; Doehle, B.P.; Pang, P.S.; Chuang, S.-M.; Ma, J.; Ding, X.; Afdhal, N.H.; Kowdley, K.V. Prevalence of resistance-associated substitutions in HCV NS5A, NS5B, or NS3 and outcomes of treatment with ledipasvir and sofosbuvir. Gastroenterology 2016, 151, 501–512.e501. [Google Scholar] [CrossRef]
- Paolucci, S.; Fiorina, L.; Mariani, B.; Gulminetti, R.; Novati, S.; Barbarini, G.; Bruno, R.; Baldanti, F. Naturally occurring resistance mutations to inhibitors of HCV NS5A region and NS5B polymerase in DAA treatment-naive patients. Virol. J. 2013, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.J.; Ghany, M.G. Current and future therapies for hepatitis C virus infection. N. Engl. J. Med. 2013, 368, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Nejabat, N.; Hosseini, S.Y.; Sarvari, J.; Gorzin, A.A.; Fattahi, M.R.; Rasoolian, M. The Investigation of Drug Resistance Substitutions in NS3 Protease Sequence of Hepatitis C Virus from Non-Responder Patients. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 2311. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zeuzem, S.; Mizokami, M.; Pianko, S.; Mangia, A.; Han, K.-H.; Martin, R.; Svarovskaia, E.; Dvory-Sobol, H.; Doehle, B.; Hedskog, C. NS5A resistance-associated substitutions in patients with genotype 1 hepatitis C virus: Prevalence and effect on treatment outcome. J. Hepatol. 2017, 66, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, A.; Sorbo, M.C.; Aragri, M.; Lenci, I.; Teti, E.; Polilli, E.; Di Maio, V.C.; Gianserra, L.; Biliotti, E.; Masetti, C. Prevalence of single and multiple natural NS3, NS5A and NS5B resistance-associated substitutions in hepatitis C virus genotypes 1–4 in Italy. Sci. Rep. 2018, 8, 8988. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Omata, M.; Lim, Y.-S.; Xie, Q.; Hou, J.L.; Jia, J.; Hedskog, C.; Martin, R.; Doehle, B.; Yang, J. HCV phylogenetic signature and prevalence of pretreatment NS5A and NS5B NI-Resistance associated substitutions in HCV-Infected patients in Mainland China. Antivir. Res. 2018, 158, 178–184. [Google Scholar] [CrossRef]
- Lenz, O.; Verbinnen, T.; Lin, T.I.; Vijgen, L.; Cummings, M.D.; Lindberg, J.; Berke, J.M.; Dehertogh, P.; Fransen, E.; Scholliers, A.; et al. In vitro resistance profile of the hepatitis C virus NS3/4A protease inhibitor TMC435. Antimicrob. Agents Chemother. 2010, 54, 1878–1887. [Google Scholar] [CrossRef]
- Jiang, M.; Mani, N.; Lin, C.; Ardzinski, A.; Nelson, M.; Reagan, D.; Bartels, D.; Zhou, Y.; Nicolas, O.; Rao, B.G.; et al. In vitro phenotypic characterization of hepatitis C virus NS3 protease variants observed in clinical studies of telaprevir. Antimicrob. Agents Chemother. 2013, 57, 6236–6245. [Google Scholar] [CrossRef]
- Lin, C.; Lin, K.; Luong, Y.P.; Rao, B.G.; Wei, Y.Y.; Brennan, D.L.; Fulghum, J.R.; Hsiao, H.M.; Ma, S.; Maxwell, J.P.; et al. In vitro resistance studies of hepatitis C virus serine protease inhibitors, VX-950 and BILN 2061: Structural analysis indicates different resistance mechanisms. J. Biol. Chem. 2004, 279, 17508–17514. [Google Scholar] [CrossRef]
- Kieffer, T.L.; De Meyer, S.; Bartels, D.J.; Sullivan, J.C.; Zhang, E.Z.; Tigges, A.; Dierynck, I.; Spanks, J.; Dorrian, J.; Jiang, M.; et al. Hepatitis C viral evolution in genotype 1 treatment-naïve and treatment-experienced patients receiving telaprevir-based therapy in clinical trials. PLoS ONE 2012, 7, e34372. [Google Scholar] [CrossRef]
- Walker, A.; Filke, S.; Lübke, N.; Obermeier, M.; Kaiser, R.; Häussinger, D.; Timm, J.; Bock, H.H. Detection of a genetic footprint of the sofosbuvir resistance-associated substitution S282T after HCV treatment failure. Virol. J. 2017, 14, 106. [Google Scholar] [CrossRef]
- Group, A.A.H.; Vallet, S.; Viron, F.; Henquell, C.; Le Guillou-Guillemette, H.; Lagathu, G.; Abravanel, F.; Trimoulet, P.; Soussan, P.; Schvoerer, E. NS3 protease polymorphism and natural resistance to protease inhibitors in French patients infected with HCV genotypes 1–5. Antivir. Ther. 2011, 16, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Morsica, G.; Andolina, A.; Merli, M.; Messina, E.; Hasson, H.; Lazzarin, A.; Uberti-Foppa, C.; Bagaglio, S. NS3 protease resistance-associated substitutions in liver tissue and plasma samples from patients infected by hepatitis C virus genotype 1A or 1B. Arch. Virol. 2017, 162, 2271–2277. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, B.F.; Campos, G.R.F.; Rodrigues, J.P.V.; Marques, N.N.; Molina, B.F.; Bittar, C.; Souza, F.F.; Martinelli, A.L.C.; Rahal, P.; Pereira, L.R.L. Baseline resistance associated substitutions in HCV genotype 1 infected cohort treated with Simeprevir, Daclatasvir and Sofosbuvir in Brazil. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.D.; Delvaux, N.; Brandão-Mello, C.E.; Nunes, E.P.; de Sousa, P.S.F.; de Souza Rodrigues, L.; Lampe, E.; do Amaral Mello, F.C. Prevalence of baseline NS3 resistance-associated substitutions (RASs) on treatment with protease inhibitors in patients infected with HCV genotype 1. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 700–706. [Google Scholar] [CrossRef]
- Dietz, J.; Müllhaupt, B.; Buggisch, P.; Graf, C.; Peiffer, K.H.; Matschenz, K.; Schattenberg, J.M.; Antoni, C.; Mauss, S.; Niederau, C.; et al. Long-term persistence of HCV resistance-associated substitutions after DAA treatment failure. J. Hepatol. 2023, 78, 57–66. [Google Scholar] [CrossRef]
- Itakura, J.; Kurosaki, M.; Kakizaki, S.; Amano, K.; Nakayama, N.; Inoue, J.; Endo, T.; Marusawa, H.; Hasebe, C.; Joko, K.; et al. Features of resistance-associated substitutions after failure of multiple direct-acting antiviral regimens for hepatitis C. JHEP Rep. 2020, 2, 100138. [Google Scholar] [CrossRef]
- Gao, M.; Nettles, R.E.; Belema, M.; Snyder, L.B.; Nguyen, V.N.; Fridell, R.A.; Serrano-Wu, M.H.; Langley, D.R.; Sun, J.H.; O’Boyle, D.R., 2nd; et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature 2010, 465, 96–100. [Google Scholar] [CrossRef]
- Fridell, R.A.; Qiu, D.; Wang, C.; Valera, L.; Gao, M. Resistance analysis of the hepatitis C virus NS5A inhibitor BMS-790052 in an in vitro replicon system. Antimicrob. Agents Chemother. 2010, 54, 3641–3650. [Google Scholar] [CrossRef]
- Meanwell, N.A.; Belema, M. The discovery and development of daclatasvir: An inhibitor of the hepatitis C virus NS5A replication complex. In HCV: The Journey from Discovery to a Cure: Volume II; Springer: Cham, Switzerland, 2019; pp. 27–55. [Google Scholar]
- Pol, S.; Corouge, M.; Vallet-Pichard, A. Daclatasvir–sofosbuvir combination therapy with or without ribavirin for hepatitis C virus infection: From the clinical trials to real life. Hepatic Med. Evid. Res. 2016, 8, 21–26. [Google Scholar] [CrossRef]
- Stedman, C. Sofosbuvir, a NS5B polymerase inhibitor in the treatment of hepatitis C: A review of its clinical potential. Ther. Adv. Gastroenterol. 2014, 7, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Flint, M.; Mullen, S.; Deatly, A.M.; Chen, W.; Miller, L.Z.; Ralston, R.; Broom, C.; Emini, E.A.; Howe, A.Y. Selection and characterization of hepatitis C virus replicons dually resistant to the polymerase and protease inhibitors HCV-796 and boceprevir (SCH 503034). Antimicrob. Agents Chemother. 2009, 53, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Soumana, D.I.; Ali, A.; Schiffer, C.A. Structural analysis of asunaprevir resistance in HCV NS3/4A protease. ACS Chem. Biol. 2014, 9, 2485–2490. [Google Scholar] [CrossRef] [PubMed]
- McPhee, F.; Friborg, J.; Levine, S.; Chen, C.; Falk, P.; Yu, F.; Hernandez, D.; Lee, M.S.; Chaniewski, S.; Sheaffer, A.K.; et al. Resistance analysis of the hepatitis C virus NS3 protease inhibitor asunaprevir. Antimicrob. Agents Chemother. 2012, 56, 3670–3681. [Google Scholar] [CrossRef]
- Welsch, C.; Domingues, F.S.; Susser, S.; Antes, I.; Hartmann, C.; Mayr, G.; Schlicker, A.; Sarrazin, C.; Albrecht, M.; Zeuzem, S.; et al. Molecular basis of telaprevir resistance due to V36 and T54 mutations in the NS3-4A protease of the hepatitis C virus. Genome Biol. 2008, 9, R16. [Google Scholar] [CrossRef] [PubMed]
- Wyles, D.L.; Luetkemeyer, A.F. Understanding Hepatitis C Virus Drug Resistance: Clinical Implications for Current and Future Regimens. Top. Antivir. Med. 2017, 25, 103–109. [Google Scholar]
- Tong, X.; Chase, R.; Skelton, A.; Chen, T.; Wright-Minogue, J.; Malcolm, B.A. Identification and analysis of fitness of resistance mutations against the HCV protease inhibitor SCH 503034. Antivir. Res. 2006, 70, 28–38. [Google Scholar] [CrossRef]
| HCV Genotype-1 | |||
|---|---|---|---|
| QSID/RSID | RAASs/Variants | Phenotype | HCV Polyprotein |
| QSID-1115239302/NP_671491.1 | S2702T | ER-Sofosbuvir | NS5B-polyprotein |
| QSID-597510766/NP_671491.1 | S2702T | ER-Sofosbuvir | NS5B-polyprotein |
| QSID-568110881/RSID-AAA72945.1 | V1196A | ER-Boceprevir | NS3-polyportein |
| QSID-908269165/RSID-AAA72945.1 | I1158V | ER-Telaprevir | NS3-polyportein |
| QSID-ATY34994/RSID-AAA72945.1 | D1194E | ER-Simeprevir | NS3-polyportein |
| QSID-ATY34994/RSID-AAA72945.1 | D1194E | ER-Simeprevir | NS3-polyportein |
| QSID-597512356/RSID-AAA72945.1 | D1194T | ER-Simeprevir | NS3-polyportein |
| QSID-530656976/RSID-AAA72945.1 | D1194G | ER-Asunaprevir | NS3-polyportein |
| QSID-333611772/RSID-AAA72945.1 | R1181K | ER to Faldaprevir and Asunaprevir | NS3-polyportein |
| QSID-568110975/RSID-AAA72945.1 | T1080S | ER -Faldaprevir, ER-Telaprevir | NS3-polyportein |
| QSID-ATY34994/RSID-AAA72945.1 | Q1106R | ER-Faldaprevir, ER-Simeprevir | NS3-polyportein |
| QSID-597512164/RSID-AAA72945.1 | D1194A | ER-Simeprevir, ER-Faldaprevir ER-Asunaprevir | NS3-polyportein |
| QSID-597511348/NP_671491.1 | S2702T | ER-Sofosbuvir | NS5B-polyprotein |
| QSID-597511368/NP_671491.1 | S2702T | ER-Sofosbuvir | NS5B-polyprotein |
| QSID-597512292/RSID-AAA72945.1 | D1194E | ER-Simeprevir | NS3-polyportein |
| QSID-597512292/RSID-AAA72945.1 | V1062A | ER-Telaprevir | NS3-polyportein |
| QSID-336039226/RSID-AAA72945.1 | D1194T | ER-Simeprevir | NS3-polyportein |
| QSID-568110888/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-333611678/RSID-AAA72945.1 | D1194G | ER-Asunaprevir | NS3-polyportein |
| QSID-597512292/RSID-AAA72945.1 | Q1106K | ER-Faldaprevir, ER-Simeprevir | NS3-polyportein |
| QSID-575502871/RSID-AAA72945.1 | T1080S | ER-Faldaprevir, ER-Telaprevir | NS3-polyportein |
| QSID-908273241/RSID- AAA72945.1 | S1148G | ES-Simeprevir | NS3-polyportein |
| QSID-AST22949/RSID-AAA72945.1 | S1148G | ES-Simeprevir | NS3-polyportein |
| QSID-575502871/RSID-AAA72945.1 | V1196A | ER-Boceprevir | NS3-polyportein |
| QSID-908268421/RSID-AAA72945.1 | Q1106K | ER-Faldaprevir, ER-Simeprevir | NS3-polyportein |
| QSID-333611598/RSID-AAA72945.1 | A1182V | ER-Faldaprevir, ER-Simeprevir | NS3-polyportein |
| QSID-333611772/RSID-AAA72945.1 | R1181K | ER-Faldaprevir, ER-Asunaprevir | NS3-polyportein |
| QSID-1042527298/RSID- NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1042527208/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1042527208/RSID-NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-1042527168/RSID-NP_671491.1 | Y2065N | ER-Daclatasvir | NS5A |
| QSID-1042527170/RSID-NP_671491.1 | Y2065N | ER-Daclatasvir | NS5A |
| QSID-1042527258/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1042527258/RSID-NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-1042527264/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1042527264/RSID-NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-1042527266/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1042527266/RSID-NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-1042527262/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1042527262/RSID-NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-1042527268/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1042527260/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1042527488/RSID-NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1115239468/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1042527490/RSID-NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1042527492/RSID-NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1042527486/RSID-NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1115239512/RSID-NP_671491.1 | M2000T | ER-Daclatasvir | NS5A |
| QSID-1115239510/RSID-NP_671491.1 | M2000T | ER-Daclatasvir | NS5A |
| QSID-1042527484/RSID-NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1042527498/RSID-NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1042527494/RSID-NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1115239524/RSID-NP_671491.1 | Y2065H | ER-Daclatasvir | NS5A |
| QSID-1042527496/RSID-NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1153219284/RSID-NP_671491.1 | M2000T | ER-Daclatasvir | NS5A |
| QSID-1115239648/RSID-NP_671491.1 | Y2065H | ER-Daclatasvir | NS5A |
| QSID-1115239656/RSID-NP_671491.1 | M2000T | ER-Daclatasvir | NS5A |
| QSID-1115239656/RSID-NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-1115239608/RSID-NP_671491.1 | Y2065H | ER-Daclatasvir | NS5A |
| QSID-1115239608/RSID-NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1115239606/RSID-NP_671491.1 | Y2065H | ER-Daclatasvir | NS5A |
| QSID-1115239606/RSID-NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1115239600/RSID-NP_671491.1 | Y2065N | ER-Daclatasvir | NS5A |
| QSID-1115239620/RSID-NP_671491.1 | Y2065H | ER-Daclatasvir | NS5A |
| QSID-1115239564/RSID-NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-1115239582/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1115239584/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1115239590/RSID-NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1115239592/RSID-NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1042527576/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1042527186/RSID-NP_671491.1 | Y2065H | ER-Daclatasvir | NS5A |
| QSID-1042527180/RSID-NP_671491.1 | Y2065H | ER-Daclatasvir | NS5A |
| QSID-1042527188/RSID-NP_671491.1 | Y2065H | ER-Daclatasvir | NS5A |
| QSID-1042527182/RSID-NP_671491.1 | Y2065H | ER-Daclatasvir | NS5A |
| QSID-1115239556/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1042527192/RSID-NP_671491.1 | Y2065H | ER-Daclatasvir | NS5A |
| QSID-1042527178/RSID-NP_671491.1 | Y2065H | ER-Daclatasvir | NS5A |
| QSID-1042527184/RSID-NP_671491.1 | Y2065H | ER-Daclatasvir | NS5A |
| QSID-1042527190/RSID-NP_671491.1 | Y2065H | ER-Daclatasvir | NS5A |
| QSID-1042527608/RSID-NP_671491.1 | L2003V | ER-Daclatasvir | NS5A |
| QSID-808181800/RSID-NP_671491.1 | Y2065N | ER-Daclatasvir | NS5A |
| QSID-808181816/RSID-NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| HCV Genotype 2 | |||
| QSID/RSID | RAASs/variants | Phenotype | HCV polyprotein |
| QSID-1152728359/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-1152728359/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-544168876/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-544168876/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-401712474/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-401712474/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-1152728369/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-1152728369/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-ATY35029/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-ATY35029/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-401712520/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-401712520/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-1152728313/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-509263121/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-1152728311/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-ATY35005/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-ATY35005/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-1152728355/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-1152728355/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-1152728371/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-1152728371/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-ATY35003/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-ATY35003/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-1152728391/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-1152728391/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-544168878/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-544168878/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-ATY35030/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-ATY35030/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-544168870/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-544168870/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-1152728397/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-1152728397/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-1152728395/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-1152728379/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-1152728379/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-576294944/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-393714879/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-401712478/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-401712478/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-544168874/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-544168874/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-1152728331/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-ATY35031/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-ATY35031/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-401712476/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-401712476/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| HCV Genotype 3 | |||
| QSID/RSID | RAASs/variants | Phenotype | HCV polyprotein |
| QSID-ART89572/NP_671491.1 | S2702T | ER-Sofosbuvir | NS5B-polyprotein |
| QSID-1152728501/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| HCV Genotype 4 | |||
| QSID/RSID | RAASs/variants | Phenotype | HCV polyprotein |
| QSID-475628336/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-475628354/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-751660972/NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-751660974/NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-751660974/NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-ATY35065/NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-ATY35065/NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-ARR74221/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-ATY35035/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-ATY35066/NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-ATY35066/NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-1042527988/NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1042527996/NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1042527990/NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1042527990/NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1042527998/NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1042528000/NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1042527992/NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1042527992/NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1042527994/NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1042527994/NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1009028115/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS5A |
| QSID-1009028115/NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1009028115/NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-1009028127/NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1009028113/NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1009028113/NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-1009028111/NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1009028111/NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-ATY35085/NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-ATY35085/NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-1009028115/RSID- NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1009028115/RSID-NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-1009028127/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1009028127/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1009028113/RSID-NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-1009028111/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1009028111/RSID-NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-ATY35085/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-ATY35085/RSID-NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-751660972/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-751660974/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-751660974/RSID-NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-ATY35065/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-ATY35065/RSID-NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-ATY35066/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-ATY35066/RSID-NP_671491.1 | Q2002R | ER-Daclatasvir | NS5A |
| QSID-1042527988/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1042527996/RSID-NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1042527990/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1042527990/RSID-NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1042527998/RSID-NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1042528000/RSID-NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1042527992/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1042527992/RSID-NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| QSID-1042527994/RSID-NP_671491.1 | L2003M | ER-Daclatasvir | NS5A |
| QSID-1042527994/RSID-NP_671491.1 | Q2002H | ER-Daclatasvir | NS5A |
| HCV Genotype 5 | |||
| QSID/RSID | RAASs/variants | Phenotype | HCV polyprotein |
| QSID-751660976/RSID-AAA72945.1 | Q1106K | ER-Faldaprevir, ER-Simeprevir | NS3-polyportein |
| QSID-ATA65699/NP_671491.1 | S2702T | ER-Sofosbuvir | NS5B-polyprotein |
| QSID-1026671962/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-1026671962/RSID-AAA72945.1 | D1194E | ER-Simeprevir | NS3-polyportein |
| QSID-751660976/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-1026671962/RSID-AAA72945.1 | Q1106K | ER-Faldaprevir, ER-Simeprevir | NS3-polyportein |
| QSID-1026671954/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-1009028101/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-1009028101/RSID-AAA72945.1 | S1148A | ES-Simeprevir | NS3-polyportein |
| QSID-1009028103/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-1009028103/RSID-AAA72945.1 | S1148A | ES-Simeprevir | NS3-polyportein |
| QSID-1009028103/RSID-AAA72945.1 | Q1106K | ER-Faldaprevir, ER-Simeprevir | NS3-polyportein |
| QSID-1026671954/RSID-AAA72945.1 | Q1106K | ER-Faldaprevir, ER-Simeprevir | NS3-polyportein |
| QSID-1009028101/RSID-AAA72945.1 | Q1106K | ER-Faldaprevir, ER-Simeprevir | NS3-polyportein |
| HCV Genotype UC | |||
| QSID/RSID | RAASs/variants | Phenotype | HCV polyprotein |
| QSID-ART89485/NP_671491.1 | Y2065H | ER-Daclatasvir | NS5A |
| QSID-ARB18146/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-844573227/RSID-AAA72945.1 | Q1106L | ER-Faldaprevir | NS3-polyportein |
| QSID-ASE05938/RSID-AAA72945.1 | S1148R | ER-Simeprevir | NS3-polyportein |
| QSID-ASE05936/RSID-AAA72945.1 | V1062L | ER-Telaprevir | NS3-polyportein |
| QSID-ART89485/RSID- NP_671491.1 | Y2065H | ER-Daclatasvir | NS5A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izhari, M.A. Molecular Mechanisms of Resistance to Direct-Acting Antiviral (DAA) Drugs for the Treatment of Hepatitis C Virus Infections. Diagnostics 2023, 13, 3102. https://doi.org/10.3390/diagnostics13193102
Izhari MA. Molecular Mechanisms of Resistance to Direct-Acting Antiviral (DAA) Drugs for the Treatment of Hepatitis C Virus Infections. Diagnostics. 2023; 13(19):3102. https://doi.org/10.3390/diagnostics13193102
Chicago/Turabian StyleIzhari, Mohammad Asrar. 2023. "Molecular Mechanisms of Resistance to Direct-Acting Antiviral (DAA) Drugs for the Treatment of Hepatitis C Virus Infections" Diagnostics 13, no. 19: 3102. https://doi.org/10.3390/diagnostics13193102
APA StyleIzhari, M. A. (2023). Molecular Mechanisms of Resistance to Direct-Acting Antiviral (DAA) Drugs for the Treatment of Hepatitis C Virus Infections. Diagnostics, 13(19), 3102. https://doi.org/10.3390/diagnostics13193102





