VOCs from Exhaled Breath for the Diagnosis of Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Data Collection
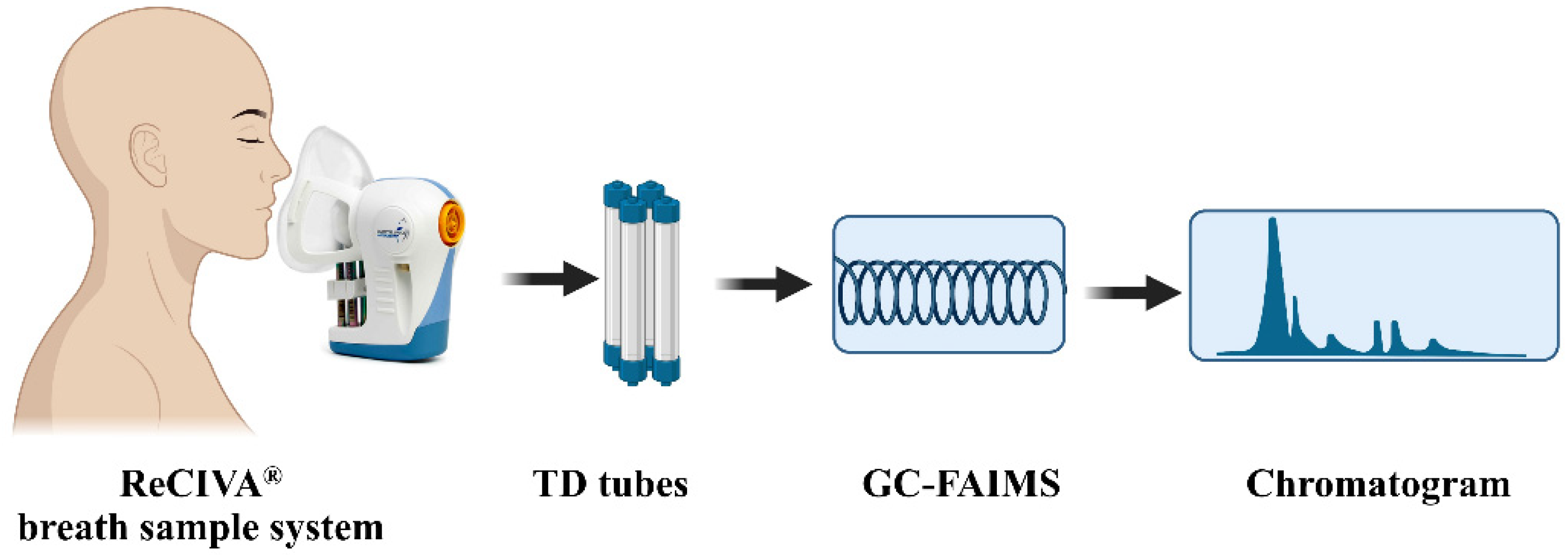
2.3. Breath Collection
2.4. VOCs Extraction and Measurement
2.5. Data Analysis
3. Results
3.1. Baseline Characteristics
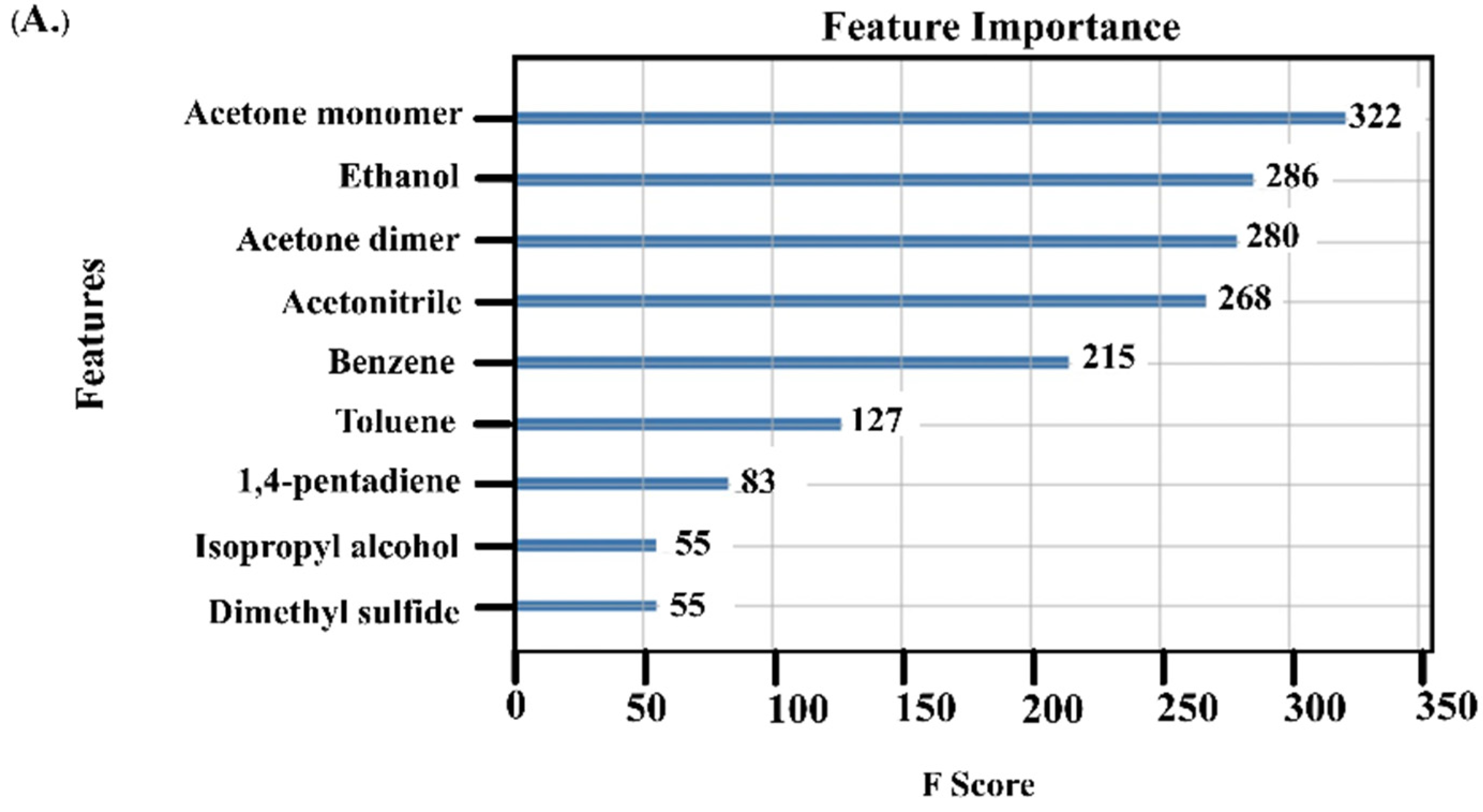
3.2. VOCs as Biomarkers for HCC Diagnosis
3.3. Performance of Acetone and AFP as Diagnostic Biomarkers
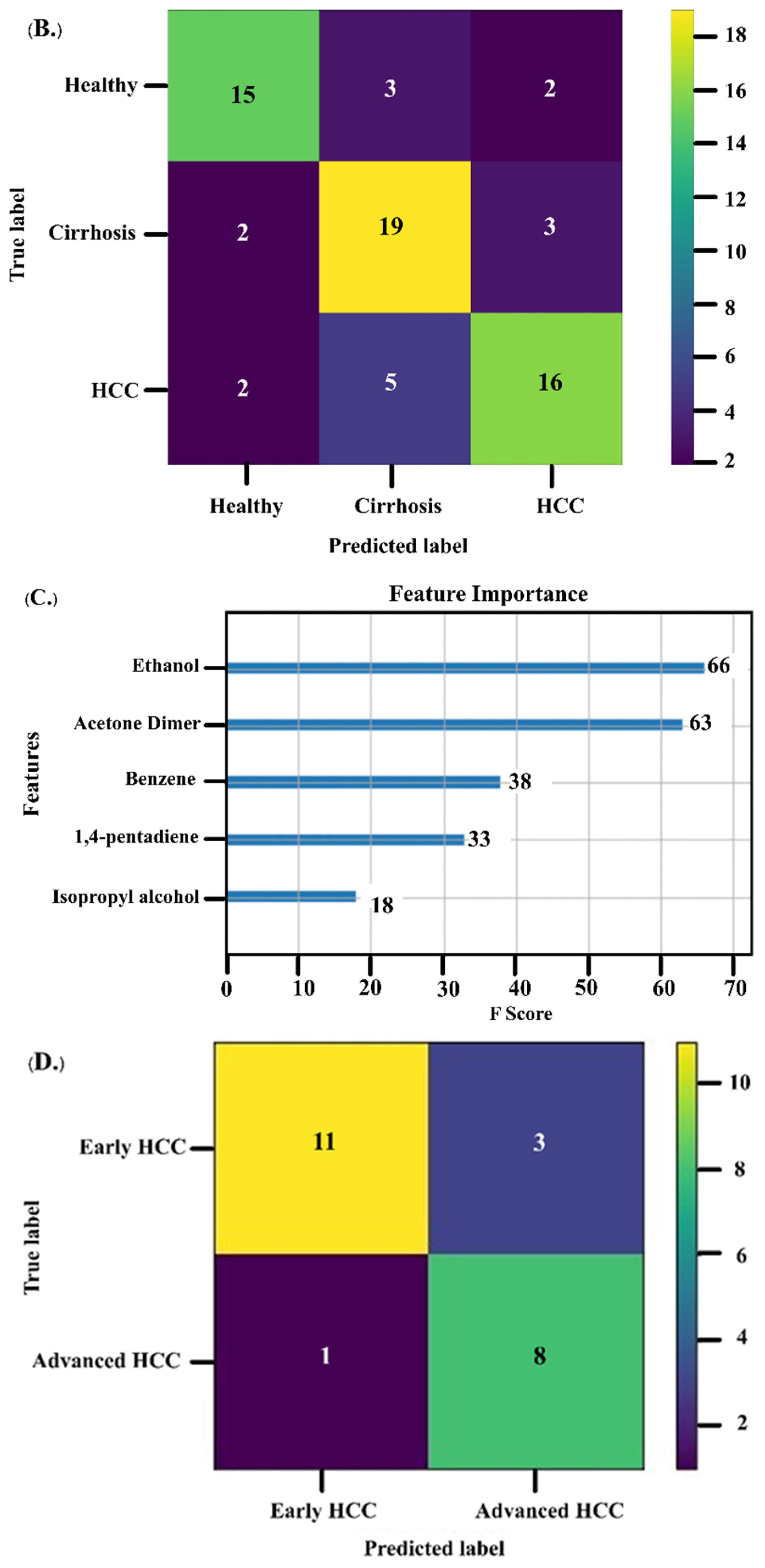
3.4. VOCs as Biomarkers for Monitoring Treatment Response
3.5. Association between VOCs and Survival of HCC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv. Cancer Res. 2021, 149, 1–61. [Google Scholar] [CrossRef]
- Lu, Q.; Li, J.; Cao, H.; Lv, C.; Wang, X.; Cao, S. Comparison of diagnostic accuracy of Midkine and AFP for detecting hepatocellular carcinoma: A systematic review and meta-analysis. Biosci. Rep. 2020, 40, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, W.-C.; Lee, C.-L.; Yang, T.-S.; Huang, C.-Y.; Teng, W.; Tseng, Y.-T.; Chen, J.-S.; Lin, Y.-C.; Hou, M.-M.; Chang, H.-H.; et al. Changes in serum α-fetoprotein level predicts treatment response and survival in hepatocellular carcinoma patients and literature review. J. Formos. Med. Assoc. 2018, 117, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718. [Google Scholar] [CrossRef] [Green Version]
- Chaiteerakij, R.; Addissie, B.D.; Roberts, L.R. Update on biomarkers of hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 2015, 13, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Osho, A.; Rich, N.E.; Singal, A.G. Role of imaging in management of hepatocellular carcinoma: Surveillance, diagnosis, and treatment response. Hepatoma Res. 2020, 6, 55–75. [Google Scholar] [CrossRef]
- Gouzerh, F.; Bessiere, J.M.; Ujvari, B.; Thomas, F.; Dujon, A.M.; Dormont, L. Odors and cancer: Current status and future directions. Biochim. et Biophys. Acta (BBA)—Rev. Cancer 2022, 1877, 188644–188662. [Google Scholar] [CrossRef]
- Hintzen, K.F.H.; Grote, J.; Wintjens, A.; Lubbers, T.; Eussen, M.M.M.; van Schooten, F.J.; Bouvy, N.D.; Peeters, A. Breath analysis for the detection of digestive tract malignancies: Systematic review. BJS Open 2021, 5, 1–12. [Google Scholar] [CrossRef]
- Dima, A.C.; Balaban, D.V.; Dima, A. Diagnostic Application of Volatile Organic Compounds as Potential Biomarkers for Detecting Digestive Neoplasia: A Systematic Review. Diagnostics 2021, 11, 2317. [Google Scholar] [CrossRef]
- Janfaza, S.; Khorsand, B.; Nikkhah, M.; Zahiri, J. Digging deeper into volatile organic compounds associated with cancer. Biol. Methods Protoc. 2019, 4, bpz014. [Google Scholar] [CrossRef] [PubMed]
- Haick, H.; Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A. Assessment, origin, and implementation of breath volatile cancer markers. Chem. Soc. Rev. 2014, 43, 1423–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cyll, K.; Ersvaer, E.; Vlatkovic, L.; Pradhan, M.; Kildal, W.; Avranden Kjaer, M.; Kleppe, A.; Hveem, T.S.; Carlsen, B.; Gill, S.; et al. Tumour heterogeneity poses a significant challenge to cancer biomarker research. Br. J. Cancer 2017, 117, 367–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardi-Agmon, I.; Abud-Hawa, M.; Liran, O.; Gai-Mor, N.; Ilouze, M.; Onn, A.; Bar, J.; Shlomi, D.; Haick, H.; Peled, N. Exhaled Breath Analysis for Monitoring Response to Treatment in Advanced Lung Cancer. J. Thorac. Oncol. 2016, 11, 827–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitiyakara, T.; Redmond, S.; Unwanatham, N.; Rattanasiri, S.; Thakkinstian, A.; Tangtawee, P.; Mingphruedhi, S.; Sobhonslidsuk, A.; Intaraprasong, P.; Kositchaiwat, C. The detection of hepatocellular carcinoma (HCC) from patients’ breath using canine scent detection: A proof-of-concept study. J. Breath Res. 2017, 11, 1–17. [Google Scholar] [CrossRef]
- Acevedo-Moreno, L.; Sasaki, K.; Firl, D.; McVey, J.; Berber, E.; Miller, C.; Lopez, R.; Grove, D.; Dweik, R.; Aucejo, F. Investigating the breath metabolome as a diagnostic tool for hepatocellular carcinoma in cirrhotic patients. HPB 2018, 20, S82–S83. [Google Scholar] [CrossRef] [Green Version]
- Qin, T.; Liu, H.; Song, Q.; Song, G.; Wang, H.Z.; Pan, Y.Y.; Xiong, F.X.; Gu, K.S.; Sun, G.P.; Chen, Z.D. The screening of volatile markers for hepatocellular carcinoma. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2247–2253. [Google Scholar] [CrossRef] [Green Version]
- Miller-Atkins, G.; Acevedo-Moreno, L.A.; Grove, D.; Dweik, R.A.; Tonelli, A.R.; Brown, J.M.; Allende, D.S.; Aucejo, F.; Rotroff, D.M. Breath Metabolomics Provides an Accurate and Noninvasive Approach for Screening Cirrhosis, Primary, and Secondary Liver Tumors. Hepatol. Commun. 2020, 4, 1041–1055. [Google Scholar] [CrossRef] [Green Version]
- Sukaram, T.; Tansawat, R.; Apiparakoon, T.; Tiyarattanachai, T.; Marukatat, S.; Rerknimitr, R.; Chaiteerakij, R. Exhaled volatile organic compounds for diagnosis of hepatocellular carcinoma. Sci. Rep. 2022, 12, 5326–5334. [Google Scholar] [CrossRef]
- Bhandari, M.P.; Veliks, V.; Stonans, I.; Padilla, M.; Suba, O.; Svare, A.; Krupnova, I.; Ivanovs, N.; Bema, D.; Mitrovics, J.; et al. Breath Sensor Technology for the Use in Mechanical Lung Ventilation Equipment for Monitoring Critically Ill Patients. Diagnostics 2022, 12, 430. [Google Scholar] [CrossRef]
- Pereira, J.; Porto-Figueira, P.; Cavaco, C.; Taunk, K.; Rapole, S.; Dhakne, R.; Nagarajaram, H.; Camara, J.S. Breath analysis as a potential and non-invasive frontier in disease diagnosis: An overview. Metabolites 2015, 5, 3–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Future Healthc. J. 2019, 6, 94–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsou, P.H.; Lin, Z.L.; Pan, Y.C.; Yang, H.C.; Chang, C.J.; Liang, S.K.; Wen, Y.F.; Chang, C.H.; Chang, L.Y.; Yu, K.L.; et al. Exploring Volatile Organic Compounds in Breath for High-Accuracy Prediction of Lung Cancer. Cancers 2021, 13, 1431. [Google Scholar] [CrossRef] [PubMed]
- Sakumura, Y.; Koyama, Y.; Tokutake, H.; Hida, T.; Sato, K.; Itoh, T.; Akamatsu, T.; Shin, W. Diagnosis by Volatile Organic Compounds in Exhaled Breath from Lung Cancer Patients Using Support Vector Machine Algorithm. Sensors 2017, 17, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Acharya, V.; Dhiman, G.; Prakasha, K.; Bahadur, P.; Choraria, A.; M, S.; J, S.; Prabhu, S.; Chadaga, K.; Viriyasitavat, W.; et al. AI-Assisted Tuberculosis Detection and Classification from Chest X-Rays Using a Deep Learning Normalization-Free Network Model. Comput. Intell. Neurosci. 2022, 2022, 2399428. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.A.; Ahmed, A.N.; Chow, M.F.; Huang, Y.F.; El-Shafie, A. Extreme gradient boosting (Xgboost) model to predict the groundwater levels in Selangor Malaysia. Ain Shams Eng. J. 2021, 12, 1545–1556. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [Green Version]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Asselman, A.; Khaldi, M.; Aammou, S. Enhancing the prediction of student performance based on the machine learning XGBoost algorithm. Interact. Learn. Environ. 2021, 30, 1–20. [Google Scholar] [CrossRef]
- Verma, A.; Yadav, D.; Singh, A.; Gupta, M.; Thapa, K.B.; Yadav, B.C. Detection of acetone via exhaling human breath for regular monitoring of diabetes by low-cost sensing device based on perovskite BaSnO3 nanorods. Sens. Actuators B Chem. 2022, 361, 131708–131720. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Liu, Y.; Cheng, S.; Duan, Y. Exhaled isopropanol: New potential biomarker in diabetic breathomics and its metabolic correlations with acetone. RSC Adv. 2017, 7, 17480–17488. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhou, W.; Wang, S.; Huang, J.; Le, Y.; Nie, S.; Wang, W.; Guo, Q. Accuracy of breath test for diabetes mellitus diagnosis: A systematic review and meta-analysis. BMJ Open Diabetes Res. Care 2021, 9, 2174–2184. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Liu, C.; Qin, M.; Zhang, X.; Xi, T.; Yuan, S.; Hao, H.; Xiong, J. Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma. Acta Pharm. Sin. B 2022, 12, 558–580. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Zhang, C.; Le, A. Glucose Metabolism in Cancer: The Warburg Effect and Beyond. Adv. Exp. Med. Biol. 2021, 1311, 3–15. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, S.; Yin, S.; Niu, W.; Xiong, W.; Tan, M.; Li, G.; Zhou, M. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget 2017, 8, 57813–57825. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, K.; Podmore, I. Current challenges in volatile organic compounds analysis as potential biomarkers of cancer. J. Biomark. 2015, 2015, 981458–981473. [Google Scholar] [CrossRef] [Green Version]
- Heaney, L.M.; Jones, D.J.; Suzuki, T. Mass spectrometry in medicine: A technology for the future? Future Sci. OA 2017, 3, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Jeong, W.K. Surveillance of hepatocellular carcinoma: Is only ultrasound enough? Clin. Mol. Hepatol. 2017, 23, 222–223. [Google Scholar] [CrossRef]
| Variables | HCC (n = 124) | Cirrhosis (n = 124) | p† | Healthy (n = 95) | p‡ |
|---|---|---|---|---|---|
| Age, years * | 62.7 ± 12.6 | 60.6 ± 9.2 | 0.126 | 59.3 ± 9.1 | 0.053 |
| Male, n (%) | 60 (48.4%) | 62 (50.0%) | 0.799 | 47 (49.5%) | 0.967 |
| Smoking, n (%) | 58 (46.8%) | 48 (38.7%) | 0.199 | 35 (36.8%) | 0.265 |
| Alcohol consumption, n (%) | 23 (18.5%) | 27 (21.8%) | 0.527 | 22 (23.2%) | 0.684 |
| Cirrhosis, n (%) | 122 (98.4%) | 124 (100.0%) | 0.156 | 0 (0.00%) | 0.016 |
| Child-Pugh class, n (%) | 0.002 | ||||
| A | 97 (78.2%) | 115 (93.5%) | 0 (0.0%) | ||
| B | 26 (21.0%) | 8 (6.5%) | 0 (0.0%) | ||
| C | 1 (0.8%) | 0 (0.0%) | 0 (0.0%) | ||
| Chronic HBV infection, n (%) | 53 (42.7%) | 42 (33.9%) | 0.151 | 0 (0.0%) | |
| Chronic HCV infection, n (%) | 34 (27.4%) | 48 (38.7%) | 0.059 | 0 (0.0%) | |
| NAFLD, n (%) | 37 (29.8%) | 32 (25.8%) | 0.479 | 0 (0.0%) | |
| Diabetes mellitus, n (%) | 45 (36.3%) | 41 (33.1%) | 0.594 | 0 (0.0%) | |
| Albumin (g/dL) * | 3.8 ± 0.6 | 4.0 ± 0.4 | 0.001 | 4.4 ± 0.2 | <0.001 |
| Total bilirubin (mg/dL) * | 1.6 ± 2.2 | 1.1 ± 0.9 | 0.018 | 0.8 ± 0.3 | 0.021 |
| AST (U/L) * | 74 ± 84 | 38 ± 36 | <0.001 | 23 ± 6 | <0.001 |
| ALT (U/L) * | 53 ±78 | 33 ± 43 | 0.014 | 24 ± 12 | 0.016 |
| Alkaline phosphatase (U/L) * | 164 ± 225 | 94 ± 48 | 0.001 | 69 ± 21 | 0.001 |
| AFP (ng/mL), median (range) | 9.9 (1.1–158,906.2) | 2.4 (1.0–18.1) | 0.008 | 3.1 (1.3–10.6) | <0.001 |
| VOCs | HCC (n = 124) | Cirrhosis (n = 124) | p† | Healthy (n = 95) | p‡ |
|---|---|---|---|---|---|
| Ethanol | 0.25 (0.12–1.01) | 0.25 (0.12–0.44) | 0.728 | 0.26 (0.12–0.42) | 0.341 |
| Acetone monomer | 3.85 (2.20–4.76) | 3.90 (2.28–4.82) | 0.559 | 4.29 (2.07–4.70) | <0.001 |
| Dimethyl sulfide | 0.29 (0.04–3.67) | 0.41 (0.08–1.81) | 0.939 | 0.28 (0.10–1.24) | 0.845 |
| 1,4-pentadiene | 0.74 (0.00–2.12) | 0.82 (0.01–3.35) | 0.389 | 0.65 (0.10–1.54) | 0.015 |
| Benzene | 0.21 (0.00–0.74) | 0.21 (0.01–1.11) | 0.199 | 0.18 (0.10–0.79) | 0.139 |
| Isopropyl alcohol | 0.18 (0.07–1.73) | 0.15 (0.08–1.22) | 0.032 | 0.15 (0.08–1.20) | 0.022 |
| Acetone dimer | 5.08 (2.84–5.83) | 4.48 (2.59–4.93) | <0.001 | 3.90 (1.32–5.03) | <0.001 |
| Acetonitrile | 0.16 (0.07–0.69) | 0.15 (0.09–0.70) | 0.426 | 0.17 (0.05–0.74) | 0.073 |
| Toluene | 0.41 (0.14–2.33) | 0.43 (0.09–2.75) | 0.782 | 0.31 (0.06–2.87) | <0.001 |
| Biomarker | Group * | Cutoff | AUC | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|---|---|
| Acetone dimer | HCC vs. non-HCC | 4.6579 | 0.816 | 83.9% | 79.4% | 70.0% | 89.6% | 81.0% |
| Early HCC vs. non-HCC | 4.6579 | 0.822 | 85.1% | 79.4% | 55.9% | 94.5%, | 80.7% | |
| HCC vs. cirrhosis | 4.6579 | 0.769 | 83.9% | 69.9% | 73.8% | 81.1% | 76.9% | |
| Early HCC vs. cirrhosis | 4.6579 | 0.775 | 85.1% | 69.9% | 60.6% | 89.6% | 75.2% | |
| AFP | HCC vs. non-HCC | 20 | 0.802 | 62.4% | 100.0% | 100.0% | 62.4% | 69.9% |
| Early HCC vs. non-HCC | 20 | 0.721 | 19.0% | 100.0% | 100.0% | 69.5%, | 68.7% | |
| HCC vs. cirrhosis | 20 | 0.806 | 40.2% | 100.0% | 100.0% | 58.8% | 67.7% | |
| Early HCC vs. cirrhosis | 20 | 0.714 | 19.0% | 100.0% | 100.0% | 66.2% | 12.6% |
| Variables | Univariate Analysis | p | Multivariate Analysis | p |
|---|---|---|---|---|
| HR (95%CI) | Adjusted HR (95%CI) | |||
| Age | 1.00 (0.95–1.05) | 0.956 | 1.03 (0.97–1.09) | 0.408 |
| Sex | 1.51 (0.48–4.76) | 0.483 | 1.05 (0.21–5.15) | 0.957 |
| AFP | 1.53 (1.09–2.17) | 0.015 | 1.39 (0.72–2.66) | 0.325 |
| HCC stage | ||||
| Early (BCLC 0-A) | reference | |||
| Advanced (BCLC B-C) | 7.07 (1.55–32.31) | 0.012 | 5.66 (0.90–35.59) | 0.065 |
| Acetone monomer | 0.54 (0.22–1.31) | 0.170 | ||
| Dimethyl sulfide | 0.79 (0.17–3.57) | 0.759 | ||
| Benzene | 0.64 (0.01–37.49) | 0.827 | ||
| Isopropyl alcohol | 4.84 (1.28–18.24) | 0.020 | 7.23 (1.36–38.54) | 0.020 |
| Acetone dimer | 1.50 (0.43–5.26) | 0.524 | ||
| Toluene | 0.64 (0.08–5.18) | 0.674 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukaram, T.; Apiparakoon, T.; Tiyarattanachai, T.; Ariyaskul, D.; Kulkraisri, K.; Marukatat, S.; Rerknimitr, R.; Chaiteerakij, R. VOCs from Exhaled Breath for the Diagnosis of Hepatocellular Carcinoma. Diagnostics 2023, 13, 257. https://doi.org/10.3390/diagnostics13020257
Sukaram T, Apiparakoon T, Tiyarattanachai T, Ariyaskul D, Kulkraisri K, Marukatat S, Rerknimitr R, Chaiteerakij R. VOCs from Exhaled Breath for the Diagnosis of Hepatocellular Carcinoma. Diagnostics. 2023; 13(2):257. https://doi.org/10.3390/diagnostics13020257
Chicago/Turabian StyleSukaram, Thanikan, Terapap Apiparakoon, Thodsawit Tiyarattanachai, Darlene Ariyaskul, Kittipat Kulkraisri, Sanparith Marukatat, Rungsun Rerknimitr, and Roongruedee Chaiteerakij. 2023. "VOCs from Exhaled Breath for the Diagnosis of Hepatocellular Carcinoma" Diagnostics 13, no. 2: 257. https://doi.org/10.3390/diagnostics13020257
APA StyleSukaram, T., Apiparakoon, T., Tiyarattanachai, T., Ariyaskul, D., Kulkraisri, K., Marukatat, S., Rerknimitr, R., & Chaiteerakij, R. (2023). VOCs from Exhaled Breath for the Diagnosis of Hepatocellular Carcinoma. Diagnostics, 13(2), 257. https://doi.org/10.3390/diagnostics13020257






