Neuroinflammatory Findings of Corneal Confocal Microscopy in Long COVID-19 Patients, 2 Years after Acute SARS-CoV-2 Infection
Abstract
:1. Introduction
2. Material and Methods
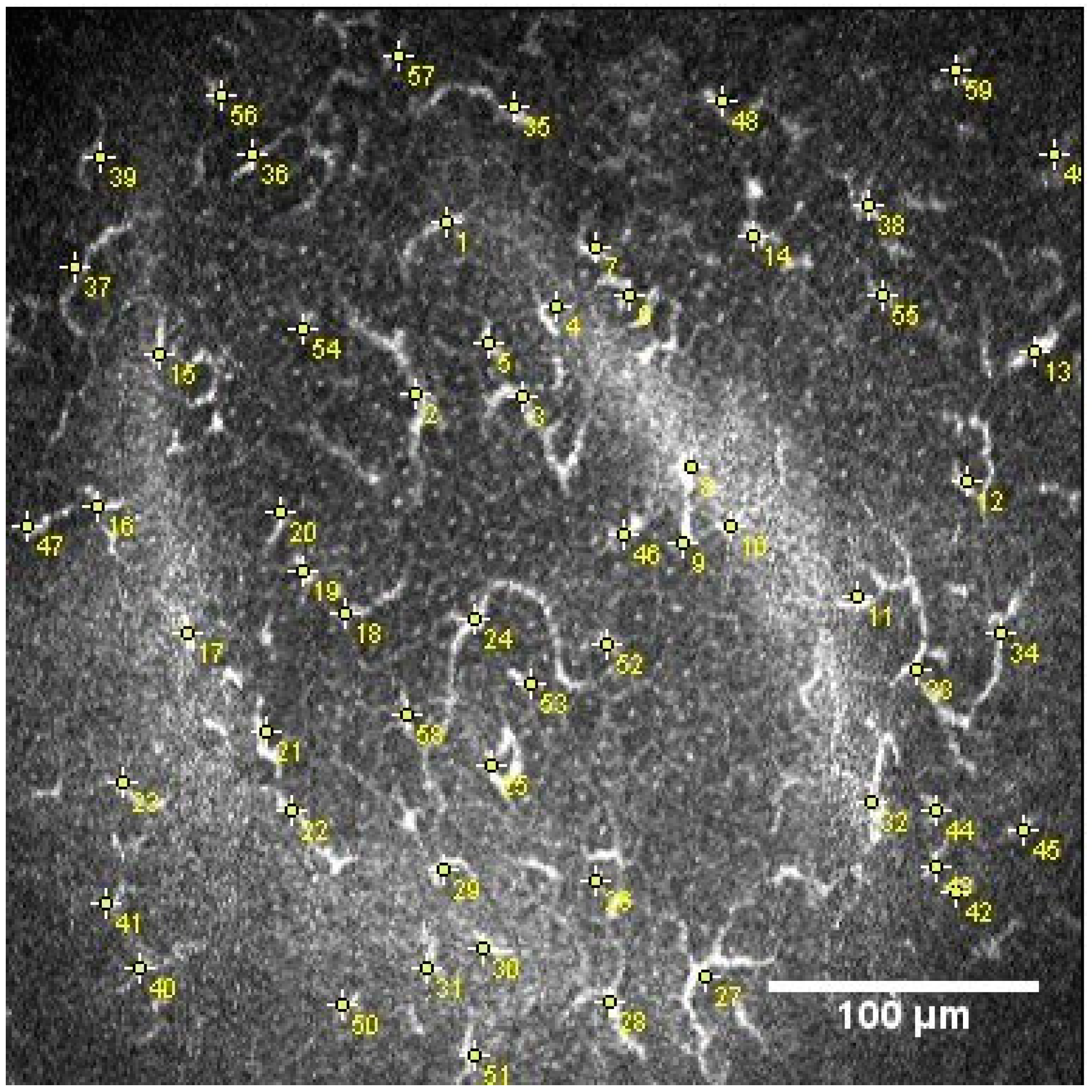
2.1. In Vivo Confocal Microscopy
2.2. Image Analysis
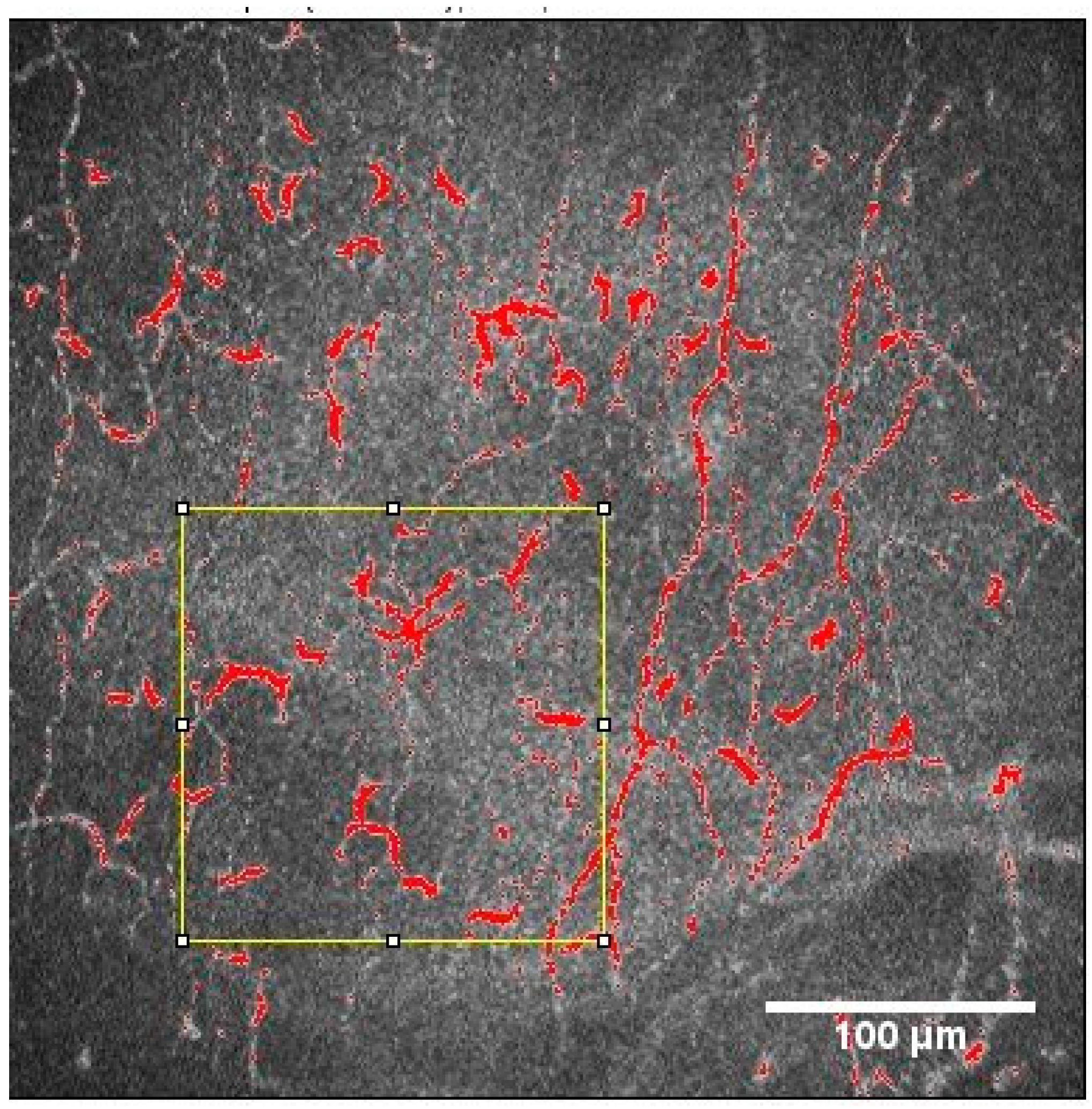
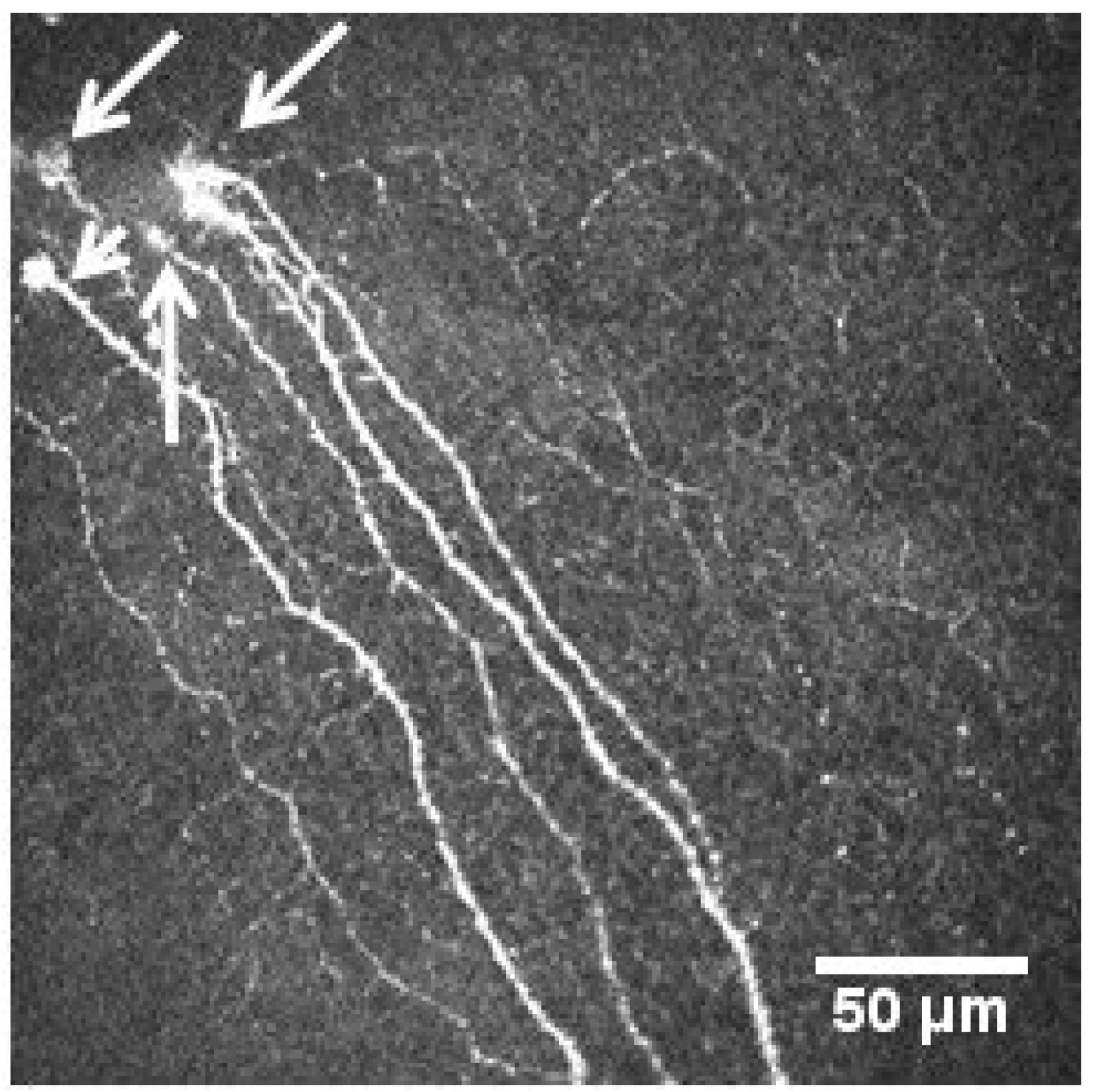
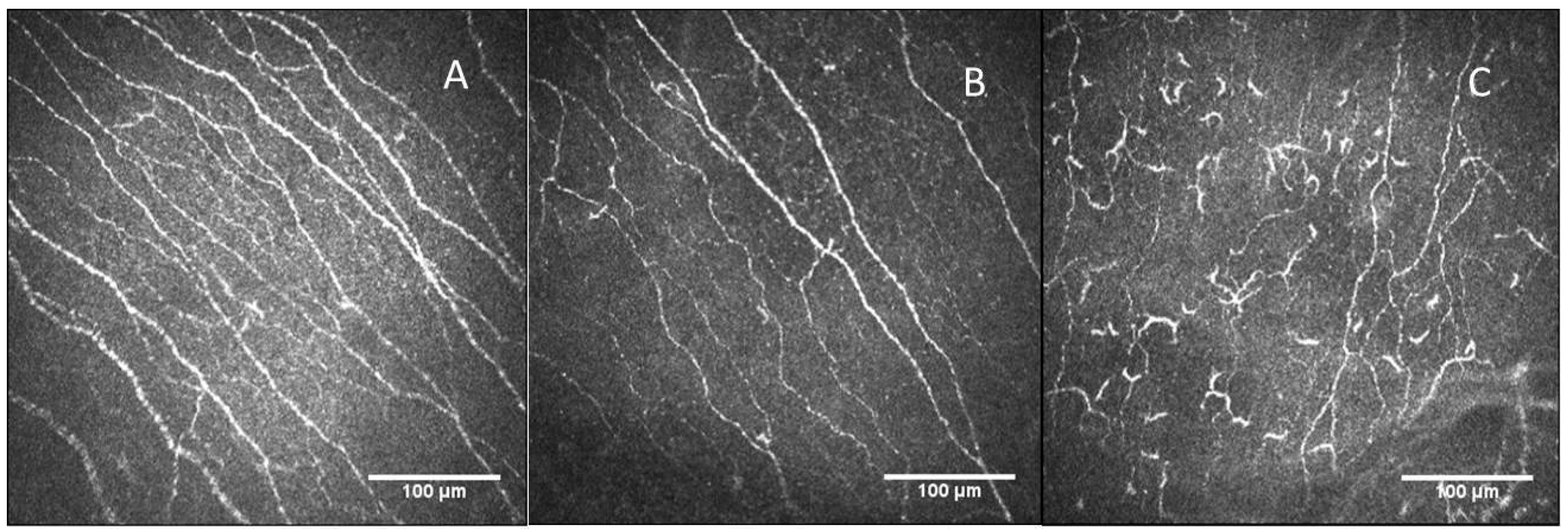
2.2.1. Nerve Plexus Morphology
2.2.2. Dendritic Cell Density and Area
2.3. Statistical Analysis
3. Results
3.1. Confocal Microscopy Findings
3.2. Relationship between Corneal Confocal Microscopy Morphology Parameters in LC-19 Patient
3.3. Corneal Confocal Microscopy Parameters in Vaccinated and Unvaccinated LC-19 Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Nasserie, T.; Hittle, M.; Goodman, S.N. Assessment of the Frequency and Variety of Persistent Symptoms Among Patients with COVID-19: A Systematic Review. JAMA Netw. Open 2021, 4, e2111417. [Google Scholar] [CrossRef] [PubMed]
- Delbressine, J.M.; Machado, F.V.C.; Goërtz, Y.M.J.; Van Herck, M.; Meys, R.; Houben-Wilke, S.; Burtin, C.; Franssen, F.M.E.; Spies, Y.; Vijlbrief, H.; et al. The Impact of Post-COVID-19 Syndrome on Self-Reported Physical Activity. Int. J. Environ. Res. Public Health 2021, 18, 6017. [Google Scholar] [CrossRef]
- Malik, P.; Patel, K.; Pinto, C.; Jaiswal, R.; Tirupathi, R.; Pillai, S.; Patel, U. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)-A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Scoppettuolo, P.; Borrelli, S.; Naeije, G. Neurological involvement in SARS-CoV-2 infection: A clinical systematic review. Brain Behav. Immun. Health 2020, 5, 100094. [Google Scholar] [CrossRef]
- Merola, A.; Rosso, M.; Romagnolo, A.; Comi, C.; Fasano, A.; Zibetti, M.; Lopez-Castellanos, J.R.; Cocito, D.; Lopiano, L.; Espay, A.J. Peripheral neuropathy as marker of severe Parkinson’s disease phenotype. Mov. Disord. 2017, 32, 1256–1258. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, I.N.; Alam, U.; Fadavi, H.; Marshall, A.; Asghar, O.; Dabbah, M.A.; Chen, X.; Graham, J.; Ponirakis, G.; Boulton, A.J.; et al. Rapid automated diagnosis of diabetic peripheral neuropathy with in vivo corneal confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, C.; Frost, S.; Jayasena, R.; Fowler, C.; Masters, C.L.; Kanagasingam, Y.; Jiao, H.; Lim, J.K.H.; Chinnery, H.R.; Downie, L.E. Morphometric Changes to Corneal Dendritic Cells in Individuals with Mild Cognitive Impairment. Front. Neurosci. 2020, 14, 556137. [Google Scholar] [CrossRef]
- Al-Aqaba, M.A.; Fares, U.; Suleman, H.; Lowe, J.; Dua, H.S. Architecture and distribution of human corneal nerves. Br. J. Ophthalmol. 2010, 94, 784–789. [Google Scholar] [CrossRef]
- Patel, D.V.; McGhee, C.N. In vivo laser scanning confocal microscopy confirms that the human corneal sub-basal nerve plexus is a highly dynamic structure. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3409–3412. [Google Scholar] [CrossRef] [PubMed]
- Marfurt, C.F.; Kingsley, R.E.; Echtenkamp, S.E. Sensory and sympathetic innervation of the mammalian cornea. A retrograde tracing study. Investig. Ophthalmol. Vis. Sci. 1989, 30, 461–472. [Google Scholar]
- Labetoulle, M.; Baudouin, C.; Calonge, M.; Merayo-Lloves, J.; Boboridis, K.G.; Akova, Y.A.; Aragona, P.; Geerling, G.; Messmer, E.M.; Benitez-Del-Castillo, J. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol. 2019, 97, 137–145. [Google Scholar] [CrossRef]
- Ponirakis, G.; Al Hamad, H.; Sankaranarayanan, A.; Khan, A.; Chandran, M.; Ramadan, M.; Tosino, R.; Gawhale, P.V.; Alobaidi, M.; AlSulaiti, E.; et al. Association of corneal nerve fiber measures with cognitive function in dementia. Ann. Clin. Transl. Neurol. 2019, 6, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, S.; Yokoo, S.; Usui, T.; Yamagami, H.; Amano, S.; Ebihara, N. Distinct populations of dendritic cells in the normal human donor corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4489–4494. [Google Scholar] [CrossRef]
- Hamrah, P.; Huq, S.O.; Liu, Y.; Zhang, Q.; Dana, M.R. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J. Leukoc. Biol. 2003, 74, 172–178. [Google Scholar] [CrossRef]
- Gao, N.; Lee, P.; Yu, F.S. Intraepithelial dendritic cells and sensory nerves are structurally associated and functional interdependent in the cornea. Sci. Rep. 2016, 6, 36414. [Google Scholar] [CrossRef] [PubMed]
- Woltsche, J.N.; Horwath-Winter, J.; Dorn, C.; Boldin, I.; Steinwender, G.; Heidinger, A.; Woltsche, N. Neuropathic Corneal Pain as Debilitating Manifestation of LONG-COVID. Ocul. Immunol. Inflamm. 2023, 31, 1216–1218. [Google Scholar] [CrossRef]
- Chinnery, H.R.; Rajan, R.; Jiao, H.; Wu, M.; Zhang, A.C.; De Silva, M.E.H.; Makrai, E.; Stepp, M.A.; Di Girolamo, N.; Downie, L.E. Identification of presumed corneal neuromas and microneuromas using laser-scanning in vivo confocal microscopy: A systematic review. Br. J. Ophthalmol. 2022, 106, 765–771. [Google Scholar] [CrossRef]
- Paltiel, A.D.; Schwartz, J.L.; Zheng, A.; Walensky, R.P. Clinical Outcomes Of A COVID-19 Vaccine: Implementation Over Efficacy. Health Aff. 2021, 40, 42–52. [Google Scholar] [CrossRef]
- Kolkedi, Z.; Csutak, A.; Szalai, E. Corneal Cellular and Neuroinflammatory Changes After SARS-CoV-2 Infection. Cornea 2022, 41, 879–885. [Google Scholar] [CrossRef]
- Bitirgen, G.; Korkmaz, C.; Zamani, A.; Ozkagnici, A.; Zengin, N.; Ponirakis, G.; Malik, R.A. Corneal confocal microscopy identifies corneal nerve fibre loss and increased dendritic cells in patients with long COVID. Br. J. Ophthalmol. 2022, 106, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://www.who.int/news-room/fact-sheets/detail/coronavirus-disease-(covid-19) (accessed on 13 March 2022).
- NICE ‘Long COVID’ Guideline. Available online: https://www.nice.org.uk/guidance/ng188/resources/covid19-rapid-guideline-managing-the-longterm-effects-of-covid19-pdf-51035515742 (accessed on 18 March 2022).
- Garcia-Gonzalez, M.; Canadas, P.; Gros-Otero, J.; Rodriguez-Perez, I.; Canones-Zafra, R.; Kozobolis, V.; Teus, M.A. Long-term corneal subbasal nerve plexus regeneration after laser in situ keratomileusis. J. Cataract. Refract. Surg. 2019, 45, 966–971. [Google Scholar] [CrossRef]
- Mirza, E.; Belviranli, S.; Gundogan, A.O.; Adam, M.; Oltulu, R. Quantitative assessment of the effect of SARS-CoV-2 on the corneal sub-basal nerve plexus of post-COVID-19 patients using in vivo confocal microscopy. Eye 2023, 37, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.J.; Movassaghi, M.; Gordy, D.; Olson, M.G.; Zhang, T.; Khurana, M.S.; Chen, Z.; Perez-Rosendahl, M.; Thammachantha, S.; Singer, E.J.; et al. Neuropathology of COVID-19 (neuro-COVID): Clinicopathological update. Free Neuropathol. 2021, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, L.; Businaro, P.; Regalbuto, S.; Gastaldi, M.; Zardini, E.; Panzeri, M.; Vegezzi, E.; Fiamingo, G.; Colombo, E.; Ravaglia, S. COVID-19 and Guillain-Barré syndrome: A single-center prospective case series with a 1-year follow-up. Medicine 2022, 101, e29704. [Google Scholar] [CrossRef] [PubMed]
- Faqihi, F.; Alharthy, A.; Memish, Z.A.; Kutsogiannis, D.J.; Brindley, P.G.; Karakitsos, D. Peripheral neuropathy in severe COVID-19 resolved with therapeutic plasma exchange. Clin. Case Rep. 2020, 8, 3234–3239. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, B.M.; Cruzat, A.; Sahin, A.; Pavan-Langston, D.; Samayoa, E.; Hamrah, P. In vivo confocal microscopy detects bilateral changes of corneal immune cells and nerves in unilateral herpes zoster ophthalmicus. Ocul. Surf. 2018, 16, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.V.; McGhee, C.N. Mapping of the normal human corneal sub-Basal nerve plexus by in vivo laser scanning confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4485–4488. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Dong, X.; Zhou, X.; Yan, L.; Wan, Q. Corneal subbasal nerve plexus changes in patients with episodic migraine: An in vivo confocal microscopy study. J. Pain Res. 2019, 12, 1489–1495. [Google Scholar] [CrossRef]
- Dermer, H.; Hwang, J.; Mittal, R.; Cohen, A.K.; Galor, A. Corneal sub-basal nerve plexus microneuromas in individuals with and without dry eye. Br. J. Ophthalmol. 2022, 106, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Bitirgen, G.; Akpinar, Z.; Malik, R.A.; Ozkagnici, A. Use of Corneal Confocal Microscopy to Detect Corneal Nerve Loss and Increased Dendritic Cells in Patients with Multiple Sclerosis. JAMA Ophthalmol. 2017, 135, 777–782. [Google Scholar] [CrossRef]
- Stettner, M.; Hinrichs, L.; Guthoff, R.; Bairov, S.; Petropoulos, I.N.; Warnke, C.; Hartung, H.P.; Malik, R.A.; Kieseier, B.C. Corneal confocal microscopy in chronic inflammatory demyelinating polyneuropathy. Ann. Clin. Transl. Neurol. 2016, 3, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Mayer, W.J.; Mackert, M.J.; Kranebitter, N.; Messmer, E.M.; Grüterich, M.; Kampik, A.; Kook, D. Distribution of antigen presenting cells in the human cornea: Correlation of in vivo confocal microscopy and immunohistochemistry in different pathologic entities. Curr. Eye Res. 2012, 37, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.; Marshall, A.; Pitceathly, R.; Fadavi, H.; Gow, D.; Roberts, M.E.; Efron, N.; Boulton, A.J.; Malik, R.A. Corneal confocal microscopy: A novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp. Neurol. 2010, 223, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.; Marshall, A.; Thompson, L.; Kenny, M.; Waldek, S.; Efron, N.; Malik, R.A. Corneal confocal microscopy: A novel noninvasive means to diagnose neuropathy in patients with Fabry disease. Muscle Nerve 2009, 40, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Thimm, A.; Carpinteiro, A.; Oubari, S.; Papathanasiou, M.; Kessler, L.; Rischpler, C.; Malik, R.A.; Reinhardt, H.C.; Rassaf, T.; Herrmann, K.; et al. Corneal confocal microscopy to detect early immune-mediated small nerve fibre loss in AL amyloidosis. Ann. Clin. Transl. Neurol. 2022, 9, 853–863. [Google Scholar] [CrossRef]
| Mean Age ± SD (Years) | Gender (F/M) | M.A.I ± SD (Months) | CNFD n/mm2 | CNFL mm/mm2 | CNBD n/mm2 | CTBD n/mm2 | DCD cells/mm2 | DCA µm2 | DCP µm | CC | Neuromas (Number of Patients) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls n = 28 | 39.4 ± 8.0 | 21/7 | X | 25.0 ± 10.7 | 33.9 ± 21.4 | 15.9 ± 4.2 | 56.8 ± 29.0 | 6.8 ± 9.9 | 30.5 ± 36.3 | 23.6 ± 28.8 | 0.1 ± 0.2 | 0 |
| Long COVID-19 n = 60 | 46.5 ± 8.0 | 53/7 | 23.4 ± 7.7 | 16.3 ± 7.7 | 24.1 ± 17.3 | 13.0 ± 5.8 | 37.1 ± 20.9 | 13.2 ± 17.6 | 95.2 ± 101.6 | 77.5 ± 85.7 | 0.4 ± 0.5 | 9 |
| p Value < 0.05 (*) | 0.065 | * | * | * | 0.1091 | * | 0.2324 | * | * | * |
| YES | NO | p Values | |
|---|---|---|---|
| Vaccinated N(%) | 47.00 (78.33%) | 13.00 (21.77%) | |
| Age (Years) | 47.5 ± 7.8 | 42.7 ± 8.2 | 0.11 |
| CNFD n/mm2 | 17.1 ± 7.8 | 13.6 ± 7.0 | 0.2192 |
| CNBD n/mm2 | 24.8 ± 18.0 | 21.6 ± 14.6 | 0.7738 |
| CNFL mm/mm2 | 12.6 ± 4.3 | 14.5 ± 9.7 | 0.7947 |
| CTBD n/mm2 | 38.5 ± 22.0 | 32.3 ± 16.1 | 0.34 |
| DCD n/mm2 | 10.1 ± 13.7 | 24.5 ± 24.9 | 0.1500 |
| DCA µm2 | 88.9 ± 100.5 | 117.8 ± 106.2 | 0.3171 |
| CDP µm | 65.2 ± 67.7 | 121.8 ± 125.8 | 0.2869 |
| CC | 0.4 ± 0.6 | 0.4 ± 0.5 | 0.83 |
| M.A.I (months) | 23.7 ± 8.4 | 22.5 ± 4.6 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cañadas, P.; Gonzalez-Vides, L.; Alberquilla García-Velasco, M.; Arriola, P.; Guemes-Villahoz, N.; Hernández-Verdejo, J.L. Neuroinflammatory Findings of Corneal Confocal Microscopy in Long COVID-19 Patients, 2 Years after Acute SARS-CoV-2 Infection. Diagnostics 2023, 13, 3188. https://doi.org/10.3390/diagnostics13203188
Cañadas P, Gonzalez-Vides L, Alberquilla García-Velasco M, Arriola P, Guemes-Villahoz N, Hernández-Verdejo JL. Neuroinflammatory Findings of Corneal Confocal Microscopy in Long COVID-19 Patients, 2 Years after Acute SARS-CoV-2 Infection. Diagnostics. 2023; 13(20):3188. https://doi.org/10.3390/diagnostics13203188
Chicago/Turabian StyleCañadas, Pilar, Leonela Gonzalez-Vides, Marta Alberquilla García-Velasco, Pedro Arriola, Noemí Guemes-Villahoz, and Jose Luis Hernández-Verdejo. 2023. "Neuroinflammatory Findings of Corneal Confocal Microscopy in Long COVID-19 Patients, 2 Years after Acute SARS-CoV-2 Infection" Diagnostics 13, no. 20: 3188. https://doi.org/10.3390/diagnostics13203188
APA StyleCañadas, P., Gonzalez-Vides, L., Alberquilla García-Velasco, M., Arriola, P., Guemes-Villahoz, N., & Hernández-Verdejo, J. L. (2023). Neuroinflammatory Findings of Corneal Confocal Microscopy in Long COVID-19 Patients, 2 Years after Acute SARS-CoV-2 Infection. Diagnostics, 13(20), 3188. https://doi.org/10.3390/diagnostics13203188






