Vitiligo Treated with Combined Piperine-Based Topical Treatment and Narrowband Ultraviolet B Therapy: Follow-Up with Reflectance Confocal Microscopy
Abstract
1. Introduction
2. Materials and Methods
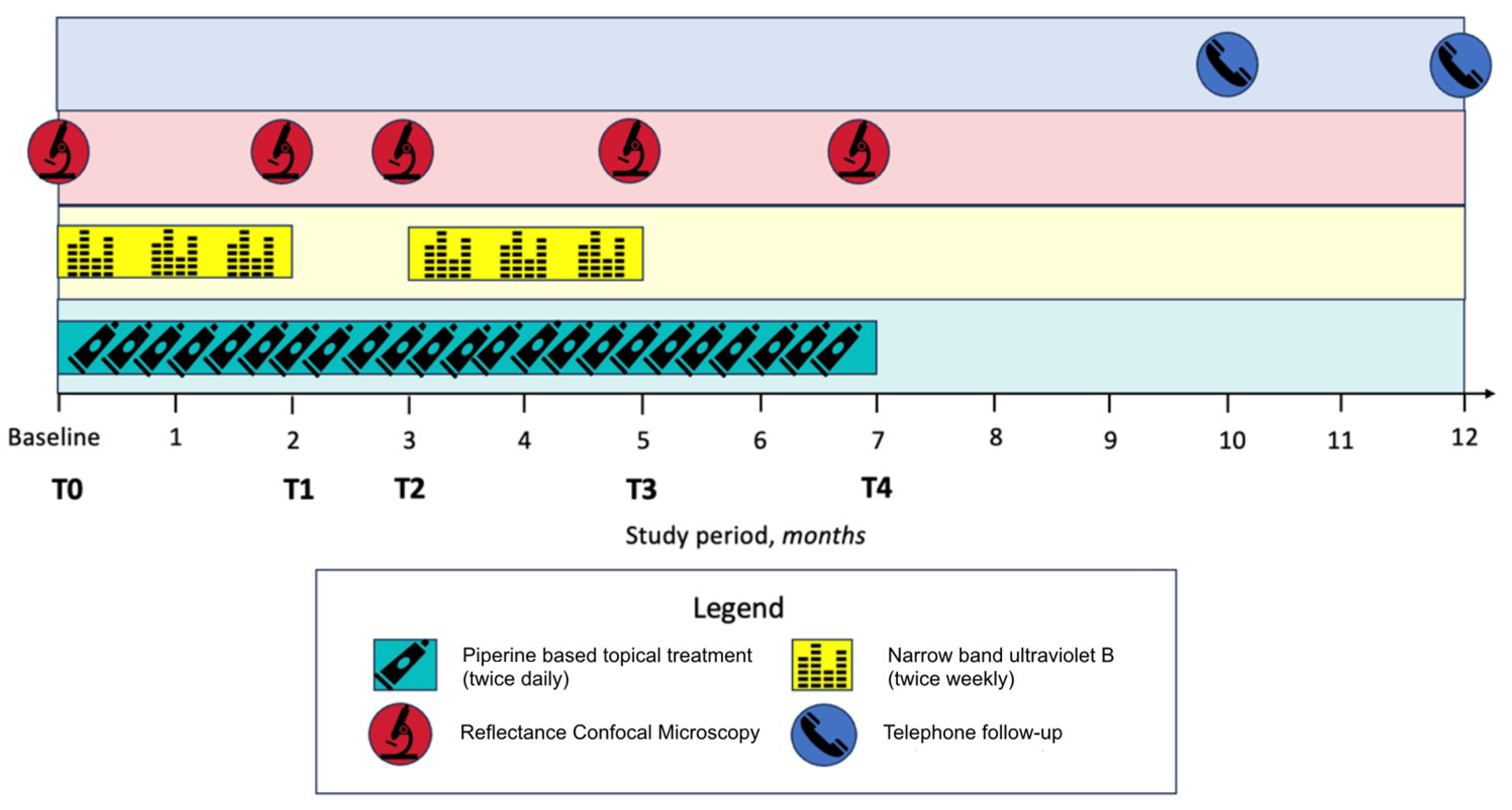
2.1. Study Design, Patient Selection and Treatment Protocol
- The presence or absence of vessels in the chalky-white target macule area;
- The Vitiligo Noticeability Scale (VNS): (1) more- (2) as- (3) slightly less- (4) less-, and (5) no longer-, noticeable [12];
- The percentage of re-pigmentation: (1) 0–24% (2) 25–49% (3) 50–74% (4) 75–100%.
2.2. RCM Imaging
2.3. Treatment Efficacy
2.4. Adverse Events
2.5. Statistical Methods
3. Results
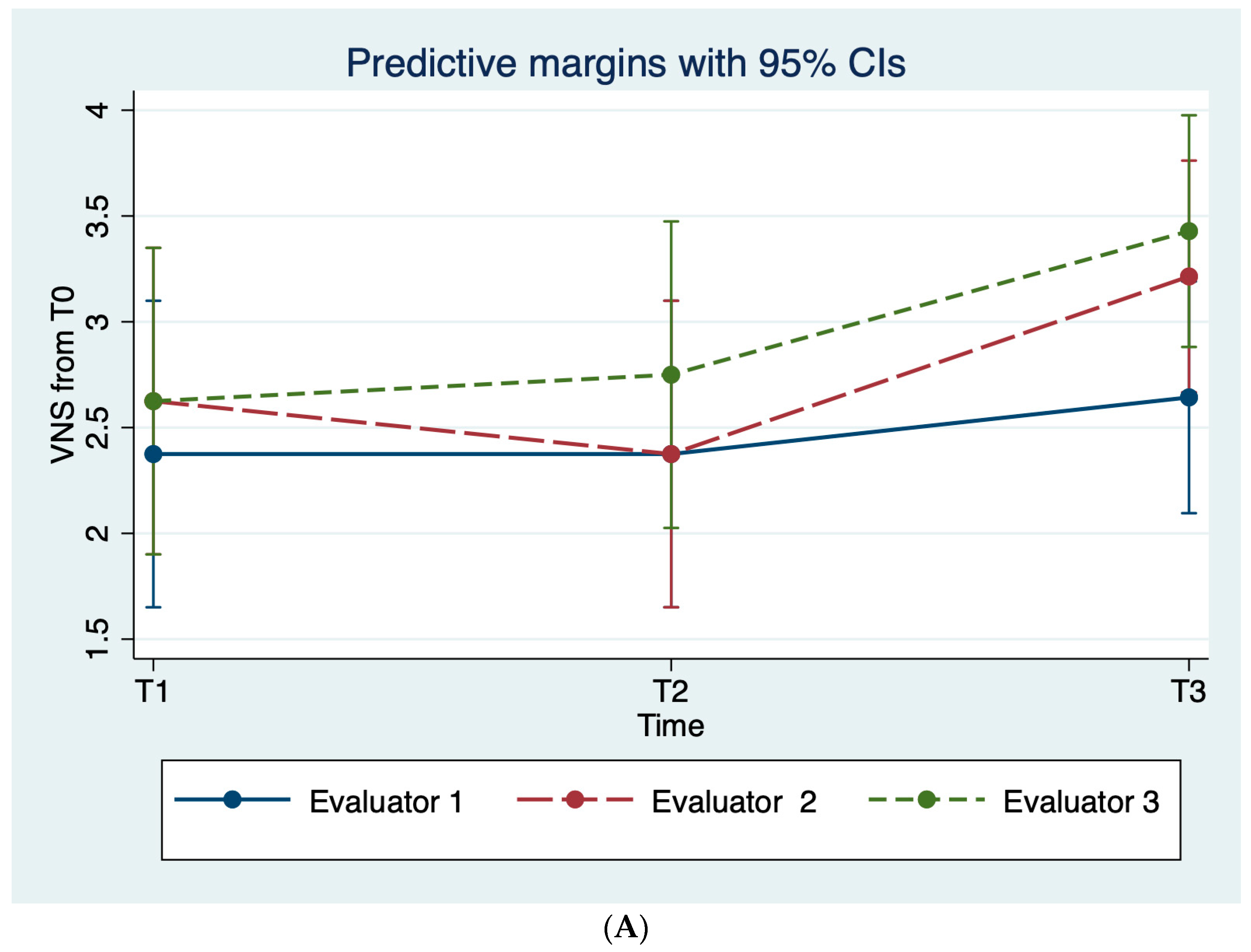
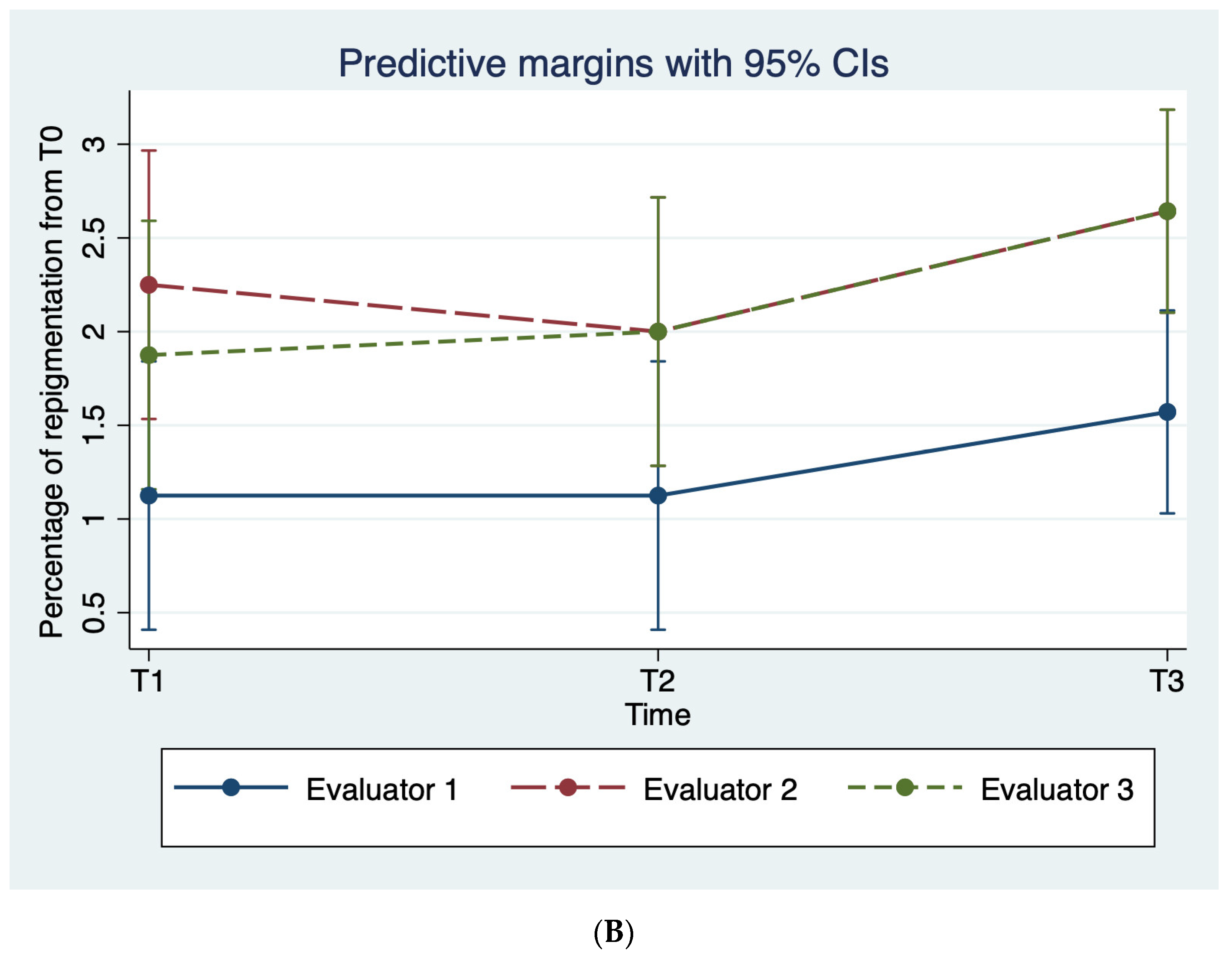
3.1. Clinical Evaluation
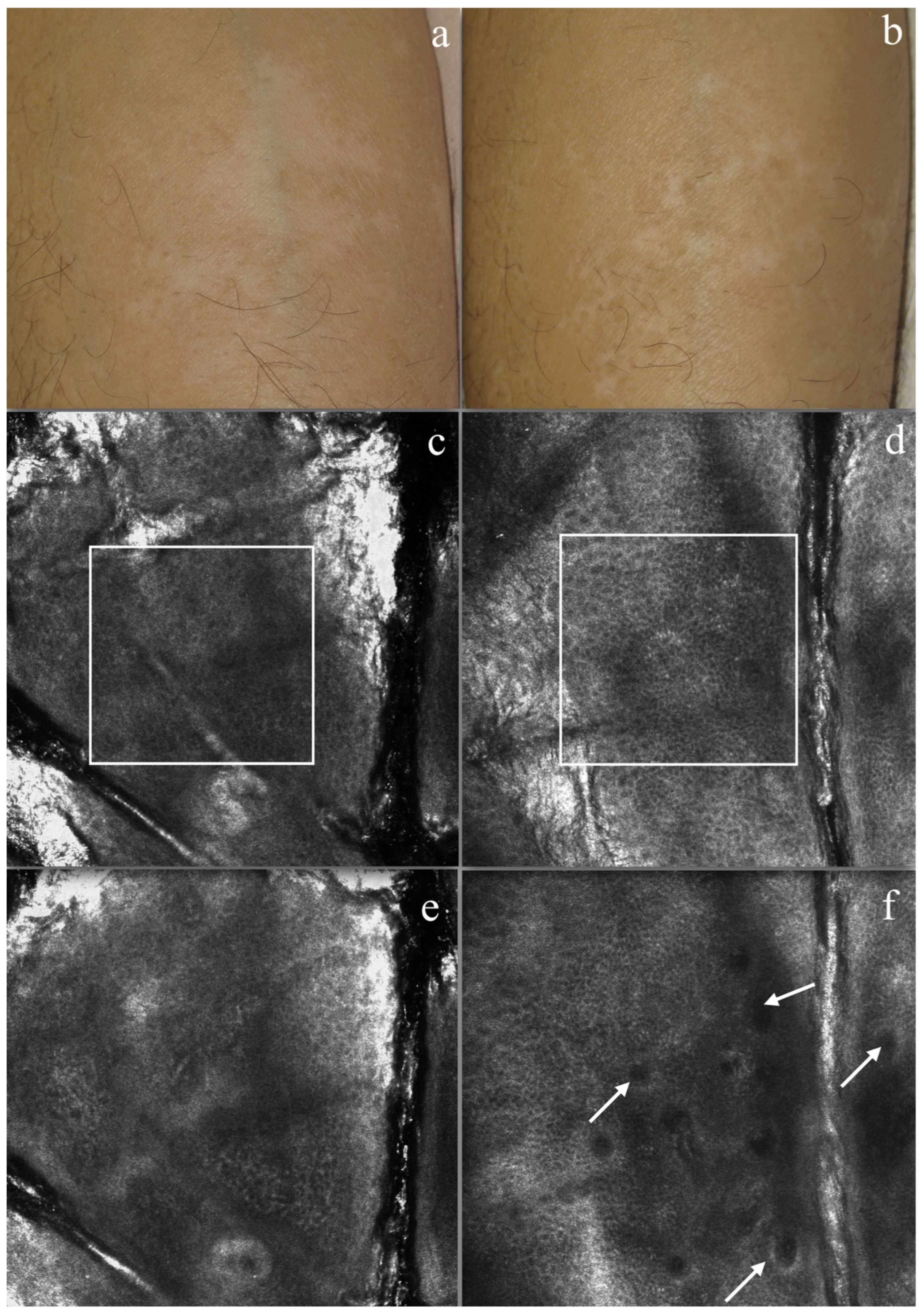
3.2. RCM Evaluation
3.3. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Picardo, M.; Dell’Anna, M.L.; Ezzedine, K.; Hamzavi, I.; Harris, J.E.; Parsad, D.; Taieb, A. Vitiligo. Nat. Rev. Dis. Prim. 2015, 1, 15011. [Google Scholar] [CrossRef]
- Bergqvist, C.; Ezzedine, K. Vitiligo: A Review. Dermatology 2020, 236, 571–592. [Google Scholar] [CrossRef]
- Ezzedine, K.; Lim, H.W.; Suzuki, T.; Katayama, I.; Hamzavi, I.; Lan, C.C.E.; Goh, B.K.; Anbar, T.; de Castro, C.S.; Lee, A.Y.; et al. Revised classification/nomenclature of vitiligo and related issues: The Vitiligo Global Issues Consensus Conference. Pigment. Cell Melanoma Res. 2012, 25, E1–E13. [Google Scholar] [CrossRef]
- Ezzedine, K.; Grimes, P.; Meurant, J.; Seneschal, J.; Léauté-Labrèze, C.; Ballanger, F.; Jouary, T.; Taïeb, C.; Taïeb, A. Living with vitiligo: Results from a national survey indicate differences between skin phototypes. Br. J. Dermatol. 2015, 173, 607–609. [Google Scholar] [CrossRef]
- Kundu, R.V.; Mhlaba, J.M.; Rangel, S.M.; Le Poole, I.C. The convergence theory for vitiligo: A reappraisal. Exp. Dermatol. 2019, 28, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Ongenae, K.; Van Geel, N.; Naeyaert, J. Evidence for an Autoimmune Pathogenesis of Vitiligo. Pigment. Cell Res. 2003, 16, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M.; Chavan, B.; Hafeez, I.; Schallreuter, K.U. Regulation of tyrosinase by tetrahydropteridines and H2O2. Biochem. Biophys. Res. Commun. 2004, 325, 1412–1417. [Google Scholar] [CrossRef]
- Telegina, T.A.; Vechtomova, Y.L.; Borzova, V.A.; Buglak, A.A. Tetrahydrobiopterin as a Trigger for Vitiligo: Phototransformation during UV Irradiation. Int. J. Mol. Sci. 2023, 24, 13586. [Google Scholar] [CrossRef]
- Tavoletti, G.; Avallone, G.; Conforti, C.; Roccuzzo, G.; Maronese, C.A.; Mattioli, M.A.; Quaglino, P.; Zalaudek, I.; Marzano, A.V.; Ribero, S.; et al. Topical ruxolitinib: A new treatment for vitiligo. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadou, V.; Atkar, R.; Batchelor, J.; McDonald, B.; Novakovic, L.; Patel, J.V.; Ravenscroft, J.; Rush, E.; Shah, D.; Shah, R.; et al. British Association of Dermatologists guidelines for the management of people with vitiligo 2021. Br. J. Dermatol. 2022, 186, 18–29. [Google Scholar] [CrossRef]
- Searle, T.; Al-Niaimi, F.; Ali, F.R. Vitiligo: An update on systemic treatments. Clin. Exp. Dermatol. 2020, 46, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, T.F.; Al-Jamal, M.; Hamzavi, I.H.; Harris, J.E.; Leone, G.; Cabrera, R.; Lim, H.W.; Pandya, A.G.; Esmat, S.M. The Vitiligo Working Group recommendations for narrowband ultraviolet B light phototherapy treatment of vitiligo. J. Am. Acad. Dermatol. 2017, 76, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Wu, S.; He, Y.; Nian, Q.; Lei, J.; Yao, Y.; Guo, J.; Zeng, J. Plant-Derived Compounds as Promising Therapeutics for Vitiligo. Front. Pharmacol. 2021, 12, 685116. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anna, M.L.; Mastrofrancesco, A.; Sala, R.; Venturini, M.; Otviani, M.; Vidolin, A.P.; Leone, G.; Calzavara, P.G.; Westerhof, W.; Picardo, M. Antioxidants and narrow band-UVB in the treatment of vitiligo: A double-blind placebo controlled trial. Clin. Exp. Dermatol. 2007, 32, 631–636. [Google Scholar] [CrossRef]
- Srinivasan, K. Black Pepper and its Pungent Principle-Piperine: A Review of Diverse Physiological Effects. Crit. Rev. Food Sci. Nutr. 2007, 47, 735–748. [Google Scholar] [CrossRef]
- Faas, L.; Venkatasamy, R.; Hider, R.; Young, A.; Soumyanath, A. In vivo evaluation of piperine and synthetic analogues as potential treatments for vitiligo using a sparsely pigmented mouse model. Br. J. Dermatol. 2008, 158, 941–950. [Google Scholar] [CrossRef]
- Thomas, K.; Batchelor, J.; Akram, P.; Chalmers, J.; Haines, R.; Meakin, G.; Duley, L.; Ravenscroft, J.; Rogers, A.; Sach, T.; et al. Randomized controlled trial of topical corticosteroid and home-based narrowband ultraviolet B for active and limited vitiligo: Results of the HI-Light Vitiligo Trial. Br. J. Dermatol. 2020, 184, 828–839. [Google Scholar] [CrossRef]
- Shafiee, A.; Hoormand, M.; Shahidi-Dadras, M.; Abadi, A. The effect of topical piperine combined with narrowband UVB on vitiligo treatment: A clinical trial study. Phytotherapy Res. 2018, 32, 1812–1817. [Google Scholar] [CrossRef]
- Batchelor, J.M.; Gran, S.; Leighton, P.; Howells, L.; Montgomery, A.A.; Tan, W.; Ahmed, I.; Thomas, K.S. Using the Vitiligo Noticeability Scale in clinical trials: Construct validity, interpretability, reliability and acceptability. Br. J. Dermatol. 2022, 187, 548–556. [Google Scholar] [CrossRef]
- Pezzini, C.; Kaleci, S.; Chester, J.; Farnetani, F.; Longo, C.; Pellacani, G. Reflectance confocal microscopy diagnostic accuracy for malignant melanoma in different clinical settings: Systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2268–2279. [Google Scholar] [CrossRef]
- Longo, C.; Farnetani, F.; Moscarella, E.; de Pace, B.; Ciardo, S.; Ponti, G.; Piana, S.; Cesinaro, A.M.; Cota, C.; Argenziano, G.; et al. Can noninvasive imaging tools potentially predict the risk of ulceration in invasive melanomas showing blue and black colors? Melanoma Res. 2013, 23, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Ardigo, M.; Malizewsky, I.; Dell’anna, M.; Berardesca, E.; Picardo, M. Preliminary evaluation of vitiligo using in vivo reflectance confocal microscopy. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Jiang, G. Progress in the application of reflectance confocal microscopy in dermatology. Adv. Dermatol. Allergol. 2021, 38, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.G.; Xu, A.E. In vivo reflectance confocal microscopy imaging of vitiligo, nevus depigmentosus and nevus anemicus. Skin Res. Technol. 2011, 17, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Ciardo, S.; Pezzini, C.; Guida, S.; Del Duca, E.; Ungar, J.; Guttman-Yassky, E.; Manfredini, M.; Farnetani, F.; Longo, C.; Pellacani, G. A plea for standardization of confocal microscopy and optical coherence tomography parameters to evaluate physiological and para-physiological skin conditions in cosmetic science. Exp. Dermatol. 2021, 30, 911–922. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Sprent, P.; Smeeton, N.C. Applied Nonparametric Statistical Methods, 3rd ed.; Chapman & Hall/CRC: New York, NY, USA, 2001; p. 463. [Google Scholar]
- Fleiss, J.L. Balanced Incomplete Block Designs for Inter-Rater Reliability Studies. Appl. Psychol. Meas. 1981, 5, 105–112. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Sayed, A.A.; Abdeen, A.; Aleya, L.; Ali, D.; Alkahtane, A.A.; Alarifi, S.; Alkahtani, S. Piperine Enhances the Antioxidant and Anti-Inflammatory Activities of Thymoquinone against Microcystin-LR-Induced Hepatotoxicity and Neurotoxicity in Mice. Oxidative Med. Cell. Longev. 2019, 2019, 1309175. [Google Scholar] [CrossRef]
- Batchelor, J.M.; Thomas, K.S.; Akram, P.; Azad, J.; Bewley, A.; Chalmers, J.R.; Cheung, S.T.; Duley, L.; Eleftheriadou, V.; Ellis, R.; et al. Home-based narrowband UVB, topical corticosteroid or combination for children and adults with vitiligo: HI-Light Vitiligo three-arm RCT. Health Technol. Assess. 2020, 24, 1–128. [Google Scholar] [CrossRef]
| Characteristic, n (%) | Total (n = 8) |
|---|---|
| Female | 3 (37.5) |
| Age, mean ± SD (range) | 48.8 ± 11.7 (33–69) |
| Macule location | |
| Upper/lower limb | 3 (37.5) |
| Trunk | 5 (62.5) |
| Fitzpatrick skin phototype | |
| II | 5 (62.5) |
| III | 2 (25.0) |
| IV | 1 (12.5) |
| Patterns/Features | T0 n = 8 | T1 n = 8 | T2 n = 8 | T3 n = 7 | T4 n = 7 | |
|---|---|---|---|---|---|---|
| Clinical | ||||||
| Chalky white target macule area | No vessels | 7 (87.5) | 1 (12.5) | 1 (12.5) | 2 (28.6) | 2 (28.6) |
| vessels | 1 (12.5) | 7 (87.5) | 7 (87.5) | 5 (71.4) | 5 (71.4) | |
| RCM | ||||||
| Irregular honeycombed pattern * | Absent | 0 (0) | 0 (0) | 2 (25) | 3 (37.5) | 3 (37.5) |
| <25% | 0 (0) | 1 (12.5) | 2 (25) | 2 (25) | 3 (37.5) | |
| 25–50% | 3 (37.5) | 4 (50) | 4 (50) | 2 (25) | 1 (12.5) | |
| >50–75% | 5 (62.5) | 3 (37.5) | 0 (0) | 0 (0) | 0 (0) | |
| Non-pigmented papillae ^° | <25% | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (50) |
| 25–50% | 0 (0) | 1 (12.5) | 3 (37.5) | 5 (62.5) | 2 (25) | |
| >50–75% | 1 (12.5) | 0 (0) | 3 (37.5) | 2 (25) | 1 (12.5) | |
| >75–100% | 7 (87.5) | 7 (87.5) | 2 (25) | 0 (0) | 0 (0) | |
| Dendritic cells | Absent | 8 (100) | 7 (87.5) | 4 (50) | 5 (62.5) | 6 (75) |
| Present | 0 (0) | 1 (12.5) | 4 (50) | 2 (25) | 1 (12.5) | |
| Vessels #$ | Absent | 7 (87.5) | 1 (12.5) | 1 (12.5) | 2 (25) | 2 (25) |
| Present | 1 (12.5) | 7 (87.5) | 7 (87.5) | 5 (62.5) | 5 (62.5) |
| Evaluators | ||||
|---|---|---|---|---|
| Value, Mean ± SD (Range) | 1 | 2 | 3 | |
| VNS | ||||
| T0–T1 | 2.37 ± 0.5 (2–3) | 2.62 ±1.5 (1–5) | 2.62 ± 0.7 (2–4) | |
| T0–T2 | 2.37 ± 0.5 (2–3) | 2.37 ±1.8 (1–4) | 2.75 ± 0.8 (2–4) | |
| T0–T3 | 2.28 ± 0.9 (1–4) | 3.14 ±1.2 (2–5) | 3.42 ± 0.9 (2–5) | |
| T0–T4 | 3.0 ± 0.8 (2–4) | 3.28 ±1.4 (1–5) | 3.42 ± 0.9 (2–5) | |
| Percentage of re-pigmentation | ||||
| T0–T1 | 1.12 ± 0.3 (1–2) | 2.25 ± 1.2 (1–4) | 1.85 ± 0.9 (1–4) | |
| T0–T2 | 1.12 ± 0.3 (1–2) | 2.0 ± 1.0 (1–4) | 2.0 ± 1.9 (1–4) | |
| T0–T3 | 1.42 ± 0.7 (1–3) | 2.57 ± 1.1 (1–4) | 2.57 ± 1.2 (1–4) | |
| T0–T4 | 1.71 ± 0.9 (1–3) | 2.71 ± 1.1 (1–4) | 2.71 ± 1.3 (1–4) | |
| Evaluators | |||
|---|---|---|---|
| Study Time Points, κ | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 |
| T0–T1 | 0.2258 | 0.6000 | 0.4286 |
| 0.0769 | 0.1304 | 0.6842 | |
| T0–T2 | 0.2258 | 0.5000 | 0.6667 |
| 0.0968 | 0.1250 | 0.7838 | |
| T0–T3 | 0.3226 | 0.1765 | 0.7586 |
| 0.1765 | 0.1714 | 0.5484 | |
| T0–T4 | 0.3438 | 0.5532 | 0.6769 |
| 0.2687 | 0.3288 | 0.7812 | |
| Overall mean agreement | 0.2171 | 0.3232 | 0.6661 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertoli, C.; Chester, J.; Cortelazzi, C.; Ciardo, S.; Manfredini, M.; Di Nuzzo, S.; Kaleci, S.; Pellacani, G.; Farnetani, F. Vitiligo Treated with Combined Piperine-Based Topical Treatment and Narrowband Ultraviolet B Therapy: Follow-Up with Reflectance Confocal Microscopy. Diagnostics 2024, 14, 494. https://doi.org/10.3390/diagnostics14050494
Bertoli C, Chester J, Cortelazzi C, Ciardo S, Manfredini M, Di Nuzzo S, Kaleci S, Pellacani G, Farnetani F. Vitiligo Treated with Combined Piperine-Based Topical Treatment and Narrowband Ultraviolet B Therapy: Follow-Up with Reflectance Confocal Microscopy. Diagnostics. 2024; 14(5):494. https://doi.org/10.3390/diagnostics14050494
Chicago/Turabian StyleBertoli, Cristina, Johanna Chester, Chiara Cortelazzi, Silvana Ciardo, Marco Manfredini, Sergio Di Nuzzo, Shaniko Kaleci, Giovanni Pellacani, and Francesca Farnetani. 2024. "Vitiligo Treated with Combined Piperine-Based Topical Treatment and Narrowband Ultraviolet B Therapy: Follow-Up with Reflectance Confocal Microscopy" Diagnostics 14, no. 5: 494. https://doi.org/10.3390/diagnostics14050494
APA StyleBertoli, C., Chester, J., Cortelazzi, C., Ciardo, S., Manfredini, M., Di Nuzzo, S., Kaleci, S., Pellacani, G., & Farnetani, F. (2024). Vitiligo Treated with Combined Piperine-Based Topical Treatment and Narrowband Ultraviolet B Therapy: Follow-Up with Reflectance Confocal Microscopy. Diagnostics, 14(5), 494. https://doi.org/10.3390/diagnostics14050494







