Computed Tomography-Based Radiomics Using Tumor and Vessel Features to Assess Resectability in Cancer of the Pancreatic Head
Abstract
:1. Introduction
2. Materials and Methods
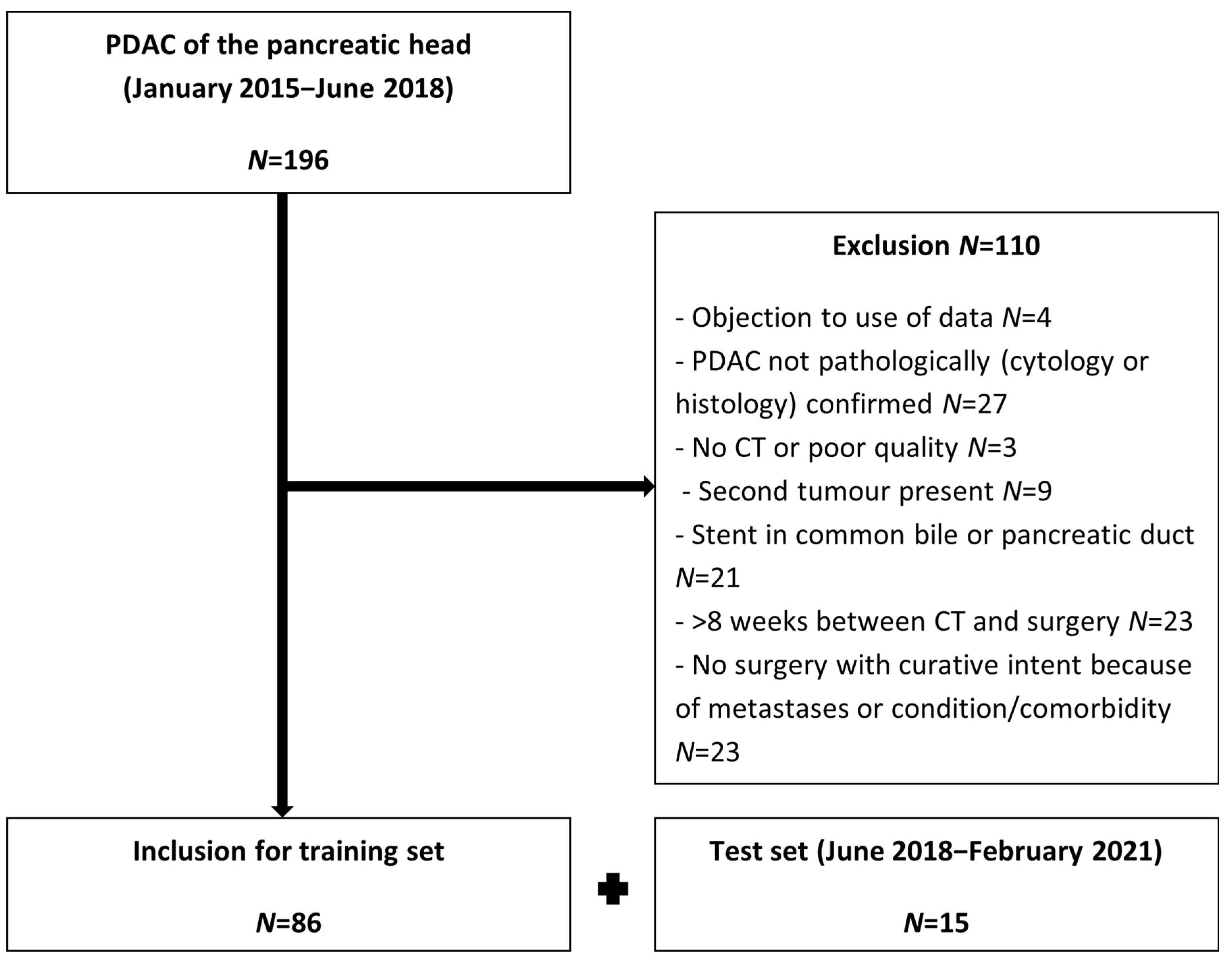
2.1. Study Population
2.2. Clinical Information
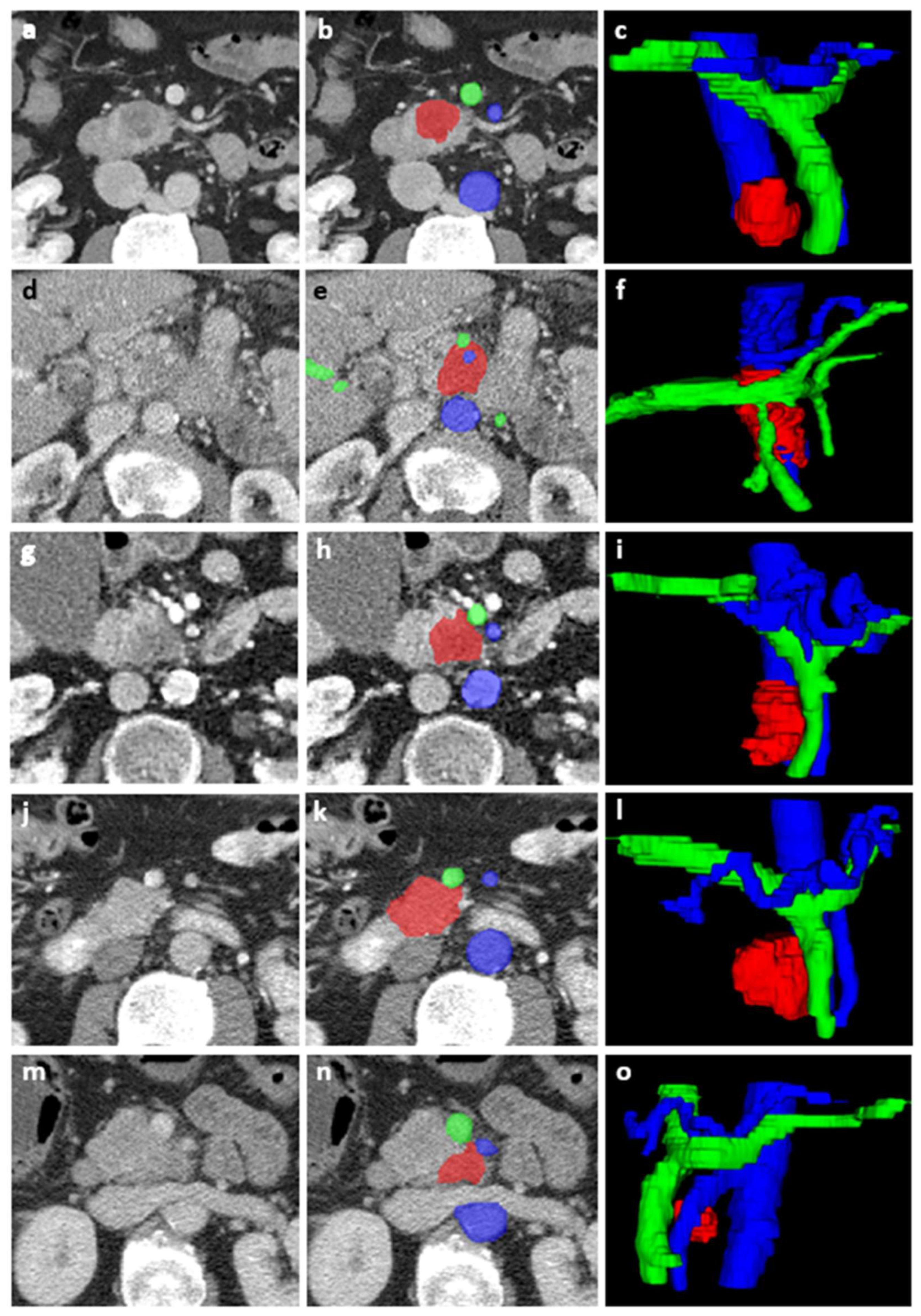
2.3. Image Acquisition and Segmentation
2.4. Tumor Features
2.5. Vessel Features
2.6. Model Development
2.7. Subset Analyses: Resection Margin Status
3. Results
3.1. Patients
3.2. Feature Extraction and Selection
3.3. Subset Analyses: Resection Margin Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Raman, S.P.; Reddy, S.; Weiss, M.J.; Manos, L.L.; Cameron, J.L.; Zheng, L.; Herman, J.M.; Hruban, R.H.; Fishman, E.K.; Wolfgang, C.L. Impact of the time interval between MDCT imaging and surgery on the accuracy of identifying metastatic disease in patients with pancreatic cancer. AJR Am. J. Roentgenol. 2015, 204, W37–W42. [Google Scholar] [CrossRef]
- van der Geest, L.G.M.; Lemmens, V.; de Hingh, I.; van Laarhoven, C.; Bollen, T.L.; Nio, C.Y.; van Eijck, C.H.J.; Busch, O.R.C.; Besselink, M.G. Nationwide outcomes in patients undergoing surgical exploration without resection for pancreatic cancer. Br. J. Surg. 2017, 104, 1568–1577. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Asbun, H.; Bain, A.; Behrman, S.W.; Benson, A.B., 3rd; Cardin, D.B.; Cha, C.; Chiorean, E.G.; et al. Pancreatic Adenocarcinoma; Version 1.2020; NCCN Clinical Practice Guidelines in Oncology; National Comprehensive Cancer Network (NCCN): New York, NY, USA, 2019. [Google Scholar]
- Versteijne, E.; van Eijck, C.H.; Punt, C.J.; Suker, M.; Zwinderman, A.H.; Dohmen, M.A.; Groothuis, K.B.; Busch, O.R.; Besselink, M.G.; de Hingh, I.H.; et al. Preoperative radiochemotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC trial): Study protocol for a multicentre randomized controlled trial. Trials 2016, 17, 127. [Google Scholar] [CrossRef]
- Isaji, S.; Murata, Y.; Kishiwada, M. New Japanese Classification of Pancreatic Cancer. In Pancreatic Cancer; Neoptolemos, J.P., Urrutia, R., Abbruzzese, J., Büchler, M.W., Eds.; Springer: New York, NY, USA, 2017; pp. 1–17. [Google Scholar] [CrossRef]
- Joo, I.; Lee, J.M.; Lee, E.S.; Son, J.Y.; Lee, D.H.; Ahn, S.J.; Chang, W.; Lee, S.M.; Kang, H.J.; Yang, H.K. Preoperative CT Classification of the Resectability of Pancreatic Cancer: Interobserver Agreement. Radiology 2019, 293, 343–349. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Attiyeh, M.A.; Chakraborty, J.; Doussot, A.; Langdon-Embry, L.; Mainarich, S.; Gonen, M.; Balachandran, V.P.; D’Angelica, M.I.; DeMatteo, R.P.; Jarnagin, W.R.; et al. Survival Prediction in Pancreatic Ductal Adenocarcinoma by Quantitative Computed Tomography Image Analysis. Ann. Surg. Oncol. 2018, 25, 1034–1042. [Google Scholar] [CrossRef]
- Cheng, S.H.; Cheng, Y.J.; Jin, Z.Y.; Xue, H.D. Unresectable pancreatic ductal adenocarcinoma: Role of CT quantitative imaging biomarkers for predicting outcomes of patients treated with chemotherapy. Eur. J. Radiol. 2019, 113, 188–197. [Google Scholar] [CrossRef]
- Bian, Y.; Jiang, H.; Ma, C.; Cao, K.; Fang, X.; Li, J.; Wang, L.; Zheng, J.; Lu, J. Performance of CT-based radiomics in diagnosis of superior mesenteric vein resection margin in patients with pancreatic head cancer. Abdom. Radiol. 2020, 45, 759–773. [Google Scholar] [CrossRef]
- Hui, B.; Qiu, J.J.; Liu, J.H.; Ke, N.W. Identification of Pancreaticoduodenectomy Resection for Pancreatic Head Adenocarcinoma: A Preliminary Study of Radiomics. Comput. Math. Methods Med. 2020, 2020, 2761627. [Google Scholar] [CrossRef] [PubMed]
- Rigiroli, F.; Hoye, J.; Lerebours, R.; Lafata, K.J.; Li, C.; Meyer, M.; Lyu, P.; Ding, Y.; Schwartz, F.R.; Mettu, N.B.; et al. CT Radiomic Features of Superior Mesenteric Artery Involvement in Pancreatic Ductal Adenocarcinoma: A Pilot Study. Radiology 2021, 301, 610–622. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, Y.; Qi, X.; Zhang, R.; Gao, X.; Xia, W.; Zhang, L. Radiomics-Assisted Presurgical Prediction for Surgical Portal Vein-Superior Mesenteric Vein Invasion in Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2020, 10, 523543. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Shi, Y.; Cao, K.; Lu, L.; Lu, J.; Song, Q.; Jin, G.; Xiao, J.; Hou, Y.; Zhang, L. DeepPrognosis: Preoperative prediction of pancreatic cancer survival and surgical margin via comprehensive understanding of dynamic contrast-enhanced CT imaging and tumor-vascular contact parsing. Med. Image Anal. 2021, 73, 102150. [Google Scholar] [CrossRef] [PubMed]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- McGraw, K.; Wong, S.P. Forming Inferences About Some Intraclass Correlation Coefficients. Psychol. Methods 1996, 1, 30–46. [Google Scholar] [CrossRef]
- Wong, J.; Baine, M.; Wisnoskie, S.; Bennion, N.; Zheng, D.; Yu, L.; Dalal, V.; Hollingsworth, M.A.; Lin, C.; Zheng, D. Effects of interobserver and interdisciplinary segmentation variabilities on CT-based radiomics for pancreatic cancer. Sci. Rep. 2021, 11, 16328. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection via the Lasso. J. R. Stat. Soc. Ser. B Methodol. 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.; Cairns, A.; Duthie, F.; Feakins, R. Dataset for the Histopathological Reporting of Carcinoma of the Pancreas, Ampulla of Vater and Common Bile Duct; The Royal College of Pathologists: London, UK, 2017. [Google Scholar]
- Kirkegård, J.; Aahlin, E.K.; Al-Saiddi, M.; Bratlie, S.O.; Coolsen, M.; de Haas, R.J.; den Dulk, M.; Fristrup, C.; Harrison, E.M.; Mortensen, M.B.; et al. Multicentre study of multidisciplinary team assessment of pancreatic cancer resectability and treatment allocation. Br. J. Surg. 2019, 106, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Y.; Luo, M.; Sun, Y.W.; Xu, Q.; Chen, W.; Zhao, G.; Wu, Z.Y. Computed tomography in diagnosing vascular invasion in pancreatic and periampullary cancers: A systematic review and meta-analysis. Hepatobiliary Pancreat. Dis. Int. 2009, 8, 457–464. [Google Scholar] [PubMed]
- Yang, R.; Lu, M.; Qian, X.; Chen, J.; Li, L.; Wang, J.; Zhang, Y. Diagnostic accuracy of EUS and CT of vascular invasion in pancreatic cancer: A systematic review. J. Cancer Res. Clin. Oncol. 2014, 140, 2077–2086. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, D.; Chen, Y.; Li, X.; Cao, P.; Cao, D. Network meta-analysis comparing neoadjuvant chemoradiation, neoadjuvant chemotherapy and upfront surgery in patients with resectable, borderline resectable, and locally advanced pancreatic ductal adenocarcinoma. Radiat. Oncol. 2019, 14, 120. [Google Scholar] [CrossRef]
- Xu, J.; Zhan, H.; Li, F.; Hu, S.; Wang, L. Neoadjuvant therapy for pancreatic cancer: Limitations and advances of response assessment (Review). Oncol. Rep. 2021, 45, 26. [Google Scholar] [CrossRef]
- Jang, J.K.; Byun, J.H.; Kang, J.H.; Son, J.H.; Kim, J.H.; Lee, S.S.; Kim, H.J.; Yoo, C.; Kim, K.P.; Hong, S.M.; et al. CT-determined resectability of borderline resectable and unresectable pancreatic adenocarcinoma following FOLFIRINOX therapy. Eur. Radiol. 2021, 31, 813–823. [Google Scholar] [CrossRef]
- Katz, M.H.; Fleming, J.B.; Bhosale, P.; Varadhachary, G.; Lee, J.E.; Wolff, R.; Wang, H.; Abbruzzese, J.; Pisters, P.W.; Vauthey, J.N.; et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012, 118, 5749–5756. [Google Scholar] [CrossRef]
- Donahue, T.R.; Isacoff, W.H.; Hines, O.J.; Tomlinson, J.S.; Farrell, J.J.; Bhat, Y.M.; Garon, E.; Clerkin, B.; Reber, H.A. Downstaging Chemotherapy and Alteration in the Classic Computed Tomography/Magnetic Resonance Imaging Signs of Vascular Involvement in Patients With Pancreaticobiliary Malignant Tumors: Influence on Patient Selection for Surgery. Arch. Surg. 2011, 146, 836–843. [Google Scholar] [CrossRef]
- Marchegiani, G.; Todaro, V.; Boninsegna, E.; Negrelli, R.; Sureka, B.; Bonamini, D.; Salvia, R.; Manfredi, R.; Pozzi Mucelli, R.; Bassi, C. Surgery after FOLFIRINOX treatment for locally advanced and borderline resectable pancreatic cancer: Increase in tumour attenuation on CT correlates with R0 resection. Eur. Radiol. 2018, 28, 4265–4273. [Google Scholar] [CrossRef]
- Kim, B.R.; Kim, J.H.; Ahn, S.J.; Joo, I.; Choi, S.-Y.; Park, S.J.; Han, J.K. CT prediction of resectability and prognosis in patients with pancreatic ductal adenocarcinoma after neoadjuvant treatment using image findings and texture analysis. Eur. Radiol. 2019, 29, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Altabella, L.; Simoni, N.; Benetti, G.; Rossi, R.; Venezia, M.; Paiella, S.; Malleo, G.; Salvia, R.; Guariglia, S.; et al. Computed tomography-based radiomic to predict resectability in locally advanced pancreatic cancer treated with chemotherapy and radiotherapy. World J. Gastrointest. Oncol. 2022, 14, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The facts and the challenges of image analysis. Eur. Radiol. Exp. 2018, 2, 36. [Google Scholar] [CrossRef]
- Yamashita, R.; Perrin, T.; Chakraborty, J.; Chou, J.F.; Horvat, N.; Koszalka, M.A.; Midya, A.; Gonen, M.; Allen, P.; Jarnagin, W.R.; et al. Radiomic feature reproducibility in contrast-enhanced CT of the pancreas is affected by variabilities in scan parameters and manual segmentation. Eur. Radiol. 2020, 30, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Da-ano, R.; Masson, I.; Lucia, F.; Doré, M.; Robin, P.; Alfieri, J.; Rousseau, C.; Mervoyer, A.; Reinhold, C.; Castelli, J.; et al. Performance comparison of modified ComBat for harmonization of radiomic features for multicenter studies. Sci. Rep. 2020, 10, 10248. [Google Scholar] [CrossRef]
- Verbeke, C.S. Resection margins in pancreatic cancer. Surg. Clin. N. Am. 2013, 93, 647–662. [Google Scholar] [CrossRef]
- Alves, N.; Schuurmans, M.; Litjens, G.; Bosma, J.S.; Hermans, J.; Huisman, H. Fully Automatic Deep Learning Framework for Pancreatic Ductal Adenocarcinoma Detection on Computed Tomography. Cancers 2022, 14, 376. [Google Scholar] [CrossRef]
- Cassinotto, C.; Chong, J.; Zogopoulos, G.; Reinhold, C.; Chiche, L.; Lafourcade, J.P.; Cuggia, A.; Terrebonne, E.; Dohan, A.; Gallix, B. Resectable pancreatic adenocarcinoma: Role of CT quantitative imaging biomarkers for predicting pathology and patient outcomes. Eur. J. Radiol. 2017, 90, 152–158. [Google Scholar] [CrossRef]
- Sandrasegaran, K.; Lin, Y.; Asare-Sawiri, M.; Taiyini, T.; Tann, M. CT texture analysis of pancreatic cancer. Eur. Radiol. 2019, 29, 1067–1073. [Google Scholar] [CrossRef]
- Yun, G.; Kim, Y.H.; Lee, Y.J.; Kim, B.; Hwang, J.H.; Choi, D.J. Tumor heterogeneity of pancreas head cancer assessed by CT texture analysis: Association with survival outcomes after curative resection. Sci. Rep. 2018, 8, 7226. [Google Scholar] [CrossRef]
- Kulkarni, A.; Carrion-Martinez, I.; Jiang, N.N.; Puttagunta, S.; Ruo, L.; Meyers, B.M.; Aziz, T.; van der Pol, C.B. Hypovascular pancreas head adenocarcinoma: CT texture analysis for assessment of resection margin status and high-risk features. Eur. Radiol. 2020, 30, 2853–2860. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Carrion-Martinez, I.; Dhindsa, K.; Alaref, A.A.; Rozenberg, R.; van der Pol, C.B. Pancreas adenocarcinoma CT texture analysis: Comparison of 3D and 2D tumor segmentation techniques. Abdom. Radiol. 2021, 46, 1027–1033. [Google Scholar] [CrossRef] [PubMed]



| Training Set (N = 86) | Test Set (N = 15) | Total (N = 101) | p-Value | |
|---|---|---|---|---|
| Age in years, mean (SD) | 71.0 (8.6) | 68.6 (9.6) | 70.6 (8.7) | 0.34 |
| Sex, N (%) | 0.1 | |||
| Male | 49 (57%) | 5 (33%) | 54 (53%) | |
| Female | 37 (43%) | 10 (67%) | 47 (47%) | |
| Resectability according to MDT, N (%) | 0.8 | |||
| Resectable | 25 (29%) 1 | 5 (33%) | 30 (30%) | |
| Borderline | 14 (16%) 1 | 3 (20%) | 17 (17%) | |
| Irresectable | 47 (54%) 1 | 7 (47%) | 54 (53%) | |
| CT-reported tumor size in mm, mean (SD) | 33.4 (15.3) | 32.6 (11.2) | 33.3 (14.7) | 0.84 |
| Time between CT and surgery in days, median (range) | 32 (8–49) | 25 (19–53) | 30 (8–53) | 0.27 |
| Surgery N (%) | 41 (48%) | 8 (53%) | 49 (49%) 1 | 0.72 |
| Resected | 31 (76%) | 7 (88%) 1 | 38 (78%) | |
| Not resected | 10 (24%) | 1 (13%) 1 | 11 (22%) | |
| No surgery | 45 (52%) | 7 (47%) | 52 (52%) 1 |
| (1) Tumor Features Model | (2) Vessel Features Model | (3) Combined Features Model |
|---|---|---|
| Shape-based—Least Axis Length | Venous Encasement Angle | Venous Encasement Angle |
| Shape-based—Major Axis Length | Arterial Encasement Angle | Arterial Encasement Angle |
| First-Order Statistics—10th Percentile | Venous Contact Length | Venous Contact Length |
| Gray Level Co-occurrence Matrix—Autocorrelation | Gray Level Dependence Matrix—Dependence Entropy | |
| Gray Level Co-occurrence Matrix—Inverse Variance | ||
| Gray Level Dependence matrix—Dependence Entropy |
| Model | Sensitivity | p-Value Sensitivity Model vs. MDT | Specificity | p-Value Specificity Model vs. MDT | PPV | NPV | AUC |
|---|---|---|---|---|---|---|---|
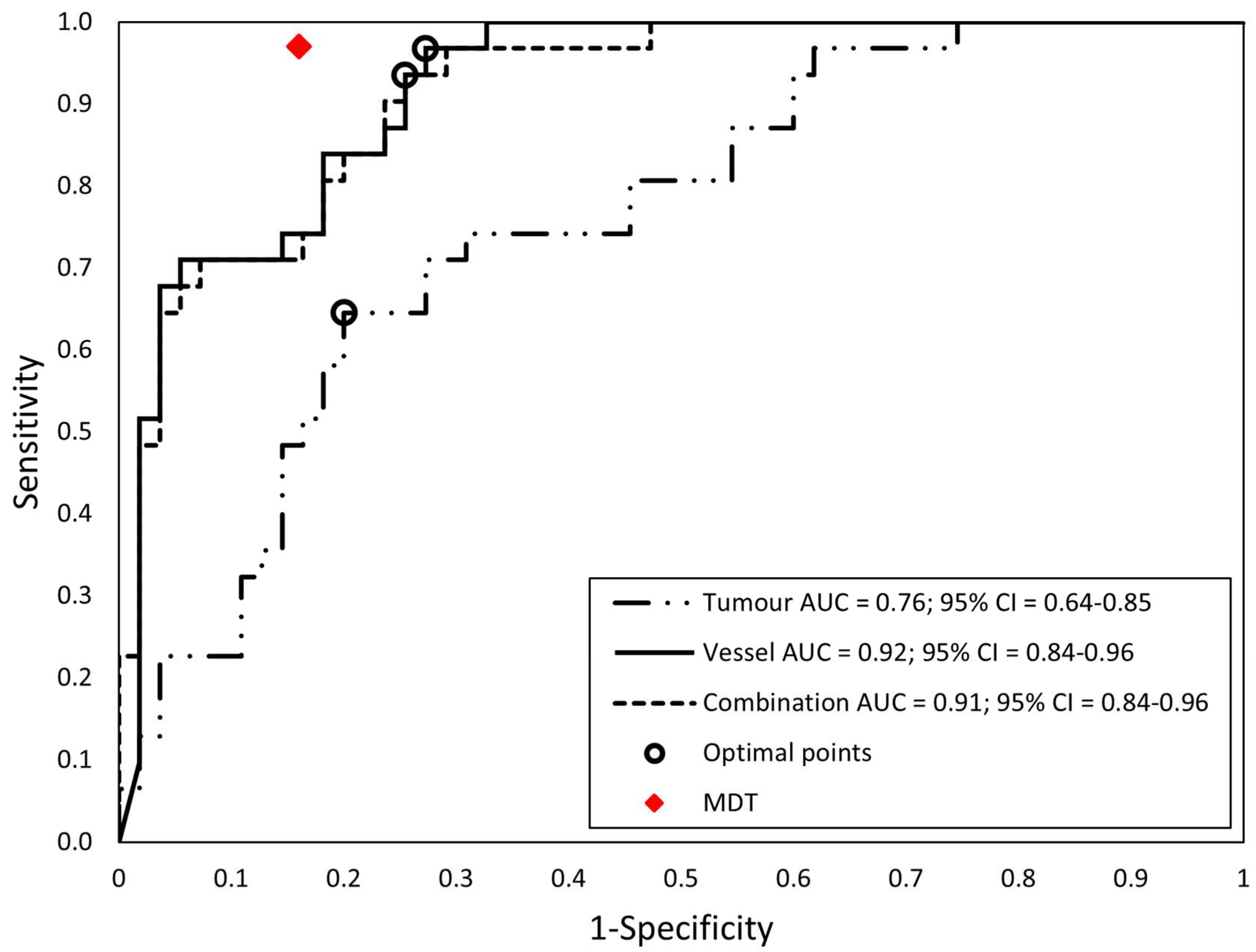
| (1) Tumor features | 0.002 * | 0.625 | 0.76 (0.64–0.85) | ||||
| Training set, N = 86 | 65% (45–81%) | 80% (67–90%) | 65% (50-77%) | 80% (71–87%) | |||
| Test set, N = 15 | 86% (42–100%) | 75% (35–97%) | 75% (47–91%) | 86% (48–97%) | |||
| (2) Vessel features | 1.000 | 0.031 * | 0.92 (0.84–0.96) | ||||
| Training set N = 86 | 97% (83–100%) | 88% (47–100%) | 67% (56–76%) | 98% (85–100%) | |||
| Test set, N = 15 | 100% (59–100%) | 73% (59–84%) | 88% (53–98%) | 100% (−) | |||
| (3) Combined features | 1.000 | 0.063 | 0.91 (0.84–0.96) | ||||
| Training set, N = 86 | 94% (79–99%) | 75% (61–85%) | 67% (57–77%) | 95% (84–99%) | |||
| Test set, N = 15 | 100% (59–100%) | 88% (47–100%) | 88% (53–98%) | 100% (−) | |||
| MDT | |||||||
| Training set, N = 86 | 97% (83–100%) | 84% (71–92%) | 77% (65–86%) | 98% (86–100%) | |||
| Test set, N = 15 | 88% (47–100%) | 100% (59–100%) | 100% (−) | 88% (53–98%) |
| Model | Sensitivity | Specificity | PPV | NPV | AUC |
|---|---|---|---|---|---|
| (1) Tumor features | 0.63 (0.42–0.82) | ||||
| Training set, N = 31 | 100% (78–100%) | 38% (15–65%) | 60% (51–69%) | 100% (−) | |
| Test set, N = 7 | 80% (28–99%) | 0% (0–84%) | 67% (56–76%) | − | |
| (2) Vessel features | 0.67 (0.43–0.85) | ||||
| Training set, N = 31 | 80% (52–96%) | 56% (30–80%) | 63% (48–76%) | 75% (50–90%) | |
| Test set, N = 7 | 80% (28–99%) | 100% (16–100%) | 100% (−) | 67% (26–92%) | |
| (3) Combined features | 0.73 (0.51–0.89) | ||||
| Training set N = 31 | 53% (27–79%) | 88% (62–98%) | 80% (50–94%) | 67% (53–78%) | |
| Test set, N = 7 | 80% (28–99%) | 50% (1–99%) | 80% (48–94%) | 50% (10–90%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litjens, G.; Broekmans, J.P.E.A.; Boers, T.; Caballo, M.; van den Hurk, M.H.F.; Ozdemir, D.; van Schaik, C.J.; Janse, M.H.A.; van Geenen, E.J.M.; van Laarhoven, C.J.H.M.; et al. Computed Tomography-Based Radiomics Using Tumor and Vessel Features to Assess Resectability in Cancer of the Pancreatic Head. Diagnostics 2023, 13, 3198. https://doi.org/10.3390/diagnostics13203198
Litjens G, Broekmans JPEA, Boers T, Caballo M, van den Hurk MHF, Ozdemir D, van Schaik CJ, Janse MHA, van Geenen EJM, van Laarhoven CJHM, et al. Computed Tomography-Based Radiomics Using Tumor and Vessel Features to Assess Resectability in Cancer of the Pancreatic Head. Diagnostics. 2023; 13(20):3198. https://doi.org/10.3390/diagnostics13203198
Chicago/Turabian StyleLitjens, Geke, Joris P. E. A. Broekmans, Tim Boers, Marco Caballo, Maud H. F. van den Hurk, Dilek Ozdemir, Caroline J. van Schaik, Markus H. A. Janse, Erwin J. M. van Geenen, Cees J. H. M. van Laarhoven, and et al. 2023. "Computed Tomography-Based Radiomics Using Tumor and Vessel Features to Assess Resectability in Cancer of the Pancreatic Head" Diagnostics 13, no. 20: 3198. https://doi.org/10.3390/diagnostics13203198
APA StyleLitjens, G., Broekmans, J. P. E. A., Boers, T., Caballo, M., van den Hurk, M. H. F., Ozdemir, D., van Schaik, C. J., Janse, M. H. A., van Geenen, E. J. M., van Laarhoven, C. J. H. M., Prokop, M., de With, P. H. N., van der Sommen, F., & Hermans, J. J. (2023). Computed Tomography-Based Radiomics Using Tumor and Vessel Features to Assess Resectability in Cancer of the Pancreatic Head. Diagnostics, 13(20), 3198. https://doi.org/10.3390/diagnostics13203198









