Study Regarding the Monitoring of Nocturnal Bruxism in Children and Adolescents Using Bruxoff Device
Abstract
:1. Introduction
2. Materials and Methods
- -
- Number of episodes of bruxism per night (SB/night);
- -
- Number of masseter muscle contractions during one night (MC/night);
- -
- Number of phasic contractions (phasic RMMA), tonic contractions (tonic RMMA), and mixed contractions (mixed RMMA);
- -
- Episodes of bruxism per hour (SB/h);
- -
- The patient’s sleep time.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lobbezoo, F.; Ahlberg, J.; Raphael, K.G.; Wetselaar, P.; Glaros, A.G.; Kato, T.; Santiago, V.; Winocur, E.; De Laat, A.; De Leeuw, R.; et al. International consensus on the assessment of bruxism: Report of a work in progress. J. Oral Rehabil. 2018, 45, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Antonio, A.G.; da Silva Pierro, V.S.; Maia, L.C. Bruxism in Children: A Warning Sign for Psychological Problems. J. Can. Dent. Assoc. 2006, 72, 155–160. [Google Scholar] [PubMed]
- Lavigne, G.J.; Khoury, S.; Abe, S.; Yamaguchi, T.; Raphael, K. Bruxism physiology and pathology: An overview for clinicians. J. Oral Rehabil. 2008, 35, 476–494. [Google Scholar] [CrossRef]
- Saczuk, K.; Lapinska, B.; Wilmont, P.; Pawlak, L.; Lukomska-Szymanska, M. The Bruxoff Device as a Screening Method for Sleep Bruxism in Dental Practice. J. Clin. Med. 2019, 8, 930. [Google Scholar] [CrossRef]
- Thorpy, M.J. ICSD—International Classification of Sleep Disorders: Diagnostic and Coding Manual; Diagnostic Classification Steering Committee, American Sleep Disorders Association: Darien, IL, USA, 1990. [Google Scholar]
- Cordray, F.E. The Relationship between Occlusion and TMD. Open J. Stomatol. 2017, 7, 35–80. [Google Scholar] [CrossRef]
- Klasser, G.D.; Rei, N.; Lavigne, G.J. Sleep bruxism etiology: The evolution of a changing paradigm. J. Can. Dent. Assoc. 2015, 81, f2. [Google Scholar]
- Serra-Negra, J.M.; Tirsa-Costa, D.; Guimarães, F.H.; Paiva, S.M.; Pordeus, I.A. Evaluation of parents/guardian knowledge about the bruxism of their children: Family knowledge of bruxism. J. Indian Soc. Pedod. Prev. Dent. 2013, 31, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Ierardo, G.; Mazur, M.; Luzzi, V.; Calcagnile, F.; Ottolenghi, L.; Polimeni, A. Treatments of sleep bruxism in children: A systematic review and meta-analysis. Cranio 2021, 39, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Carra, M.C.; Huynh, N.; Lavigne, G. Sleep bruxism: A compre- hensive overview for the dental clinician interested in sleep medicine. Dent. Clin. N. Am. 2012, 56, 387–413. [Google Scholar] [CrossRef]
- Castroflorio, T.; Mesin, L.; Tartaglia, G.M.; Sforza, C.; Farina, D. Use of electromyographic and electrocardiographic signals to detect sleep bruxism episodes in a natural environment. IEEE J. Biomed. Health Inform. 2013, 17, 994–1001. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Abe, S.; Rompré, P.H.; Manzini, C.; Lavigne, G.J. Comparison of ambulatory and polysomnographic recording of jaw muscle activity during sleep in normal subjects. J. Oral Rehabil. 2012, 39, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Vlăduțu, D.E.; Ionescu, M.; Mercuț, R.; Noveri, L.; Lăzărescu, G.; Popescu, S.M.; Scrieciu, M.; Manolea, H.O.; Iacov Crăițoiu, M.M.; Ionescu, A.G.; et al. Ecological Momentary Assessment of Masseter Muscle Activity in Patients with Bruxism. Int. J. Environ. Res. Public Health 2022, 20, 581. [Google Scholar] [CrossRef] [PubMed]
- User Manual of Bruxoff Device. Available online: https://www.bruxoff.com/wp-content/uploads/2012/10/Bruxoff-Users-Manual-v.1.1.pdf (accessed on 19 September 2023).
- Manfredini, D.; Ahlberg, J.; Castroflorio, T.; Poggio, C.E.; Guarda-Nardini, L.; Lobbezoo, F. Diagnostic accuracy of portable instrumental devices to measure sleep bruxism: A systematic literature review of polysomnographic studies. J. Oral Rehabil. 2014, 41, 836–842. [Google Scholar] [CrossRef]
- Lavigne, G.J.; Huynh, N.; Kato, T.; Okura, K.; Adachi, K.; Yao, D.; Sessle, B. Genesis of sleep bruxism: Motor and autonomic-cardiac interactions. Arch. Oral Biol. 2007, 52, 381–384. [Google Scholar] [CrossRef]
- Castroflorio, T.; Deregibus, A.; Bargellini, A.; Debernardi, C.; Manfredini, D. Detection of sleep bruxism: Comparison between an electromyographic and electrocardiographic portable holter and polysomnography. J. Oral Rehabil. 2014, 41, 163–169. [Google Scholar] [CrossRef]
- Carra, M.C.; Huynh, N.; Lavigne, G.J. Diagnostic accuracy of sleep bruxism scoring in absence of audio-video recording: A pilot study. Sleep Breath. 2015, 19, 183–190. [Google Scholar] [CrossRef]
- Farina, D.; Cescon, C. Concentric-ring electrode systems for noninvasive detection of single motor unit activity. IEEE Trans. Biomed. Eng. 2001, 48, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Baad-Hansen, L.; Thymi, M.; Lobbezoo, F.; Svensson, P. To what extent is bruxism associated with musculoskeletal signs and symptoms? A systematic review. J. Oral Rehabil. 2019, 46, 845–861. [Google Scholar] [CrossRef]
- Wetselaar, P.; Vermaire, E.J.H.; Lobbezoo, F.; Schuller, A.A. The prevalence of awake bruxism and sleep bruxism in the Dutch adult population. J. Oral Rehabil. 2019, 46, 617–623. [Google Scholar] [CrossRef]
- Wieckiewicz, M.; Zietek, M.; Smardz, J.; Zenczak-Wieckiewicz, D.; Grychowska, N. Mental status as a common factor for masticatory muscle pain: A systematic review. Front. Psychol. 2017, 8, 646. [Google Scholar] [CrossRef]
- Manfredini, D.; Winocur, E.; Guarda-Nardini, L.; Paesani, D.; Lobbezoo, F. Epidemiology of bruxism in adults: A systematic review of the literature. J. Orofac. Pain 2013, 27, 99–110. [Google Scholar] [CrossRef]
- Khoury, S.; Carra, M.C.; Huynh, N.; Montplaisir, J.; Lavigne, G.J. Sleep Bruxism-Tooth Grinding Prevalence, Characteristics and Familial Aggregation: A Large Cross-Sectional Survey and Polysomnographic Validation. Sleep 2016, 39, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Emodi Perlman, A.; Lobbezoo, F.; Zar, A.; Friedman Rubin, P.; van Selms, M.K.A.; Winocur, E. Self-Reported bruxism and associated factors in Israeli adolescents. J. Oral Rehabil. 2016, 43, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, P.; Carpinelli, L.; Savarese, G. Perceived stress and bruxism in university students. BMC Res. Notes 2016, 9, 514. [Google Scholar] [CrossRef]
- Shetty, S.; Pitti, V.; Babu, C.L.S.; Kumar, G.P.S.; Deepthi, B.C. Bruxism: A literature review. J. Indian Prosthodont. Soc. 2010, 10, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Deregibus, A.; Castroflorio, T.; Bargellini, A.; Debernardi, C. Reliability of a portable device for the detection of sleep bruxism. Clin. Oral Investig. 2014, 18, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- De La Hoz-Aizpurua, J.L.; Díaz-Alonso, E.; Latouche-Arbizu, R.; Mesa-Jiménez, J. Sleep bruxism. Conceptual review and update. Med. Oral Patol. Oral Cir. Bucal 2011, 16, 231–239. [Google Scholar] [CrossRef]
- Yoshida, Y.; Suganuma, T.; Takaba, M.; Ono, Y.; Abe, Y.; Yoshizawa, S.; Sakai, T.; Yoshizawa, A.; Nakamura, H.; Kawana, F.; et al. Association between patterns of jaw motor activity during sleep and clinical signs and symptoms of sleep bruxism. J. Sleep Res. 2017, 26, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Svensson, P.; Jadidi, F.; Arima, T.; Baad-Hansen, L.; Sessle, B.J. Relationships between craniofacial pain and bruxism. J. Oral Rehabil. 2008, 35, 524–547. [Google Scholar] [CrossRef]
- Castroflorio, T.; Bargellini, A.; Rossini, G.; Cugliari, G.; Deregibus, A.; Manfredini, D. Agreement between clinical and portable EMG/ECG diagnosis of sleep bruxism. J. Oral Rehabil. 2015, 42, 759–764. [Google Scholar] [CrossRef]
- Pattussi, M.P.; Grossi, M.L.; Fagondes, S.C.; Saueressig, A.C.; Mainieri, V.C. Validation of the Bitestrip versus polysomnography in the diagnosis of patients with a clinical history of sleep bruxism. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 612–617. [Google Scholar]
- Shochat, T.; Gavish, A.; Arons, E.; Hadas, N.; Molotsky, A.; Lavie, P.; Oksenberg, A. Validation of the BiteStrip screener for sleep bruxism. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 104, 32–39. [Google Scholar] [CrossRef]
- Yachida, W.; Arima, T.; Castrillon, E.E.; Baad-Hansen, L.; Ohata, N.; Svensson, P. Diagnostic validity of self-reported measures of sleep bruxism using an ambulatory single-channel EMG device. J. Prosthodont. Res. 2016, 60, 250–257. [Google Scholar] [CrossRef]
- Needham, R.; Davies, S.J. Use of the Grindcare® device in the management of nocturnal bruxism: A pilot study. Br. Dent. J. 2013, 215, E1. [Google Scholar] [CrossRef]
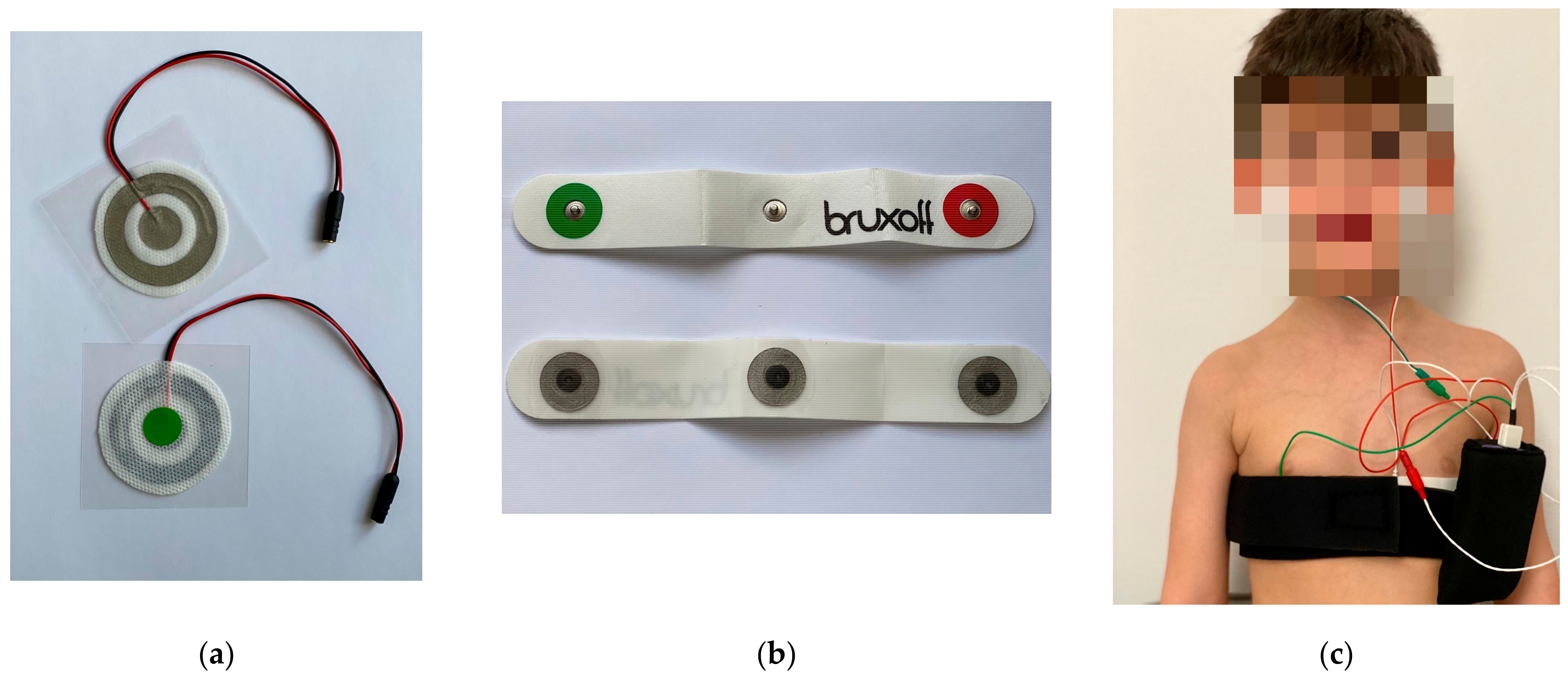
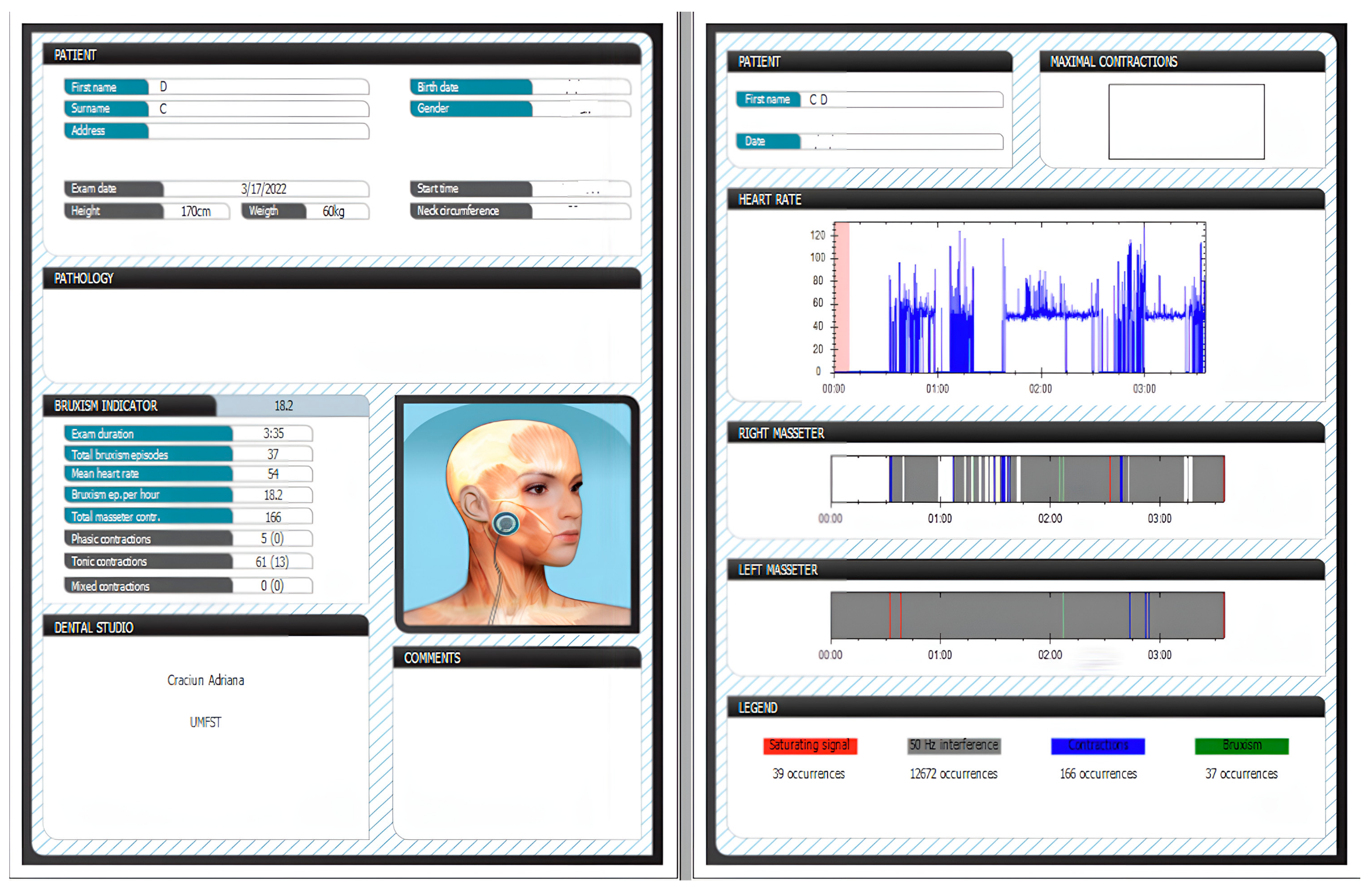
| Age | Sleeping Time (min.) | SB/night | Heart Rate | SB/h | MC/night | Phasic RMMA | Tonic RMMA | Mixed RMMA | |
|---|---|---|---|---|---|---|---|---|---|
| Minimum | 5.000 | 58.00 | 0.000 | 57.00 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Median | 11.00 | 71.00 | 8.000 | 77.00 | 4.000 | 33.00 | 8.000 | 8.500 | 1.000 |
| Maximum | 16.00 | 592.0 | 237.0 | 98.00 | 54.00 | 448.0 | 147.0 | 176.0 | 117.0 |
| Mean | 10.08 | 200.6 | 42.29 | 77.97 | 12.30 | 124.0 | 34.47 | 47.47 | 16.31 |
| Std. Deviation | 3.451 | 201.4 | 71.22 | 12.97 | 14.88 | 154.2 | 45.73 | 63.22 | 32.40 |
| Lower 95% CI of mean | 8.916 | 162.2 | 28.70 | 75.50 | 9.462 | 94.56 | 25.75 | 35.41 | 10.13 |
| Upper 95% CI of mean | 11.25 | 239.1 | 55.87 | 80.45 | 15.14 | 153.4 | 43.20 | 59.53 | 22.49 |
| Age | Sleeping Time (min.) | SB/night | Heart Rate | SB/h | MC/night | Phasic RMMA | Tonic RMMA | Mixed RMMA | |
|---|---|---|---|---|---|---|---|---|---|
| Minimum | 5.000 | 60.00 | 0.000 | 54.00 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Median | 15.00 | 75.00 | 0.000 | 74.00 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Maximum | 19.00 | 455.0 | 50.00 | 86.00 | 24.60 | 225.0 | 15.00 | 83.00 | 15.00 |
| Mean | 12.47 | 179.4 | 15.47 | 72.33 | 5.467 | 59.27 | 3.867 | 25.33 | 3.000 |
| Std. Deviation | 5.617 | 152.0 | 19.62 | 10.38 | 8.378 | 79.84 | 5.375 | 32.13 | 5.625 |
| Lower 95% CI of mean | 9.356 | 133.7 | 9.573 | 69.21 | 2.950 | 35.28 | 2.252 | 15.68 | 1.310 |
| Upper 95% CI of mean | 15.58 | 225.1 | 21.36 | 75.45 | 7.984 | 83.25 | 5.482 | 34.99 | 4.690 |
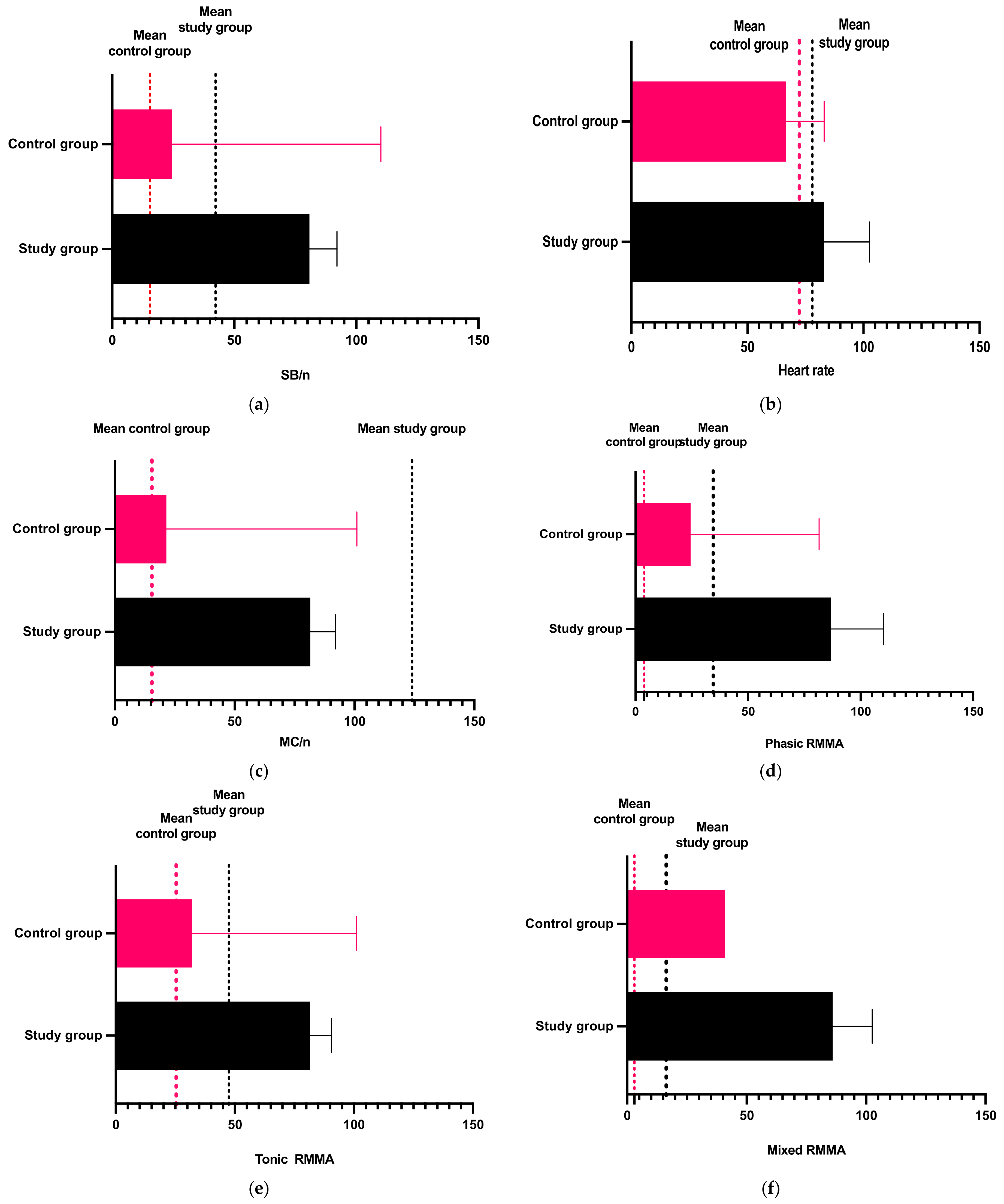
| Control Group | SB/night | Heart Rate | MC/night | Phasic RMMA | Tonic RMMA | Mixed RMMA | |
|---|---|---|---|---|---|---|---|
| Study Group | |||||||
| p value | 0.0069 | 0.0228 | 0.0001 | <0.0001 | 0.0069 | 0.0017 | |
| Statistic significance | ** | * | *** | **** | ** | ** | |
| Mann-Whitney U | 1769 | 1863 | 1499 | 1301 | 1782 | 1710 | |
| Difference between medians | −8.000 | −3.000 | −33.000 | −8.000 | −8.500 | −1.000 | |
| Parameters | r | p Value | Summary | |
|---|---|---|---|---|
| SB/night | Heart rate | 0.1795 | 0.0631 | Weak positive correlation ns |
| MC/night | 0.8765 | <0.0001 | Strong positive correlation **** | |
| Phasic RMMA | 0.8754 | <0.0001 | Strong positive correlation **** | |
| Tonic RMMA | 0.9201 | <0.0001 | Strong positive correlation **** | |
| Mixed RMMA | 0.8021 | <0.0001 | Strong positive correlation **** | |
| Heart rate | MC/night | −0.0497 | 0.6095 | Negative correlation/no correlation ns |
| Phasic RMMA | −0.1821 | 0.0592 | Negative correlation/no correlation ns | |
| Tonic RMMA | −0.0287 | 0.7681 | Negative correlation/no correlation ns | |
| Mixed RMMA | −0.1463 | 0.1309 | Negative correlation/no correlation ns | |
| MC/night | Phasic RMMA | 0.9400 | <0.0001 | Strong positive correlation **** |
| Tonic RMMA | 0.9475 | <0.0001 | Strong positive correlation **** | |
| Mixed RMMA | 0.8580 | <0.0001 | Strong positive correlation **** | |
| Phasic RMMA | Tonic RMMA | 0.9448 | <0.0001 | Strong positive correlation **** |
| Mixed RMMA | 0.9081 | <0.0001 | Strong positive correlation **** | |
| Tonic RMMA | Mixed RMMA | 0.9156 | <0.0001 | Strong positive correlation **** |
| Minimum | Median | Maximum | Mean | Std. Deviation | Lower 95% CI of Mean | Upper 95% CI of Mean | ||
|---|---|---|---|---|---|---|---|---|
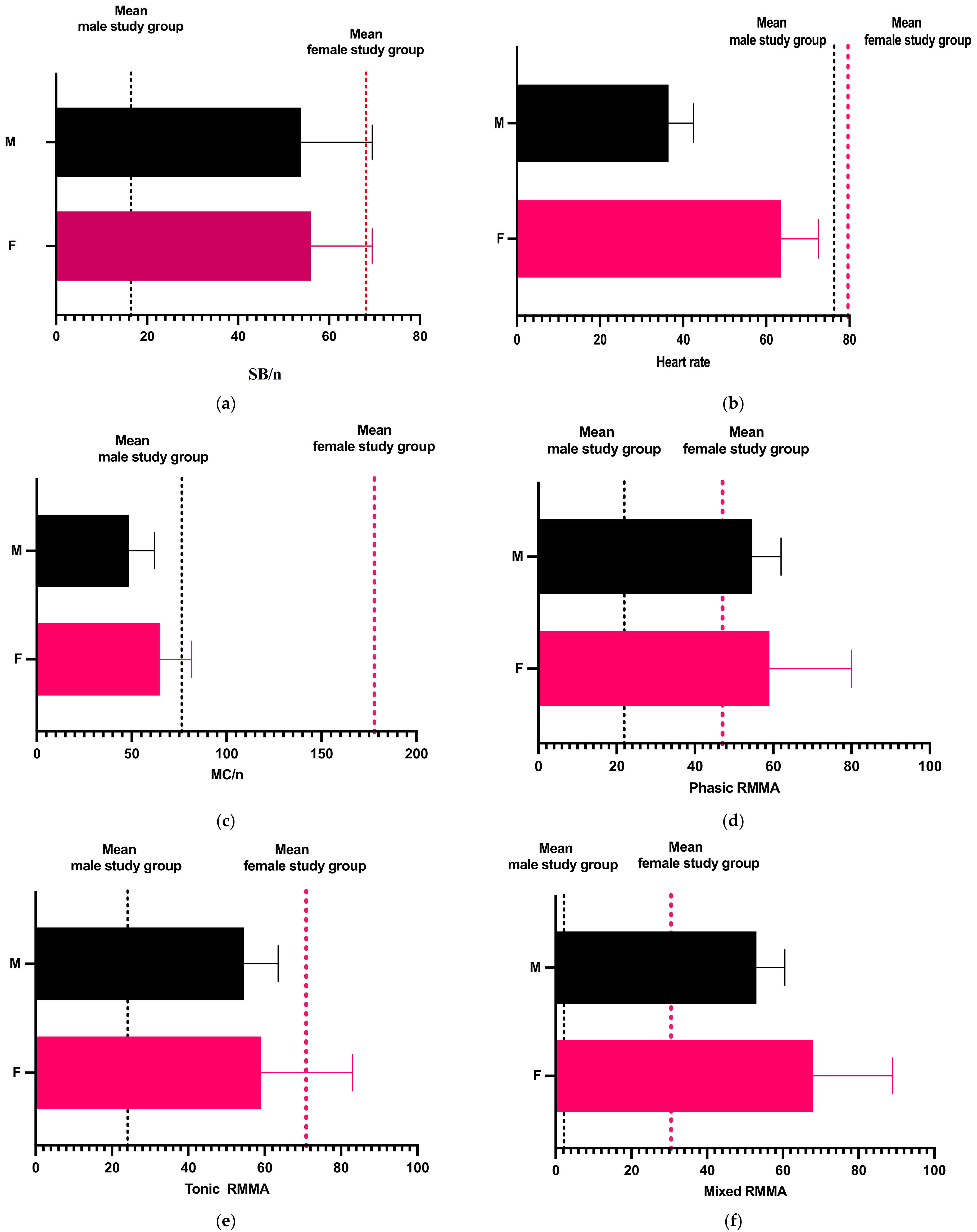
| Female study group | Age | 6 | 12 | 16 | 11.33 | 3.009 | 10.51 | 12.15 |
| Sleeping time (min.) | 60 | 239.5 | 592 | 293.8 | 233.9 | 229.9 | 357.6 | |
| SB/night | 0 | 10.5 | 237 | 68.11 | 92.57 | 42.84 | 93.38 | |
| Heart rate | 57 | 80.5 | 98 | 79.61 | 12.28 | 76.26 | 82.96 | |
| SB/h | 0 | 5.75 | 25.1 | 10.08 | 10.36 | 7.256 | 12.91 | |
| MC/night | 0 | 157.5 | 448 | 177.9 | 176.6 | 129.7 | 226.1 | |
| Phasic RMMA | 0 | 26.5 | 147 | 47.06 | 54.16 | 32.27 | 61.84 | |
| Tonic RMMA | 0 | 55 | 176 | 70.83 | 72.78 | 50.97 | 90.7 | |
| Mixed RMMA | 0 | 12.5 | 117 | 30.44 | 41.29 | 19.17 | 41.71 | |
| Male study group | Age | 5 | 8.5 | 14 | 8.833 | 3.369 | 7.914 | 9.753 |
| Sleeping time (min.) | 58 | 66 | 341 | 107.5 | 97.37 | 80.92 | 134.1 | |
| SB/night | 0 | 8 | 55 | 16.46 | 17.66 | 11.64 | 21.28 | |
| Heart rate | 58 | 72 | 95 | 76.33 | 13.55 | 72.63 | 80.03 | |
| SB/h | 0 | 4 | 54 | 14.52 | 18.16 | 9.561 | 19.47 | |
| MC/night | 0 | 27 | 318 | 70.06 | 104.4 | 41.56 | 98.55 | |
| Phasic RMMA | 0 | 8 | 96 | 21.89 | 31.09 | 13.4 | 30.37 | |
| Tonic RMMA | 0 | 8.5 | 123 | 24.11 | 40.75 | 12.99 | 35.23 | |
| Mixed RMMA | 0 | 1 | 8 | 2.167 | 2.612 | 1.454 | 2.88 |
| Male Study Group | SB/night | Heart Rate | MC/night | Phasic RMMA | Tonic RMMA | Mixed RMMA | |
|---|---|---|---|---|---|---|---|
| Female Study Group | |||||||
| p value | 0.5457 | 0.0433 | 0.0307 | 0.4243 | 0.0973 | 0.0174 | |
| Statistical significance | ns | * | * | ns | ns | * | |
| Mann–Whitney U | 1359 | 1130 | 1107 | 1328 | 1193 | 1089 | |
| Difference between medians | −2.500 | −8.500 | −130.5 | −18.50 | −46.50 | −11.50 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crăciun, A.E.; Cerghizan, D.; Jánosi, K.M.; Popșor, S.; Bica, C.I. Study Regarding the Monitoring of Nocturnal Bruxism in Children and Adolescents Using Bruxoff Device. Diagnostics 2023, 13, 3233. https://doi.org/10.3390/diagnostics13203233
Crăciun AE, Cerghizan D, Jánosi KM, Popșor S, Bica CI. Study Regarding the Monitoring of Nocturnal Bruxism in Children and Adolescents Using Bruxoff Device. Diagnostics. 2023; 13(20):3233. https://doi.org/10.3390/diagnostics13203233
Chicago/Turabian StyleCrăciun, Adriana Elena, Diana Cerghizan, Kinga Mária Jánosi, Sorin Popșor, and Cristina Ioana Bica. 2023. "Study Regarding the Monitoring of Nocturnal Bruxism in Children and Adolescents Using Bruxoff Device" Diagnostics 13, no. 20: 3233. https://doi.org/10.3390/diagnostics13203233
APA StyleCrăciun, A. E., Cerghizan, D., Jánosi, K. M., Popșor, S., & Bica, C. I. (2023). Study Regarding the Monitoring of Nocturnal Bruxism in Children and Adolescents Using Bruxoff Device. Diagnostics, 13(20), 3233. https://doi.org/10.3390/diagnostics13203233






