Deep-Learning-Based Automated Rotator Cuff Tear Screening in Three Planes of Shoulder MRI
Abstract
1. Introduction
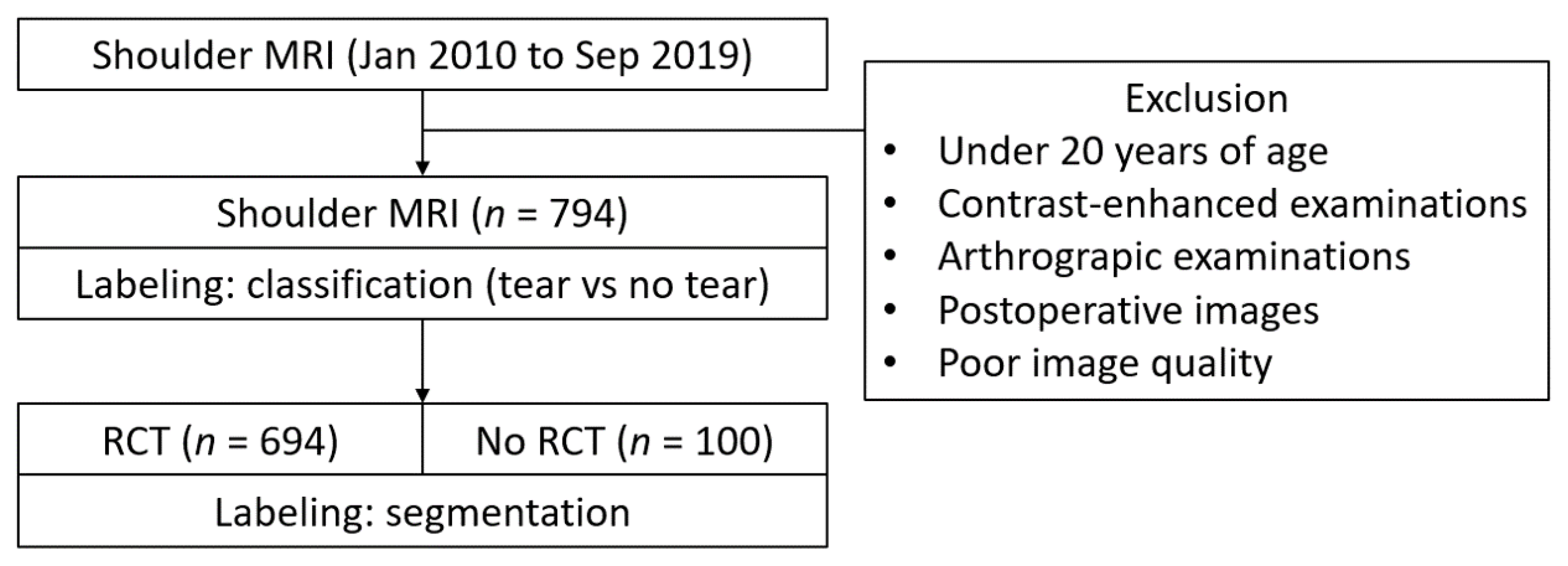
2. Materials and Methods
2.1. Image Labeling
2.2. Model Implementation
2.3. Statistical Analysis
3. Results
3.1. Subject Demographics
3.2. Performance of the Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maruvada, S.; Madrazo-Ibarra, A.; Varacallo, M. Anatomy, Rotator Cuff. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Zoga, A.C.; Kamel, S.I.; Hynes, J.P.; Kavanagh, E.C.; O’Connor, P.J.; Forster, B.B. The Evolving Roles of MRI and Ultrasound in First-Line Imaging of Rotator Cuff Injuries. AJR Am. J. Roentgenol. 2021, 217, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Takagishi, K.; Osawa, T.; Yanagawa, T.; Nakajima, D.; Shitara, H.; Kobayashi, T. Prevalence and risk factors of a rotator cuff tear in the general population. J. Shoulder Elbow Surg. 2010, 19, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Via, A.G.; De Cupis, M.; Spoliti, M.; Oliva, F. Clinical and biological aspects of rotator cuff tears. Muscles Ligaments Tendons J. 2013, 3, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Jaap Willems, W. Rotator cuff tear: A detailed update. Asia Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2015, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.Y.; Kwon, Y.S.; Choi, S. Current Concepts and Recent Trends in Arthroscopic Treatment of Large to Massive Rotator Cuff Tears: A Review. Clin. Shoulder Elb. 2019, 22, 50–57. [Google Scholar] [CrossRef]
- Morag, Y.; Jacobson, J.A.; Miller, B.; De Maeseneer, M.; Girish, G.; Jamadar, D. MR imaging of rotator cuff injury: What the clinician needs to know. RadioGraphics 2006, 26, 1045–1065. [Google Scholar] [CrossRef]
- Sharma, G.; Bhandary, S.; Khandige, G.; Kabra, U. MR Imaging of Rotator Cuff Tears: Correlation with Arthroscopy. J. Clin. Diagn. Res. 2017, 11, TC24–TC27. [Google Scholar] [CrossRef]
- Ahn, K.S.; Bae, B.; Jang, W.Y.; Lee, J.H.; Oh, S.; Kim, B.H.; Lee, S.W.; Jung, H.W.; Lee, J.W.; Sung, J.; et al. Assessment of rapidly advancing bone age during puberty on elbow radiographs using a deep neural network model. Eur. Radiol. 2021, 31, 8947–8955. [Google Scholar] [CrossRef]
- Lee, K.C.; Choi, I.C.; Kang, C.H.; Ahn, K.S.; Yoon, H.; Lee, J.J.; Kim, B.H.; Shim, E. Clinical Validation of an Artificial Intelligence Model for Detecting Distal Radius, Ulnar Styloid, and Scaphoid Fractures on Conventional Wrist Radiographs. Diagnostics 2023, 13, 1657. [Google Scholar] [CrossRef]
- Zhang, B.; Jia, C.; Wu, R.; Lv, B.; Li, B.; Li, F.; Du, G.; Sun, Z.; Li, X. Improving rib fracture detection accuracy and reading efficiency with deep learning-based detection software: A clinical evaluation. Br. J. Radiol. 2021, 94, 20200870. [Google Scholar] [CrossRef]
- Saeed, M.U.; Dikaios, N.; Dastgir, A.; Ali, G.; Hamid, M.; Hajjej, F. An automated deep learning approach for spine segmentation and vertebrae recognition using computed tomography images. Diagnostics 2023, 13, 2658. [Google Scholar] [CrossRef] [PubMed]
- Medina, G.; Buckless, C.G.; Thomasson, E.; Oh, L.S.; Torriani, M. Deep learning method for segmentation of rotator cuff muscles on MR images. Skeletal Radiol. 2021, 50, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Familiari, F.; Galasso, O.; Massazza, F.; Mercurio, M.; Fox, H.; Srikumaran, U.; Gasparini, G. Artificial intelligence in the management of rotator cuff tears. Int. J. Environ. Res. Public Health 2022, 19, 16779. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ro, K.; You, S.; Nam, B.R.; Yook, S.; Park, H.S.; Yoo, J.C.; Park, E.; Cho, K.; Cho, B.H.; et al. Development of an automatic muscle atrophy measuring algorithm to calculate the ratio of supraspinatus in supraspinous fossa using deep learning. Comput. Methods Programs Biomed. 2019, 182, 105063. [Google Scholar] [CrossRef]
- Ro, K.; Kim, J.Y.; Park, H.; Cho, B.H.; Kim, I.Y.; Shim, S.B.; Choi, I.Y.; Yoo, J.C. Deep-learning framework and computer assisted fatty infiltration analysis for the supraspinatus muscle in MRI. Sci. Rep. 2021, 11, 15065. [Google Scholar] [CrossRef] [PubMed]
- Riem, L.; Feng, X.; Cousins, M.; DuCharme, O.; Leitch, E.B.; Werner, B.C.; Sheean, A.J.; Hart, J.; Antosh, I.J.; Blemker, S.S. A Deep Learning Algorithm for Automatic 3D Segmentation of Rotator Cuff Muscle and Fat from Clinical MRI Scans. Radiol. Artif. Intell. 2023, 5, e220132. [Google Scholar] [CrossRef] [PubMed]
- Hess, H.; Ruckli, A.C.; Bürki, F.; Gerber, N.; Menzemer, J.; Burger, J.; Schär, M.; Zumstein, M.A.; Gerber, K. Deep-Learning-Based Segmentation of the Shoulder from MRI with Inference Accuracy Prediction. Diagnostics 2023, 13, 1668. [Google Scholar] [CrossRef]
- Gupta, P.; Haeberle, H.S.; Zimmer, Z.R.; Levine, W.N.; Williams, R.J.; Ramkumar, P.N. Artificial intelligence-based applications in shoulder surgery leaves much to be desired: A systematic review. JSES Rev. Rep. Tech. 2023, 3, 189–200. [Google Scholar] [CrossRef]
- Hahn, S.; Yi, J.; Lee, H.J.; Lee, Y.; Lee, J.; Wang, X.; Fung, M. Comparison of deep learning-based reconstruction of PROPELLER Shoulder MRI with conventional reconstruction. Skeletal Radiol. 2023, 52, 1545–1555. [Google Scholar] [CrossRef]
- Kim, M.; Park, H.M.; Kim, J.Y.; Kim, S.H.; Hoeke, S.; De Neve, W. MRI-based diagnosis of rotator cuff tears using deep learning and weighted linear combinations. In Proceedings of the Machine Learning for Healthcare Conference, PMLR 2020, Virtual Event, 7–8 August 2020; pp. 292–308. [Google Scholar]
- Sezer, A.; Sezer, H.B. Capsule network-based classification of rotator cuff pathologies from MRI. Comput. Electr. Eng. 2019, 80, 106480. [Google Scholar] [CrossRef]
- Shim, E.; Kim, J.Y.; Yoon, J.P.; Ki, S.Y.; Lho, T.; Kim, Y.; Chung, S.W. Automated rotator cuff tear classification using 3D convolutional neural network. Sci. Rep. 2020, 10, 15632. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Chepelev, L.; Nisha, Y.; Sathiadoss, P.; Rybicki, F.J.; Sheikh, A.M. Evaluation of a deep learning method for the automated detection of supraspinatus tears on MRI. Skeletal Radiol. 2022, 51, 1765–1775. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Wang, C.N.; Ou, Y.K.; Fu, J. Combined image enhancement, feature extraction, and classification protocol to improve detection and diagnosis of rotator-cuff tears on MR imaging. Magn. Reson. Med. Sci. 2014, 13, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Redmon, J.; Farhadi, A. YOLO 9000: Better, faster, stronger. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 7263–7271. [Google Scholar]
- Reis, D.; Kupec, J.; Hong, J.; Daoudi, A. Real-Time Flying Object Detection with YOLOv8. arXiv 2023, arXiv:2305.09972. [Google Scholar]
- Zhang, Z. Improved adam optimizer for deep neural networks. In Proceedings of the 2018 IEEE/ACM 26th International Symposium on Quality of Service (IWQoS), Banff, AB, Canada, 4–6 June 2018; pp. 1–2. [Google Scholar] [CrossRef]
- Gyftopoulos, S.; Lin, D.; Knoll, F.; Doshi, A.M.; Rodrigues, T.C.; Recht, M.P. Artificial Intelligence in Musculoskeletal Imaging: Current Status and Future Directions. AJR Am. J. Roentgenol. 2019, 213, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, D.; Lee, K.J.; Kang, Y.; Ahn, J.M.; Lee, E.; Lee, J.W.; Kang, H.S. Ruling out rotator cuff tear in shoulder radiograph series using deep learning: Redefining the role of conventional radiograph. Eur. Radiol. 2020, 30, 2843–2852. [Google Scholar] [CrossRef]
- Lee, K.; Kim, J.Y.; Lee, M.H.; Choi, C.H.; Hwang, J.Y. Imbalanced Loss-Integrated Deep-Learning-Based Ultrasound Image Analysis for Diagnosis of Rotator-Cuff Tear. Sensors 2021, 21, 2214. [Google Scholar] [CrossRef]
- Taghizadeh, E.; Truffer, O.; Becce, F.; Eminian, S.; Gidoin, S.; Terrier, A.; Farron, A.; Büchler, P. Deep learning for the rapid automatic quantification and characterization of rotator cuff muscle degeneration from shoulder CT datasets. Eur. Radiol. 2021, 31, 181–190. [Google Scholar] [CrossRef]
- Goh, C.K.; Peh, W.C. Pictorial essay: Pitfalls in magnetic resonance imaging of the shoulder. Can. Assoc. Radiol. J. 2012, 63, 247–259. [Google Scholar] [CrossRef]
- Marcon, G.F.; Macedo, T.A. Artifacts and pitfalls in shoulder magnetic resonance imaging. Radiol. Bras. 2015, 48, 242–248. [Google Scholar] [CrossRef]
- Takeuchi, N.; Kozono, N.; Nishii, A.; Matsuura, K.; Ishitani, E.; Onizuka, T.; Mizuki, Y.; Kimura, T.; Yuge, H.; Uchimura, T.; et al. Prevalence and predisposing factors of neuropathic pain in patients with rotator cuff tears. J. Orthop. Sci. 2023. [Google Scholar] [CrossRef] [PubMed]
- Neyton, L.; Daggett, M.; Kruse, K.; Walch, G. The hidden lesion of the subscapularis: Arthroscopically revisited. Arthrosc. Tech. 2016, 5, e877–e881. [Google Scholar] [CrossRef][Green Version]
- Qureshi, R.; Ragab, M.G.; Abdulkader, S.J.; Alqushaib, A.; Sumiea, E.H.; Alhussian, H. A Comprehensive Systematic Review of YOLO for Medical Object Detection (2018 to 2023). TechRxiv 2023. [Google Scholar] [CrossRef]
- Inui, A.; Mifune, Y.; Nishimoto, H.; Mukohara, S.; Fukuda, S.; Kato, T.; Furukawa, T.; Tanaka, S.; Kusunose, M.; Takigami, S.; et al. Detection of elbow OCD in the ultrasound image by artificial intelligence using YOLOv8. Appl. Sci. 2023, 13, 7623. [Google Scholar] [CrossRef]
- Kufel, J.; Bargieł-Łączek, K.; Koźlik, M.; Czogalik, Ł.; Dudek, P.; Magiera, M.; Bartnikowska, W.; Lis, A.; Paszkiewicz, I.; Kocot, S.; et al. Chest X-ray Foreign Objects Detection Using Artificial Intelligence. J. Clin. Med. 2023, 12, 5841. [Google Scholar] [CrossRef] [PubMed]
- Terzi, D.S.; Azginoglu, N. In-Domain Transfer Learning Strategy for Tumor Detection on Brain MRI. Diagnostics 2023, 13, 2110. [Google Scholar] [CrossRef]
- Longo, U.G.; De Salvatore, S.; Zollo, G.; Calabrese, G.; Piergentili, I.; Loppini, M.; Denaro, V. Magnetic resonance imaging could precisely define the mean value of tendon thickness in partial rotator cuff tears. BMC Musculoskelet. Disord. 2023, 24, 718. [Google Scholar] [CrossRef]
- Kim, H.; Shin, K.; Kim, H.; Lee, E.S.; Chung, S.W.; Koh, K.H.; Kim, N. Can deep learning reduce the time and effort required for manual segmentation in 3D reconstruction of MRI in rotator cuff tears? PLoS ONE 2022, 17, e0274075. [Google Scholar] [CrossRef]



| Subjects | Training | Tuning | Testing | ||
|---|---|---|---|---|---|
| No RCT (n = 100) | Number of Patches | 1511 | 150 | 391 | |
| Plane | Axial | 566 | 51 | 152 | |
| Coronal | 362 | 37 | 86 | ||
| Sagittal | 583 | 62 | 153 | ||
| RCT (n = 694) | Number of Patches | 6427 | 795 | 1534 | |
| Plane | Axial | 753 | 237 | 435 | |
| Coronal | 2415 | 289 | 547 | ||
| Sagittal | 2233 | 269 | 552 | ||
| AUC | Sensitivity | Specificity | Precision | Accuracy | F1 Score | |
|---|---|---|---|---|---|---|
| ALL | 0.94 | 98% | 91% | 98% | 96% | 97% |
| Axial | 0.71 | 51% | 100% | 100% | 58% | 68% |
| Sagittal | 0.70 | 72% | 63% | 92% | 70% | 81% |
| Coronal | 0.68 | 48% | 95% | 98% | 55% | 64% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-C.; Cho, Y.; Ahn, K.-S.; Park, H.-J.; Kang, Y.-S.; Lee, S.; Kim, D.; Kang, C.H. Deep-Learning-Based Automated Rotator Cuff Tear Screening in Three Planes of Shoulder MRI. Diagnostics 2023, 13, 3254. https://doi.org/10.3390/diagnostics13203254
Lee K-C, Cho Y, Ahn K-S, Park H-J, Kang Y-S, Lee S, Kim D, Kang CH. Deep-Learning-Based Automated Rotator Cuff Tear Screening in Three Planes of Shoulder MRI. Diagnostics. 2023; 13(20):3254. https://doi.org/10.3390/diagnostics13203254
Chicago/Turabian StyleLee, Kyu-Chong, Yongwon Cho, Kyung-Sik Ahn, Hyun-Joon Park, Young-Shin Kang, Sungshin Lee, Dongmin Kim, and Chang Ho Kang. 2023. "Deep-Learning-Based Automated Rotator Cuff Tear Screening in Three Planes of Shoulder MRI" Diagnostics 13, no. 20: 3254. https://doi.org/10.3390/diagnostics13203254
APA StyleLee, K.-C., Cho, Y., Ahn, K.-S., Park, H.-J., Kang, Y.-S., Lee, S., Kim, D., & Kang, C. H. (2023). Deep-Learning-Based Automated Rotator Cuff Tear Screening in Three Planes of Shoulder MRI. Diagnostics, 13(20), 3254. https://doi.org/10.3390/diagnostics13203254







