Association between Corneal Higher-Order Aberrations Evaluated with a Videokeratographer and Corneal Surface Abnormalities in Dry Eye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Items for Examination
2.3. Evaluation of Subjective Symptoms
2.4. Evaluation of Tear Volume and TF Stability
2.5. Evaluation of HOAs Using VK
2.6. Evaluation of FBUT and CED
2.7. Environmental Conditions
2.8. Statistical Analysis
3. Results
3.1. Subjective and Objective Parameters
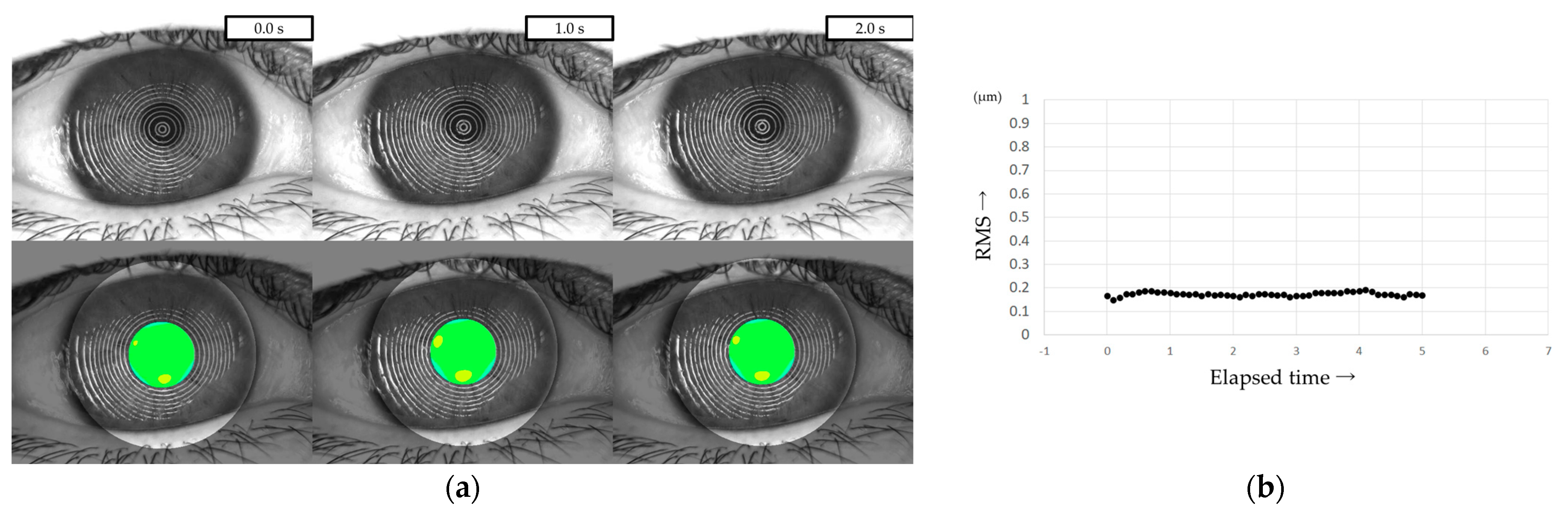
3.2. Continuous Measurement of HOAs with VK
3.3. Relationship between HOAs and Other Parameters
3.4. Factors Determining HOAs
3.5. Representative Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rieger, G. The importance of the precorneal tear film for the quality of optical imaging. Br. J. Ophthalmol. 1992, 76, 157–158. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Tsubota, K.; Yokoi, N.; Shimazaki, J.; Watanabe, H.; Dogru, M.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. New perspectives on dry eye definition and diagnosis: A consensus report by the Asia Dry Eye Society. Ocul. Surf. 2017, 15, 65–76. [Google Scholar] [CrossRef]
- Huang, F.C.; Tseng, S.H.; Shih, M.H.; Chen, F.K. Effect of artificial tears on corneal surface regularity, contrast sensitivity, and glare disability in dry eyes. Ophthalmology 2002, 109, 1934–1940. [Google Scholar] [CrossRef]
- Puell, M.C.; Benítez-del-Castillo, J.M.; Martínez-de-la-Casa, J.; Sánchez-Ramos, C.; Vico, E.; Pérez-Carrasco, M.J.; Pedraza, C.; del-Hierro, A. Contrast sensitivity and disability glare in patients with dry eye. Acta. Ophthalmol. Scand. 2006, 84, 527–531. [Google Scholar] [CrossRef]
- Koh, S.; Maeda, N.; Ikeda, C.; Asonuma, S.; Ogawa, M.; Hiraoka, T.; Oshika, T.; Nishida, K. The effect of ocular surface regularity on contrast sensitivity and straylight in dry eye. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2647–2651. [Google Scholar] [CrossRef]
- Szczotka-Flynn, L.B.; Maguire, M.G.; Ying, G.S.; Lin, M.C.; Bunya, V.Y.; Dana, R.; Asbell, P.A. Dry Eye Assessment and Management (DREAM) Study Research Group. Impact of dry eye on visual acuity and contrast sensitivity: Dry eye assessment and management study. Optom. Vis. Sci. 2019, 96, 387–396. [Google Scholar] [CrossRef]
- Ishida, R.; Kojima, T.; Dogru, M.; Kaido, M.; Matsumoto, Y.; Tanaka, M.; Goto, E.; Tsubota, K. The application of a new continuous functional visual acuity measurement system in dry eye syndromes. Am. J. Ophthalmol. 2005, 139, 253–258. [Google Scholar] [CrossRef]
- Kaido, M.; Dogru, M.; Ishida, R.; Tsubota, K. Concept of functional visual acuity and its applications. Cornea 2007, 26, S29–S35. [Google Scholar] [CrossRef]
- Kaido, M.; Ishida, R.; Dogru, M.; Tsubota, K. The relation of functional visual acuity measurement methodology to tear functions and ocular surface status. Jpn. J. Ophthalmol. 2011, 55, 451–459. [Google Scholar] [CrossRef]
- Liu, Z.; Pflugfelder, S.C. Corneal surface regularity and the effect of artificial tears in aqueous tear deficiency. Ophthalmology 1999, 106, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.; Maeda, N.; Ogawa, M.; Asonuma, S.; Takai, Y.; Maruyama, K.; Klyce, S.D.; Nishida, K. Fourier analysis of corneal irregular astigmatism due to the anterior corneal surface in dry eye. Eye Contact Lens 2019, 45, 188–194. [Google Scholar] [CrossRef]
- Liang, J.; Grimm, B.; Goelz, S.; Bille, J.F. Objective measurement of wave aberrations of the human eye with the use of a Hartmann-Shack wave-front sensor. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 1994, 11, 1949–1957. [Google Scholar] [CrossRef]
- Thibos, L.N.; Hong, X. Clinical applications of the Shack-Hartmann aberrometer. Optom. Vis. Sci. 1999, 76, 817–825. [Google Scholar] [CrossRef]
- Montés-Micó, R.; Cáliz, A.; Alió, J.L. Wavefront analysis of higher order aberrations in dry eye patients. J. Refract. Surg. 2004, 20, 243–247. [Google Scholar] [CrossRef]
- Montés-Micó, R.; Alió, J.L.; Charman, W.N. Dynamic changes in the tear film in dry eyes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1615–1619. [Google Scholar] [CrossRef]
- Koh, S.; Maeda, N.; Hirohara, Y.; Mihashi, T.; Bessho, K.; Hori, Y.; Inoue, T.; Watanabe, H.; Fujikado, T.; Tano, Y. Serial measurements of higher-order aberrations after blinking in patients with dry eye. Investig. Ophthalmol. Vis. Sci. 2008, 49, 133–138. [Google Scholar] [CrossRef]
- Koh, S.; Maeda, N.; Ikeda, C.; Asonuma, S.; Mitamura, H.; Oie, Y.; Soma, T.; Tsujikawa, M.; Kawasaki, S.; Nishida, K. Ocular forward light scattering and corneal backward light scattering in patients with dry eye. Investig. Ophthalmol. Vis. Sci. 2014, 18, 6601–6606. [Google Scholar] [CrossRef]
- Diaz-Valle, D.; Arriola-Villalobos, P.; García-Vidal, S.E.; Sánchez-Pulgarín, M.; Borrego Sanz, L.; Gegúndez-Fernández, J.A.; Benitez-Del-Castillo, J.M. Effect of lubricating eyedrops on ocular light scattering as a measure of vision quality in patients with dry eye. J. Cataract Refract. Surg. 2012, 38, 1192–1197. [Google Scholar] [CrossRef]
- Montés-Micó, R.; Alió, J.L.; Charman, W.N. Postblink changes in the ocular modulation transfer function measured by a double-pass method. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4468–4473. [Google Scholar] [CrossRef]
- Kobashi, H.; Kamiya, K.; Yanome, K.; Igarashi, A.; Shimizu, K. Longitudinal assessment of optical quality and intraocular scattering using the double-pass instrument in normal eyes and eyes with short tear breakup time. PLoS ONE 2013, 8, e82427. [Google Scholar] [CrossRef] [PubMed]
- Kaido, M.; Matsumoto, Y.; Shigeno, Y.; Ishida, R.; Dogru, M.; Tsubota, K. Corneal fluorescein staining correlates with visual function in dry eye patients. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9516–9522. [Google Scholar] [CrossRef]
- Ferrer-Blasco, T.; García-Lázaro, S.; Montés-Micó, R.; Cerviño, A.; González-Méijome, J.M. Dynamic changes in the air-tear film interface modulation transfer function. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Denoyer, A.; Rabut, G.; Baudouin, C. Tear film aberration dynamics and vision-related quality of life in patients with dry eye disease. Ophthalmology 2012, 119, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Németh, J.; Erdélyi, B.; Csákány, B.; Gáspár, P.; Soumelidis, A.; Kahlesz, F.; Lang, Z. High-speed videotopographic measurement of tear film build-up time. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1783–1790. [Google Scholar]
- Iskander, D.R.; Collins, M.J. Applications of high-speed videokeratoscopy. Clin. Exp. Optom. 2005, 88, 223–231. [Google Scholar] [CrossRef]
- Goto, T.; Zheng, X.; Klyce, S.D.; Kataoka, H.; Uno, T.; Karon, M.; Tatematsu, Y.; Bessyo, T.; Tsubota, K.; Ohashi, Y. A new method for tear film stability analysis using videokeratography. Am. J. Ophthalmol. 2003, 135, 607–612. [Google Scholar] [CrossRef]
- Goto, T.; Zheng, X.; Okamoto, S.; Ohashi, Y. Tear film stability analysis system: Introducing a new application for videokeratography. Cornea 2004, 23 (Suppl. S8), S65–S70. [Google Scholar] [CrossRef]
- Kojima, T.; Ishida, R.; Dogru, M.; Goto, E.; Takano, Y.; Matsumoto, Y.; Kaido, M.; Ohashi, Y.; Tsubota, K. A new noninvasive tear stability analysis system for the assessment of dry eyes. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1369–1374. [Google Scholar] [CrossRef]
- Szczesna-Iskander, D.H.; Iskander, D.R. Future directions in non-invasive measurements of tear film surface kinetics. Optom. Vis. Sci. 2012, 89, 749–759. [Google Scholar] [CrossRef]
- Llorens-Quintana, C.; Szczesna-Iskander, D.; Iskander, D.R. Supporting dry eye diagnosis with a new method for noninvasive tear film quality assessment. Optom. Vis. Sci. 2019, 96, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Kusada, N.; Yokoi, N.; Kato, H.; Furusawa, Y.; Sakai, R.; Sotozono, C. Evaluation of dry eye with videokeratographer using a newly developed indicator. Am. J. Ophthalmol. 2023, 252, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Sakane, Y.; Yamaguchi, M.; Yokoi, N.; Uchino, M.; Dogru, M.; Oishi, T.; Ohashi, Y.; Ohashi, Y. Development and validation of the Dry Eye-Related Quality-of-Life Score questionnaire. JAMA Ophthalmol. 2013, 131, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Bron, A.J.; Tiffany, J.M.; Maruyama, K.; Komuro, A.; Kinoshita, S. Relationship between tear volume and tear meniscus curvature. Arch. Ophthalmol. 2004, 122, 1265–1269. [Google Scholar] [CrossRef]
- Yokoi, N.; Takehisa, Y.; Kinoshita, S. Correlation of tear lipid layer interference patterns with the diagnosis and severity of dry eye. Am. J. Ophthalmol. 1996, 122, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Georgiev, G.A.; Kato, H.; Komuro, A.; Sonomura, Y.; Sotozono, C.; Tsubota, K.; Kinoshita, S. Classification of Fluorescein Breakup Patterns: A Novel Method of Differential Diagnosis for Dry Eye. Am. J. Ophthalmol. 2017, 180, 72–85. [Google Scholar] [CrossRef]
- Lemp, M.A. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995, 21, 221–232. [Google Scholar]
- Thibos, L.N.; Hong, X.; Bradley, A.; Cheng, X. Statistical variation of aberration structure and image quality in a normal population of healthy eyes. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2002, 19, 2329–2348. [Google Scholar] [CrossRef]
- Koh, S.; Maeda, N.; Kuroda, T.; Hori, Y.; Watanabe, H.; Fujikado, T.; Tano, Y.; Hirohara, Y.; Mihashi, T. Effect of tear film break-up on higher-order aberrations measured with wavefront sensor. Am. J. Ophthalmol. 2002, 134, 115–117. [Google Scholar] [CrossRef]
- Koh, S.; Maeda, N.; Hirohara, Y.; Mihashi, T.; Ninomiya, S.; Bessho, K.; Watanabe, H.; Fujikado, T.; Tano, Y. Serial measurements of higher-order aberrations after blinking in normal subjects. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3318–3324. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Yokoi, N.; Watanabe, H.; Dogru, M.; Kojima, T.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. Members of The Asia Dry Eye Society. A new perspective on dry eye classification: Proposal by the Asia Dry Eye Society. Eye Contact Lens 2020, 46 (Suppl. S1), S2–S13. [Google Scholar] [CrossRef] [PubMed]
- Koh, S. Mechanisms of Visual Disturbance in Dry Eye. Cornea 2016, 35, S83–S88. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.; Maeda, N.; Ninomiya, S.; Watanabe, H.; Fujikado, T.; Tano, Y.; Hirohara, Y.; Mihashi, T. Paradoxical increase of visual impairment with punctal occlusion in a patient with mild dry eye. J. Cataract Refract. Surg. 2006, 32, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Mihashi, T.; Hirohara, Y.; Koh, S.; Ninomiya, S.; Maeda, N.; Fujikado, T. Tear film break-up time evaluated by real-time Hartmann-Shack wavefront sensing. Jpn. J. Ophthalmol. 2006, 50, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.; Fatt, I.; Radke, C.J. Deposition and thinning of the human tear film. J. Colloid Interface Sci. 1996, 184, 44–51. [Google Scholar] [CrossRef] [PubMed]
- King-Smith, P.E.; Fink, B.A.; Hill, R.M.; Koelling, K.W.; Tiffany, J.M. The thickness of the tear film. Curr. Eye Res. 2004, 29, 357–368. [Google Scholar] [CrossRef]
- Goto, E.; Tseng, S.C. Kinetic analysis of tear interference images in aqueous tear deficiency dry eye before and after punctal occlusion. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1897–1905. [Google Scholar] [CrossRef]
- Sharma, A. Breakup and dewetting of the corneal mucus layer. An update. Adv. Exp. Med. Biol. 1998, 438, 273–280. [Google Scholar] [CrossRef]
- Argüeso, P. Glycobiology of the ocular surface: Mucins and lectins. Jpn. J. Ophthalmol. 2013, 57, 150–155. [Google Scholar] [CrossRef]
- Mengher, L.S.; Bron, A.J.; Tonge, S.R.; Gilbert, D.J. Effect of fluorescein instillation on the pre-corneal tear film stability. Curr. Eye Res. 1985, 4, 9–12. [Google Scholar] [CrossRef]
- Toda, I.; Shimazaki, J.; Tsubota, K. Dry eye with only decreased tear break-up time is sometimes associated with allergic conjunctivitis. Ophthalmology 1995, 102, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Shigeyasu, C.; Yamada, M.; Yokoi, N.; Kawashima, M.; Suwaki, K.; Uchino, M.; Hiratsuka, Y.; Tsubota, K.; Decs-J Study Group. Characteristics and utility of fluorescein breakup patterns among dry eyes in clinic-based settings. Diagnostics 2020, 10, 711. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.; Tung, C.I.; Inoue, Y.; Jhanji, V. Effects of tear film dynamics on quality of vision. Br. J. Ophthalmol. 2018, 102, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Kusada, N.; Kato, H.; Furusawa, Y.; Sotozono, C.; Georgiev, G.A. Successful detection of the characteristics of tear film breakup appearing immediately after eye opening by videokeratography with a newly-developed indicator. Diagnostics 2023, 13, 240. [Google Scholar] [CrossRef]
- Nichols, K.K.; Nichols, J.J.; Mitchell, G.L. The lack of association between signs and symptoms in patients with dry eye disease. Cornea 2004, 23, 762–770. [Google Scholar] [CrossRef]
- Mizuno, Y.; Yamada, M.; Miyake, Y. Dry Eye Survey Group of the National Hospital Organization of Japan. Association between clinical diagnostic tests and health-related quality of life surveys in patients with dry eye syndrome. Jpn. J. Ophthalmol. 2010, 54, 259–265. [Google Scholar] [CrossRef]
- Dougherty, B.E.; Nichols, J.J.; Nichols, K.K. Rasch analysis of the Ocular Surface Disease Index (OSDI). Investig. Ophthalmol. Vis. Sci. 2011, 52, 8630–8635. [Google Scholar] [CrossRef]
- Goto, E.; Yagi, Y.; Matsumoto, Y.; Tsubota, K. Impaired functional visual acuity of dry eye patients. Am. J. Ophthalmol. 2002, 133, 181–186. [Google Scholar] [CrossRef]
- Koh, S.; Maeda, N.; Hori, Y.; Inoue, T.; Watanabe, H.; Hirohara, Y.; Mihashi, T.; Fujikado, T.; Tano, Y. Effects of suppression of blinking on quality of vision in borderline cases of evaporative dry eye. Cornea 2008, 27, 275–278. [Google Scholar] [CrossRef]
- Mangione, C.M.; Lee, P.P.; Gutierrez, P.R.; Spritzer, K.; Berry, S.; Hays, R.D. National Eye Institute Visual Function Questionnaire Field Test Investigators. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch. Ophthalmol. 2001, 119, 1050–1058. [Google Scholar] [CrossRef]
- Buehren, T.; Collins, M.J.; Iskander, D.R.; Davis, B.; Lingelbach, B. The stability of corneal topography in the post-blink interval. Cornea 2001, 20, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Shimizu, E.; Yagi-Yaguchi, Y.; Tomida, D.; Satake, Y.; Shimazaki, J. A novel entity of corneal diseases with irregular posterior corneal surfaces: Concept and clinical relevance. Cornea 2017, 36 (Suppl. S1), S53–S59. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.H.; Ji, Y.S.; Oh, H.J.; Yoon, K.C. Higher order aberrations of the corneal surface after laser subepithelial keratomileusis. Korean J. Ophthalmol. 2014, 28, 285–291. [Google Scholar] [CrossRef] [PubMed]




| HOAs(0) | HOAs(1) | HOAs(2) | HOAs(3) | HOAs(4) | HOAs(5) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| versus | r | p | r | p | r | p | r | p | r | p | r | p | ||||||
| TMR | −0.203 | * | 0.047 | −0.141 | * | 0.170 | −0.146 | * | 0.157 | −0.141 | * | 0.172 | −0.162 | * | 0.116 | −0.050 | * | 0.626 |
| IG | 0.434 | ** | <0.001 | 0.439 | ** | <0.001 | 0.378 | ** | <0.001 | 0.387 | ** | <0.001 | 0.364 | ** | <0.001 | 0.361 | ** | <0.001 |
| SG | 0.348 | ** | <0.001 | 0.431 | ** | <0.001 | 0.419 | ** | <0.001 | 0.435 | ** | <0.001 | 0.413 | ** | <0.001 | 0.391 | ** | <0.001 |
| NIBUT | −0.292 | * | 0.004 | −0.414 | * | <0.001 | −0.417 | * | <0.001 | −0.311 | * | 0.002 | −0.379 | * | <0.001 | −0.161 | * | 0.117 |
| FBUT | −0.284 | * | 0.005 | −0.345 | * | <0.001 | −0.386 | * | <0.001 | −0.313 | * | 0.002 | −0.370 | * | <0.001 | −0.175 | * | 0.089 |
| CED score | 0.503 | ** | <0.001 | 0.536 | ** | <0.001 | 0.517 | ** | <0.001 | 0.537 | ** | <0.001 | 0.523 | ** | <0.001 | 0.500 | ** | <0.001 |
| summary score of DEQS | −0.136 | * | 0.187 | −0.052 | * | 0.614 | −0.167 | * | 0.103 | −0.075 | * | 0.469 | −0.021 | * | 0.839 | −0.158 | * | 0.124 |
| HOAs(1) | RC | SRC | SE | t-Value | p-Value | |
| Intercept | 0.347 | 0 | 0.066 | 5.25 | <0.001 | * |
| NIBUT | −0.028 | −0.227 | 0.012 | −2.41 | 0.018 | * |
| CED score | 0.144 | 0.436 | 0.031 | 4.62 | <0.001 | * |
| HOAs(2) | RC | SRC | SE | t-Value | p-Value | |
| Intercept | 0.323 | 0 | 0.046 | 7.05 | <0.001 | * |
| NIBUT | −0.021 | −0.255 | 0.008 | −2.64 | 0.010 | * |
| CED score | 0.084 | 0.376 | 0.021 | 3.89 | <0.001 | * |
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age, Sex | 47, Female | 79, Female | 77, Female |
| FBUP | Random break | Line break | Spot break |
| Subtypes of DE | IEDE | ADDE | DWDE |
| Summary score of DEQS | 91.7 | 26.7 | 20.0 |
| TMR (mm) | 0.273 | 0.095 | 0.245 |
| IG | 4 | 2 | 2 |
| SG | 1 | 2 | 1 |
| NIBUT (s) | 8.31 | 3.32 | 0 |
| FBUT (s) | 4 | 2 | 0 |
| CED score | 0 | 1 | 0 |
| HOAs(0) (RMS, µm) | 0.104 | 0.546 | 0.408 |
| HOAs(1) (RMS, µm) | 0.110 | 0.430 | 0.696 |
| HOAs(2) (RMS, µm) | 0.064 | 0.473 | 0.765 |
| HOAs(3) (RMS, µm) | 0.076 | 0.420 | 0.701 |
| HOAs(4) (RMS, µm) | 0.078 | 0.428 | 0.573 |
| HOAs(5) (RMS, µm) | 0.107 | 0.408 | 0.791 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusada, N.; Yokoi, N.; Sotozono, C. Association between Corneal Higher-Order Aberrations Evaluated with a Videokeratographer and Corneal Surface Abnormalities in Dry Eye. Diagnostics 2023, 13, 3319. https://doi.org/10.3390/diagnostics13213319
Kusada N, Yokoi N, Sotozono C. Association between Corneal Higher-Order Aberrations Evaluated with a Videokeratographer and Corneal Surface Abnormalities in Dry Eye. Diagnostics. 2023; 13(21):3319. https://doi.org/10.3390/diagnostics13213319
Chicago/Turabian StyleKusada, Natsuki, Norihiko Yokoi, and Chie Sotozono. 2023. "Association between Corneal Higher-Order Aberrations Evaluated with a Videokeratographer and Corneal Surface Abnormalities in Dry Eye" Diagnostics 13, no. 21: 3319. https://doi.org/10.3390/diagnostics13213319
APA StyleKusada, N., Yokoi, N., & Sotozono, C. (2023). Association between Corneal Higher-Order Aberrations Evaluated with a Videokeratographer and Corneal Surface Abnormalities in Dry Eye. Diagnostics, 13(21), 3319. https://doi.org/10.3390/diagnostics13213319






