Multimodal Diagnostics of Changes in Rat Lungs after Vaping
Abstract
:1. Introduction
2. Materials and Methods
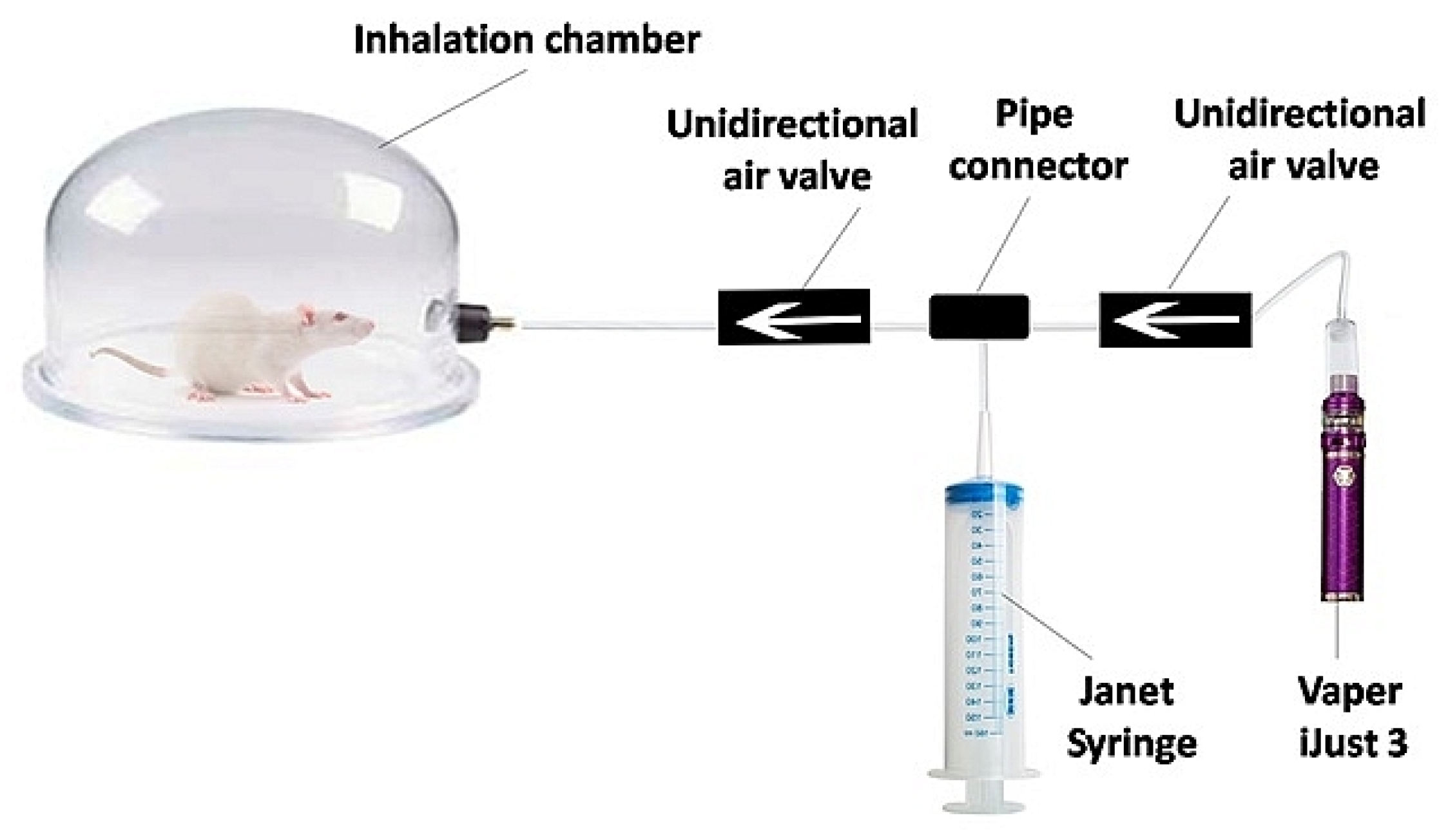
2.1. E-Cigarette Liquid and Experimental Setup
2.2. Animal Study Protocols and Lung Samples Preparation
2.3. The Spectroscopy and Imaging Techniques
2.4. Histopathological Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chronic Respiratory Diseases—World Health Organization (WHO). Available online: https://www.who.int/health-topics/chronic-respiratory-diseases#tab=tab_1 (accessed on 5 April 2023).
- Exarchos, K.P.; Gkrepi, G.; Kostikas, K.; Gogali, A. Recent Advances of Artificial Intelligence Applications in Interstitial Lung Diseases. Diagnostics 2023, 13, 2303. [Google Scholar] [CrossRef] [PubMed]
- Pacurari, A.C.; Bhattarai, S.; Muhammad, A.; Avram, C.; Mederle, A.O.; Rosca, O.; Bratosin, F.; Bogdan, I.; Fericean, R.M.; Biris, M.; et al. Diagnostic Accuracy of Machine Learning AI Architectures in Detection and Classification of Lung Cancer: A Systematic Review. Diagnostics 2023, 13, 2145. [Google Scholar] [CrossRef] [PubMed]
- Benbelkacem, S.; Zenati-Henda, N.; Zerrouki, N.; Oulefki, A.; Agaian, S.; Masmoudi, M.; Bentaleb, A.; Liew, A. Tumor Lung Visualization and Localization through Virtual Reality and Thermal Feedback Interface. Diagnostics 2023, 13, 567. [Google Scholar] [CrossRef]
- Maggi, L.; Biava, A.M.; Fiorelli, S.; Coluzzi, F.; Ricci, A.; Rocco, M. Lung Ultrasound: A Diagnostic Leading Tool for SARS-CoV-2 Pneumonia: A Narrative Review. Diagnostics 2021, 11, 2381. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.; Inzamam, W.; Banday, M.K.; Rasool, S.R.; Bhat, M.H.; Vladulescu, C.; Al-Misned, F.A.; El-Serehy, H.A. Lung Ultrasonography Is an Acceptable Imaging Modality to Diagnose COVID-19 and Effectively Correlates with HRCT Chest—A Prospective Study. Diagnostics 2023, 13, 2091. [Google Scholar] [CrossRef] [PubMed]
- Perri, A.; Fattore, S.; Prontera, G.; Patti, M.L.; Sbordone, A.; Tana, M.; D’Andrea, V.; Vento, G. Lung Ultrasound in the Early Diagnosis and Management of the Mild Form of Meconium Aspiration Syndrome: A Case Report. Diagnostics 2023, 13, 719. [Google Scholar] [CrossRef]
- Rea, G.; Sverzellati, N.; Bocchino, M.; Lieto, R.; Milanese, G.; D’Alto, M.; Bocchini, G.; Maniscalco, M.; Valente, T.; Sica, G. Beyond Visual Interpretation: Quantitative Analysis and Artificial Intelligence in Interstitial Lung Disease Diagnosis “Expanding Horizons in Radiology”. Diagnostics 2023, 13, 2333. [Google Scholar]
- Ozawa, Y.; Ohno, Y.; Nagata, H.; Tamokami, K.; Nishikimi, K.; Oshima, Y.; Hamabuchi, N.; Mat-suyama, T.; Ueda, T.; Toyama, H. Advances for Pulmonary Functional Imaging: Dual-Energy Computed Tomography for Pulmonary Functional Imaging. Diagnostics 2023, 13, 2295. [Google Scholar] [CrossRef]
- Mascalchi, M.; Picozzi, G.; Puliti, D.; Diciotti, S.; Deliperi, A.; Romei, C.; Falaschi, F.; Pistelli, F.; Grazzini, M.; Van-nucchi, L.; et al. Lung Cancer Screening with Low-Dose CT: What We Have Learned in Two Decades of ITALUNG and What Is Yetto Be Addressed. Diagnostics 2023, 13, 2197. [Google Scholar]
- Guglielmo, P.; Marturano, F.; Bettinelli, A.; Sepulcri, M.; Pasello, G.; Gregianin, M.; Paiusco, M.; Evangelista, L. Additional Value of PET and CT Image-Based Features in the Detection of Occult Lymph Node Metastases in Lung Cancer: A Systematic Review of the Literature. Diagnostics 2023, 13, 2153. [Google Scholar]
- Iqbal, U.; Imtiaz, R.; Saudagar, A.K.J.; Alam, K.A. CRV-NET: Robust Intensity Recognition of Coronavirus in Lung Computerized Tomography Scan Images. Diagnostics 2023, 13, 1783. [Google Scholar] [CrossRef]
- Nardone, V.; Belfiore, M.P.; DeChiara, M.; DeMarco, G.; Patanè, V.; Balestrucci, G.; Buono, M.; Salvarezza, M.; DiGuida, G.; D’Angiolella, D.; et al. CARdioimaging in Lung Cancer PatiEnts Undergoing Radical Radio Therapy: CARE-RTTrial. Diagnostics 2023, 13, 1717. [Google Scholar] [CrossRef]
- Farahat, I.S.; Sharafeldeen, A.; Elsharkawy, M.; Soliman, A.; Mahmoud, A.; Ghazal, M.; Taher, F.; Bilal, M.; AbdelRazek, A.A.K.; Aladrousy, W.; et al. The Role of 3D CT Imaging in the Accurate Diagnosis of Lung Function in Coronavirus Patients. Diagnostics 2022, 12, 696. [Google Scholar] [CrossRef]
- Lancaster, H.L.; Heuvelmans, M.A.; Oudkerk, M. Low-dose computed tomography lung cancer screening: Clinical evidence and implementation research. J. Intern. Med. 2022, 292, 68–80. [Google Scholar] [CrossRef]
- Liszewski, M.C.; Ciet, P.; Winant, A.J.; Lee, E.Y. Lung and large airway imaging: Magnetic resonance imaging versus computed tomography. Pediatr. Radiol. 2022, 52, 1814–1825. [Google Scholar] [CrossRef] [PubMed]
- Newman, B. Magnetic resonance imaging for congenital lung malformations. Pediatr. Radiol. 2022, 52, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Gulhane, A.V.; Chen, D.L. Overview of positron emission tomography in functional imaging of the lungs for diffuse lung diseases. Brit. J. Radiol. 2022, 95, 20210824. [Google Scholar] [CrossRef] [PubMed]
- Al Rasheedi, M.; Han, S.; Thygesen, H.; Neilson, M.; Hendry, F.; Alkarn, A.; Maclay, J.D.; Leung, H.Y. A Comparative Evaluation of Mediastinal Nodal SUV max and Derived Ratios from 18F-FDG PET/CT Imaging to Predict Nodal Metastases inNon-Small Cell Lung Cancer. Diagnostics 2023, 13, 1209. [Google Scholar] [CrossRef]
- Marciniak, T. Biometric Technologies Based on Optical Coherence Tomography. Sensors 2023, 23, 3753. [Google Scholar] [CrossRef]
- Liu, H.-C.; Lin, M.-H.; Ting, C.-H.; Wang, Y.-M.; Sun, C.-W. Intraoperative application of optical coherence tomography for lung tumor. J. Biophotonics 2023, 16, e202200344. [Google Scholar] [CrossRef]
- Nandy, S.; Berigei, S.R.; Keyes, C.M.; Muniappan, A.; Auchincloss, H.G.; Lanuti, M.; Roop, B.W.; Shih, A.R.; Colby, T.V.; Medoff, B.D.; et al. Polarization-Sensitive Endobronchial Optical Coherence Tomography for Microscopic Imaging of Fibrosis in Interstitial Lung Disease. Am. J. Respir. Crit. Care Med. 2022, 206, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Kohlfaerber, T.; Pieper, M.; Münter, M.; Holzhausen, C.; Ahrens, M.; Idel, C.; Bruchhage, K.L.; Leichtle, A.; König, P.; Hüttmann, G.; et al. Dynamic microscopic optical coherence tomography to visualize the morphological and functional micro-anatomy of the airways. Biomed. Opt. Express 2022, 13, 3211–3223. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, C.; Gaertner, M.; Koch, E. Optical Coherence Tomography (OCT) for Time-Resolved Imaging of Alveolar Dynamics in Mechanically Ventilated Rats. Appl. Sci. 2017, 7, 287. [Google Scholar] [CrossRef]
- Sfayyih, A.H.; Sabry, A.H.; Jameel, S.M.; Sulaiman, N.; Raafat, S.M.; Humaidi, A.J.; Kubaiaisi, Y.M.A. Acoustic-Based DeepLearning Architectures for Lung Disease Diagnosis: A Comprehensive Overview. Diagnostics 2023, 13, 1748. [Google Scholar] [CrossRef]
- Alsheikhy, A.A.; Said, Y.; Shawly, T.; Alzahrani, A.K.; Lahza, H. A CAD System for Lung Cancer Detection Using Hybrid Deep Learning Techniques. Diagnostics 2023, 13, 1174. [Google Scholar] [CrossRef]
- Said, Y.; Alsheikhy, A.A.; Shawly, T.; Lahza, H. Medical Images Segmentation for Lung Cancer Diagnosis Based on Deep Learning Architectures. Diagnostics 2023, 13, 546. [Google Scholar] [CrossRef]
- Li, R.; Xiao, C.; Huang, Y.; Hassan, H.; Huang, B. Deep Learning Applications in Computed Tomography Images for Pulmonary Nodule Detection and Diagnosis: A Review. Diagnostics 2022, 12, 298. [Google Scholar] [CrossRef]
- Yu, T.; Zhu, D.; Oliveira, L.; Genina, E.A.; Bashkatov, A.N.; Tuchin, V.V. Tissue optical clearing mechanisms. In Handbook of Tissue Optical Clearing: New Prospects in Optical Imaging; Tuchin, V., Dan, Z., Genina, E.A., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 3–30. [Google Scholar]
- Bashkatov, A.N.; Berezin, K.V.; Dvoretskiy, K.N.; Chernavina, M.L.; Genina, E.A.; Genin, V.D.; Kochubey, V.I.; Lazareva, E.N.; Pravdin, A.B.; Shvachkina, M.E.; et al. Measurement of tissue optical properties in the context of tissue optical clearing. J. Biomed. Opt. 2018, 23, 091416. [Google Scholar] [CrossRef]
- Yu, T.; Zhu, J.; Li, D.; Zhu, D. Physical and chemical mechanisms of tissue optical clearing. iScience 2021, 24, 102178. [Google Scholar] [CrossRef]
- Quirk, B.C.; McLaughlin, R.A.; Pagnozzi, A.M.; Kennedy, B.F.; Noble, P.B.; Sampson, D.D. Optofluidic needle probe integrating targeted delivery of fluid with optical coherence tomography imaging. Opt. Lett. 2014, 39, 2888–2891. [Google Scholar] [CrossRef]
- Pichardo, A.H.; Amadeo, F.; Wilm, B.; Lévy, R.; Ressel, L.; Murray, P.; Sée, V. Optical Tissue Clearing to Study the Intra-Pulmonary Biodistribution of Intravenously Delivered Mesenchymal Stromal Cells and Their Interactions with Host Lung Cells. Int. J. Mol. Sci. 2022, 23, 14171. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Yang, Z.; Li, X. Tissue clearing technique: Recent progress and biomedical applications. J. Anat. 2021, 238, 489–507. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.R.; Voigt, F.F.; Shepherd, D.P.; Huisken, J. Tutorial: Practical considerations for tissue clearing and imaging. Nat. Protoc. 2021, 16, 2732–2748. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Wu, H.; Liu, L.; Lin, J.; Zhang, S. Organic solvent-based tissue clearing techniques and their applications. J. Biophotonics 2021, 14, e202000413. [Google Scholar] [CrossRef] [PubMed]
- Ariel, P. A beginner’s guide to tissue clearing. Int. J. Biochem. Cell Biol. 2017, 84, 35–39. [Google Scholar] [CrossRef]
- Costantini, I.; Cicchi, R.; Silvestri, L.; Vanzi, F.; Pavone, F.S. In-vivo and ex-vivo optical clearing methods for biological tissues: Review. Biomed. Opt. Express 2019, 10, 5251–5267. [Google Scholar] [CrossRef]
- Glycerin. In The MAK-Collection for Occupational Health and Safety. MAK Value Documentation, 2007. Available online: https://onlinelibrary.wiley.com/action/showCitFormats?doi=10.1002%2F3527600418.mb5681kske4215 (accessed on 5 April 2023).
- Werley, M.S.; McDonald, P.; Lilly, P.; Kirkpatrick, D.; Wallery, J.; Byron, P.; Venitz, J. Non-clinical safety and pharmacokinetic evaluations of propylene glycol aerosol in Sprague-Dawley rats and Beagle dogs. Toxicology 2011, 287, 76–90. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Kramek-Romanowska, K. Predicted Deposition of E-Cigarette Aerosol in the Human Lungs. J. Aerosol Med. Pulm. Drug Deliv. 2016, 29, 299–309. [Google Scholar] [CrossRef]
- Snow, S.J.; McGee, M.A.; Henriquez, A.; Richards, J.E.; Schladweiler, M.C.; Ledbetter, A.D.; Kodavanti, U.P. Respiratory Effects and Systemic Stress Response Following Acute Acrolein Inhalation in Rats. Toxicol. Sci. 2017, 158, 454–464. [Google Scholar] [CrossRef]
- Sharp, P.; Villano, J. The Laboratory Rat, 2nd ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group, An Informa Business: Boca Raton, FL, USA, 2013; 377p. [Google Scholar]
- Prahl, S.A. The Adding-Doubling Method. In Optical-Thermal Response of Laser-Irradiated Tissue; Welch, A.J., van Gemert, M.J.C., Eds.; Plenum Press: New York, NY, USA, 1995; pp. 101–129. [Google Scholar]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D Appl. Phys. 2005, 38, 2543–2555. [Google Scholar] [CrossRef]
- Thompson, J.H.; Richter, W.R. Hematoxylin-eosin staining adapted to automatic tissue processing. Biotech. Histochem. 1960, 35, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Farid, S.A.; Mahmoud, O.M.; Salem, N.A.; Abdel-Alrahman, G.; Hafez, G.A. Long term effects of maternal protein restriction on post natal lung alveoli development of rat off spring. Folia Morphol. 2015, 74, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Zickri, M.B.; Fadl, S.G.; Metwally, H.G. Comparative Study between Intravenous and Intraperitoneal Stem Cell Therapy in Amiodarone Induced Lung Injury in Rat. Int. J. Stem Cells 2014, 7, 1–11. [Google Scholar] [CrossRef]
- Irvin, C.G.; Bates, J.H. Measuring the lung function in the mouse: The challenge of size. Respir. Res. 2003, 4, 4. [Google Scholar] [CrossRef]
- Gotts, J.E.; Jordt, S.E.; McConnell, R.; Tarran, R. What are the respiratory effects of e-cigarettes? BMJ 2019, 366, l5275. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems. Public Health Consequences of E-Cigarettes; Eaton, D.L., Kwan, L.Y., Stratton, K., Eds.; National Academies Press: Washington, DC, USA, 2018. [Google Scholar]
- Scott, A.; Lugg, S.T.; Aldridge, K.; Lewis, K.E.; Bowden, A.; Mahida, R.Y.; Grudzinska, F.S.; Dosanjh, D.; Parekh, D.; Foronjy, R. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax 2018, 73, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Higham, A.; Rattray, N.J.; Dewhurst, J.A.; Trivedi, D.K.; Fowler, S.J.; Goodacre, R.; Singh, D. Electronic cigarette exposure triggers neutrophil inflammatory responses. Respir. Res. 2016, 17, 56. [Google Scholar] [CrossRef]
- Wieslander, G.; Norbäck, D.; Lindgren, T. Experimental exposure to propylene glycol mist in aviation emergency training: Acute ocular and respiratory effects. Occup. Environ. Med. 2001, 58, 649–655. [Google Scholar] [CrossRef]
- Reidel, B.; Radicioni, G.; Clapp, P.W.; Ford, A.A.; Abdelwahab, S.; Rebuli, M.E.; Haridass, P.; Alexis, N.E.; Jaspers, I.; Kesimer, M. E-Cigarette Use Causes a Unique Innate Immune Response in the Lung, Involving Increased Neutrophilic Activation and Altered Mucin Secretion. Am. J. Respir. Crit. Care Med. 2018, 197, 492–501. [Google Scholar] [CrossRef]
- Bucharskaya, A.B.; Yanina, I.Y.; Atsigeida, S.V.; Genin, V.D.; Lazareva, E.N.; Navolokin, N.A.; Dyachenko, P.A.; Tuchina, D.K.; Tuchina, E.S.; Genina, E.A.; et al. Optical clearing and testing of lung tissue using inhalation aerosols: Prospects for monitoring the action of viral infections. Biophys. Rev. 2022, 14, 1005–1022. [Google Scholar] [CrossRef]
- Marchesini, R.; Bertoni, A.; Andreola, S.; Melloni, E.; Sichirollo, A.E. Extinction and absorption coefficients and scattering phase functions of human tissues in vitro. Appl. Opt. 1989, 28, 2318–2324. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Thiboutot, J.; Park, H.C.; Li, A.; Loube, J.; Mitzner, W.; Yarmus, L.; Brown, R.H.; Li, X. Direct visualization and quantitative imaging of small airway anatomy in vivo using deep learning assisted diffractive OCT. IEEE Trans. Biomed. Eng. 2022, 70, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Beek, J.F.; Blokland, P.; Posthumus, P.; Aalders, M.; Pickering, J.W.; Sterenborg, H.J.C.M.; van Gemert, M.J.C. In vitro double-integrating-sphere optical properties of tissues between 630 and 1064 nm. Phys. Med. Biol. 1997, 42, 2255–2261. [Google Scholar] [CrossRef]
- Mosca, S.; Lanka, P.; Stone, N.; Sekar, S.K.V.; Matousek, P.; Valentini, G.; Pifferi, A. Optical characterization of porcine tissues from various organs in the 650–1100 nm range using time-domain diffuse spectroscopy. Biomed. Opt. Express 2020, 11, 1697–1706. [Google Scholar] [CrossRef]
- Genina, E.A.; Bashkatov, A.N.; Larin, K.V.; Tuchin, V.V. Light-tissue interaction at optical clearing. In Laser Imaging and Manipulation in Cell Biology; Pavone, F.S., Ed.; Wiley-VCH Verlag GmbH &Co.K Ga A: Weinheim, Germany, 2010; pp. 115–164. [Google Scholar]
- Beek, J.F.; van Staveren, H.J.; Posthumus, P.; Sterenborg, H.J.C.M.; van Gemert, M.J.C. The optical properties of lung as a function of respiration. Phys. Med. Biol. 1997, 42, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- Tuchin, V.V. Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnostics, 3rd ed.; PM254, SPIE Press: Bellingham, WA, USA, 2015; 988p. [Google Scholar]
- Tuchin, V.V. (Ed.) Handbook of Optical Biomedical Diagnostics, 2nd ed.; SPIE Press: Bellingham, WA, USA, 2016. [Google Scholar]
- Rowell, T.R.; Reeber, S.L.; Lee, S.L.; Harris, R.A.; Nethery, R.C.; Herring, A.H.; Glish, G.L.; Tarran, R. Flavored e-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L52–L66. [Google Scholar] [CrossRef]
- Sassano, M.F.; Davis, E.S.; Keating, J.E.; Zorn, B.T.; Kochar, T.K.; Wolfgang, M.C.; Glish, G.L.; Tarran, R. Evaluation of e-liquid toxicity using an open-source high-through put screening assay. PLoS Biol. 2018, 16, e2003904. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanina, I.Y.; Genin, V.D.; Genina, E.A.; Mudrak, D.A.; Navolokin, N.A.; Bucharskaya, A.B.; Kistenev, Y.V.; Tuchin, V.V. Multimodal Diagnostics of Changes in Rat Lungs after Vaping. Diagnostics 2023, 13, 3340. https://doi.org/10.3390/diagnostics13213340
Yanina IY, Genin VD, Genina EA, Mudrak DA, Navolokin NA, Bucharskaya AB, Kistenev YV, Tuchin VV. Multimodal Diagnostics of Changes in Rat Lungs after Vaping. Diagnostics. 2023; 13(21):3340. https://doi.org/10.3390/diagnostics13213340
Chicago/Turabian StyleYanina, Irina Yu., Vadim D. Genin, Elina A. Genina, Dmitry A. Mudrak, Nikita A. Navolokin, Alla B. Bucharskaya, Yury V. Kistenev, and Valery V. Tuchin. 2023. "Multimodal Diagnostics of Changes in Rat Lungs after Vaping" Diagnostics 13, no. 21: 3340. https://doi.org/10.3390/diagnostics13213340
APA StyleYanina, I. Y., Genin, V. D., Genina, E. A., Mudrak, D. A., Navolokin, N. A., Bucharskaya, A. B., Kistenev, Y. V., & Tuchin, V. V. (2023). Multimodal Diagnostics of Changes in Rat Lungs after Vaping. Diagnostics, 13(21), 3340. https://doi.org/10.3390/diagnostics13213340










