Updates in Diagnostic Imaging for Infectious Keratitis: A Review
Abstract
1. Introduction
2. Slit Lamp Biomicroscopy
3. Optical Coherence Tomography
3.1. Types of AS-OCT
3.1.1. Spectral Domain OCT
3.1.2. Swept Source
4. In Vivo Confocal Microscopy
5. Artificial Intelligence—Deep Learning Methods
5.1. Background
5.2. Deep Learning Models in Infectious Keratitis
5.3. Future Perspectives
5.4. Limitations
6. Conclusions
7. The Literature Search
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ung, L.; Acharya, N.R.; Agarwal, T.; Alfonso, E.C.; Bagga, B.; Bispo, P.J.; Burton, M.J.; Dart, J.K.; Doan, T.; Fleiszig, S.M.; et al. Infectious corneal ulceration: A proposal for neglected tropical disease status. Bull. World Health Organ. 2019, 97, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Y.; Kong, X.; Wolle, M.; Gasquet, N.; Ssekasanvu, J.; Mariotti, S.P.; Bourne, R.; Taylor, H.; Resnikoff, S.; West, S. Global trends in blindness and vision impairment resulting from corneal opacity 1984–2020: A meta-analysis. Ophthalmology 2023, 130, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Aguas, M.; Khoo, P.; Watson, S.L. Infectious keratitis: A review. Clin. Exp. Ophthalmol. 2022, 50, 543–562. [Google Scholar] [CrossRef]
- Ngo, J.; Khoo, P.; Watson, S.L. Improving the efficiency and the technique of the corneal scrape procedure via an evidence based instructional video at a quaternary referral eye hospital. Curr. Eye Res. 2020, 45, 529–534. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Ho, C.S.; Cairns, J.; Elsahn, A.; Al-Aqaba, M.; Boswell, T.; Said, D.G.; Dua, H.S. 12-year analysis of incidence, microbiological profiles and in vitro antimicrobial susceptibility of infectious keratitis: The nottingham infectious keratitis study. Br. J. Ophthalmol. 2021, 105, 328–333. [Google Scholar] [CrossRef]
- Maberly, J. Evaluating Severity of Microbial Keratitis Using Optical Coherence Tomography. Ph.D. Thesis, The University of Sidney, Sidney, Australia, 2021. [Google Scholar]
- Allan, B.D.; Dart, J.K. Strategies for the management of microbial keratitis. Br. J. Ophthalmol. 1995, 79, 777–786. [Google Scholar] [CrossRef]
- Khoo, P.; McCluskey, P.; Cabrera-Aguas, M.; Watson, S.L. Bacterial eye infections. In Encyclopedia of Infection and Immunity; Rezaei, N., Ed.; Elsevier: Oxford, UK, 2022; pp. 204–218. [Google Scholar]
- Cabrera-Aguas, M.; Khoo, P.; McCluskey, P.; Watson, S.L. Viral ocular infections. In Encyclopedia of Infection and Immunity; Rezaei, N., Ed.; Elsevier: Oxford, UK, 2022; pp. 219–233. [Google Scholar]
- White, M.L.; Chodosh, J. Herpes Simplex Virus Keratitis: A Treatment Guideline; Hoskins Center for Quality Eye Care, American Academy of Ophthalmology: San Francisco, CA, USA, 2014. [Google Scholar]
- Azher, T.N.; Yin, X.T.; Tajfirouz, D.; Huang, A.J.; Stuart, P.M. Herpes simplex keratitis: Challenges in diagnosis and clinical management. Clin. Ophthalmol. 2017, 11, 185–191. [Google Scholar] [CrossRef]
- Thomas, P.A. Current perspectives on ophthalmic mycoses. Clin. Microbiol. Rev. 2003, 16, 730–797. [Google Scholar] [CrossRef]
- Maharana, P.; Sharma, N.; Nagpal, R.; Jhanji, V.; Das, S.; Vajpayee, R. Recent advances in diagnosis and management of mycotic keratitis. Indian J. Ophthalmol. 2016, 64, 346–357. [Google Scholar]
- Cabrera-Aguas, M.; Khoo, P.; McCluskey, P.; Watson, S.L. Fungal ocular infections. In Encyclopedia of Infection and Immunity; Rezaei, N., Ed.; Elsevier: Oxford, UK, 2022; pp. 234–245. [Google Scholar]
- Dart, J.K.; Saw, V.P.; Kilvington, S. Acanthamoeba keratitis: Diagnosis and treatment update 2009. Am. J. Ophthalmol. 2009, 148, 487–499.e2. [Google Scholar] [CrossRef] [PubMed]
- Höllhumer, R.; Keay, L.; Watson, S.L. Acanthamoeba keratitis in australia: Demographics, associated factors, presentation and outcomes: A 15-year case review. Eye 2020, 34, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Khoo, P.; Cabrera-Aguas, M.; Watson, S.L. Parasitic eye infections. In Encyclopedia of Infection and Immunity; Rezaei, N., Ed.; Elsevier: Oxford, UK, 2022; pp. 246–258. [Google Scholar]
- Ting, D.S.J.; Gopal, B.P.; Deshmukh, R.; Seitzman, G.D.; Said, D.G.; Dua, H.S. Diagnostic armamentarium of infectious keratitis: A comprehensive review. Ocul. Surf. 2022, 23, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Wiki, A.A.o.O.-E. Slit Lamp Examination. Available online: https://eyewiki.aao.org/Slit_Lamp_Examination (accessed on 29 September 2023).
- Muth, D.R.; Blaser, F.; Foa, N.; Scherm, P.; Mayer, W.J.; Barthelmes, D.; Zweifel, S.A. Smartphone slit lamp imaging-usability and quality assessment. Diagnostics 2023, 13, 423. [Google Scholar] [CrossRef]
- Mukherjee, B.; Nair, A.G. Principles and practice of external digital photography in ophthalmology. Indian J. Ophthalmol. 2012, 60, 119–125. [Google Scholar] [CrossRef]
- Chhablani, J.; Kaja, S.; Shah, V.A. Smartphones in ophthalmology. Indian J. Ophthalmol. 2012, 60, 127. [Google Scholar]
- Store, G. Pixel 8 Specifications. Available online: https://store.google.com/au/product/pixel_8_specs?hl=en-US&pli=1 (accessed on 29 September 2023).
- Roy, S.; Pantanowitz, L.; Amin, M.; Seethala, R.R.; Ishtiaque, A.; Yousem, S.A.; Parwani, A.V.; Cucoranu, l.; Hartman, D.J. Smartphone adapters for digital photomicrography. J. Pathol. Inform. 2014, 5, 24. [Google Scholar] [CrossRef]
- Konstantopoulos, A.; Yadegarfar, G.; Fievez, M.; Anderson, D.F.; Hossain, P. In vivo quantification of bacterial keratitis with optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2011, 52, 1093–1097. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Rollins, A.M.; Roth, J.E.; Yazdanfar, S.; Westphal, V.; Bardenstein, D.S.; Izatt, J.A. Real-time optical coherence tomography of the anterior segment at 1310 nm. Arch. Ophthalmol. 2001, 119, 1179–1185. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Said, D.G.; Dua, H.S. Interface haze after descemet stripping automated endothelial keratoplasty. JAMA Ophthalmol. 2019, 137, 1201–1202. [Google Scholar] [CrossRef]
- Darren Shu Jeng, T.; Sathish, S.; Jean-Pierre, D. Epithelial ingrowth following laser in situ keratomileusis (LASIK): Prevalence, risk factors, management and visual outcomes. BMJ Open. Ophthalmol. 2018, 3, e000133. [Google Scholar]
- Almaazmi, A.; Said, D.G.; Messina, M.; Alsaadi, A.; Dua, H.S. Mechanism of fluid leak in non-traumatic corneal perforations: An anterior segment optical coherence tomography study. Br. J. Ophthalmol. 2020, 104, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ren, S.W.; Dai, L.J.; Zhang, B.; Gu, Y.W.; Pang, C.J.; Wang, Y. Bacterial keratitis following small incision lenticule extraction. Infect. Drug. Resist. 2022, 15, 4585–4593. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.; Brar, S.; Nagesh, B.N. Management of infectious keratitis following uneventful small-incision lenticule extraction using a multimodal approach—A case report. Indian J. Ophthalmol. 2020, 68, 3064. [Google Scholar] [PubMed]
- Geevarghese, A.; Wollstein, G.; Ishikawa, H.; Schuman, J.S. Optical coherence tomography and glaucoma. Annu. Rev. Vis. Sci. 2021, 7, 693–726. [Google Scholar] [CrossRef]
- Unterhuber, A.; Povazay, B.; Bizheva, K.; Hermann, B.; Sattmann, H.; Stingl, A.; Le, T.; Seefeld, M.; Menzel, R.; Preusser, M.; et al. Advances in broad bandwidth light sources for ultrahigh resolution optical coherence tomography. Phys. Med. Biol. 2004, 49, 1235–1246. [Google Scholar] [CrossRef]
- Soliman, W.; Fathalla, A.M.; El-Sebaity, D.M.; Al-Hussaini, A.K. Spectral domain anterior segment optical coherence tomography in microbial keratitis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 549–553. [Google Scholar] [CrossRef]
- Yamazaki, N.; Kobayashi, A.; Yokogawa, H.; Ishibashi, Y.; Oikawa, Y.; Tokoro, M.; Sugiyama, K. In vivo imaging of radial keratoneuritis in patients with acanthamoeba keratitis by anterior-segment optical coherence tomography. Ophthalmology 2014, 121, 2153–2158. [Google Scholar] [CrossRef]
- Oliveira, M.A.; Rosa, A.; Soares, M.; Gil, J.; Costa, E.; Quadrado, M.J.; Murta, J. Anterior segment optical coherence tomography in the early management of microbial keratitis: A cross-sectional study. Acta Med. Port. 2020, 33, 318–325. [Google Scholar] [CrossRef]
- Schuman, J.S. Spectral domain optical coherence tomography for glaucoma (an aos thesis). Trans. Am. Ophthalmol. Soc. 2008, 106, 426–458. [Google Scholar]
- Adhi, M.; Liu, J.J.; Qavi, A.H.; Grulkowski, I.; Lu, C.D.; Mohler, K.J.; Ferrara, D.; Kraus, M.F.; Baumal, C.R.; Witkin, A.J.; et al. Choroidal analysis in healthy eyes using swept-source optical coherence tomography compared to spectral domain optical coherence tomography. Am. J. Ophthalmol. 2014, 157, 1272–1281.e1. [Google Scholar] [CrossRef] [PubMed]
- Kostanyan, T.; Wollstein, G.; Schuman, J.S. Evaluating glaucoma damage: Emerging imaging technologies. Expert Rev. Ophthalmol. 2015, 10, 183–195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Donovan, C.; Arenas, E.; Ayyala, R.S.; Margo, C.E.; Espana, E.M. Fungal keratitis: Mechanisms of infection and management strategies. Surv. Ophthalmol. 2021, 67, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Brasnu, E.; Bourcier, T.; Dupas, B.; Degorge, S.; Rodallec, T.; Laroche, L.; Borderie, V.; Baudouin, C. In vivo confocal microscopy in fungal keratitis. Br. J. Ophthalmol. 2007, 91, 588–591. [Google Scholar] [CrossRef]
- Kumar, R.L.; Cruzat, A.; Hamrah, P. Current state of in vivo confocal microscopy in management of microbial keratitis. Semin. Ophthalmol. 2010, 25, 166–170. [Google Scholar] [CrossRef]
- Kanavi, M.R.; Javadi, M.; Yazdani, S.; Mirdehghanm, S. Sensitivity and specificity of confocal scan in the diagnosis of infectious keratitis. Cornea 2007, 26, 782–786. [Google Scholar] [CrossRef]
- Wang, Y.E.; Tepelus, T.C.; Vickers, L.A.; Baghdasaryan, E.; Gui, W.; Huang, P.; Irvine, J.A.; Sadda, S.; Hsu, H.Y.; Lee, O.L. Role of in vivo confocal microscopy in the diagnosis of infectious keratitis. Int. Ophthalmol. 2019, 39, 2865–2874. [Google Scholar] [CrossRef]
- Vaddavalli, P.K.; Garg, P.; Sharma, S.; Sangwan, V.S.; Rao, G.N.; Thomas, R. Role of confocal microscopy in the diagnosis of fungal and acanthamoeba keratitis. Ophthalmology 2011, 118, 29–35. [Google Scholar] [CrossRef]
- Goh, J.W.Y.; Harrison, R.; Hau, S.; Alexander, C.L.; Tole, D.M.; Avadhanam, V.S. Comparison of in vivo confocal microscopy, pcr and culture of corneal scrapes in the diagnosis of acanthamoeba keratitis. Cornea 2018, 37, 480–485. [Google Scholar] [CrossRef]
- Chidambaram, J.D.; Prajna, N.V.; Larke, N.L.; Palepu, S.; Lanjewar, S.; Shah, M.; Elakkiya, S.; Lalitha, P.; Carnt, N.; Vesaluoma, M.H.; et al. Prospective study of the diagnostic accuracy of the in vivo laser scanning confocal microscope for severe microbial keratitis. Ophthalmology 2016, 123, 2285–2293. [Google Scholar] [CrossRef]
- Villani, E.; Baudouin, C.; Efron, N.; Hamrah, P.; Kojima, T.; Patel, S.V.; Pflugfelder, S.C.; Zhivov, A.; Dogru, M. In vivo confocal microscopy of the ocular surface: From bench to bedside. Curr. Eye Res. 2014, 39, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Foo, V.H.; Yang, L.W.Y.; Sia, J.T.; Ang, M.; Lin, H.; Chodosh, J.; Mehta, J.S.; Ting, D.S.W. Artificial intelligence for anterior segment diseases: Emerging applications in ophthalmology. Br. J. Ophthalmol. 2021, 105, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Y.; Zhang, H.; Samusak, A.; Rao, H.; Xiao, C.; Abula, M.; Cao, Q.; Dai, Q. Artificial intelligence-assisted diagnosis of ocular surface diseases. Front. Cell. Dev. Biol. 2023, 11, 1133680. [Google Scholar] [CrossRef] [PubMed]
- Buisson, M.; Navel, V.; Labbe, A.; Watson, S.L.; Baker, J.S.; Murtagh, P.; Chiambaretta, F.; Dutheil, F. Deep learning versus ophthalmologists for screening for glaucoma on fundus examination: A systematic review and meta-analysis. Clin. Exp. Ophthalmol. 2021, 49, 1027–1038. [Google Scholar] [CrossRef]
- Rampat, R.; Deshmukh, R.; Chen, X.; Ting, D.S.W.; Said, D.G.; Dua, H.S.; Ting, D.S.J. Artificial intelligence in cornea, refractive surgery, and cataract: Basic principles, clinical applications, and future directions. Asia Pac. J. Ophthalmol. 2021, 10, 268–281. [Google Scholar] [CrossRef]
- Kuo, M.-T.; Hsu, B.W.-Y.; Lin, Y.-S.; Fang, P.-C.; Yu, H.-J.; Chen, A.; Yu, M.-S.; Tseng, V.S. Comparisons of deep learning algorithms for diagnosing bacterial keratitis via external eye photographs. Sci. Rep. 2021, 11, 24227. [Google Scholar] [CrossRef]
- Redd, T.K.; Prajna, N.V.; Srinivasan, M.; Lalitha, P.; Krishnan, T.; Rajaraman, R.; Venugopal, A.; Acharya, N.; Seitzman, G.D.; Lietman, T.M.; et al. Image-based differentiation of bacterial and fungal keratitis using deep convolutional neural networks. Ophthalmol. Sci. 2022, 2, 100119. [Google Scholar] [CrossRef]
- Wang, L.; Chen, K.; Wen, H.; Zheng, Q.; Chen, Y.; Pu, J.; Chen, W. Feasibility assessment of infectious keratitis depicted on slit-lamp and smartphone photographs using deep learning. Int. J. Med. Inform. 2021, 155, 104583. [Google Scholar] [CrossRef]
- Hung, N.; Shih, A.K.; Lin, C.; Kuo, M.-T.; Hwang, Y.-S.; Wu, W.-C.; Kuo, C.-F.; Kang, E.Y.; Hsiao, C.-H. Using slit-lamp images for deep learning-based identification of bacterial and fungal keratitis: Model development and validation with different convolutional neural networks. Diagnostics 2021, 11, 1246. [Google Scholar] [CrossRef]
- Koyama, A.; Miyazaki, D.; Nakagawa, Y.; Ayatsuka, Y.; Miyake, H.; Ehara, F.; Sasaki, S.I.; Shimizu, Y.; Inoue, Y. Determination of probability of causative pathogen in infectious keratitis using deep learning algorithm of slit-lamp images. Sci. Rep. 2021, 11, 22642. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Wang, S.; Wei, Z.; Zhang, Y.; Wang, Z.; Chen, K.; Ou, Z.; Liang, Q. Deep learning-based classification of infectious keratitis on slit-lamp images. Ther. Adv. Chronic Dis. 2022, 13, 20406223221136071. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.-T.; Hsu, B.W.-Y.; Yin, Y.-K.; Fang, P.-C.; Lai, H.-Y.; Chen, A.; Yu, M.-S.; Tseng, V.S. A deep learning approach in diagnosing fungal keratitis based on corneal photographs. Sci. Rep. 2020, 10, 14424. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.T.; Hsu, B.W.; Lin, Y.S.; Fang, P.C.; Yu, H.J.; Hsiao, Y.T.; Tseng, V.S. Deep learning approach in image diagnosis of pseudomonas keratitis. Diagnostics 2022, 12, 2948. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Thammasudjarit, R.; Jongkhajornpong, P.; Attia, J.; Thakkinstian, A. Deep learning for discrimination between fungal keratitis and bacterial keratitis: Deepkeratitis. Cornea 2022, 41, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Matai, H.D.; Raman, S.; Kumar, S.; Ravichandran, S.; Swaminathan, S.; Rani Alex, J.S. Advances in the diagnosis of herpes simplex stromal necrotising keratitis: A feasibility study on deep learning approach. Indian J. Ophthalmol. 2022, 70, 3279–3283. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Hu, S.; Sun, Y.; Wang, Y.; Xu, P.; Ye, J. Class-aware attention network for infectious keratitis diagnosis using corneal photographs. Comput. Biol. Med. 2022, 151 Pt A, 106301. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Huang, G.; Liu, Z.; Van Der Maaten, L.; Weinberger, K.Q. Densely connected convolutional networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 4700–4708. [Google Scholar]
- Sandler, M.; Howard, A.; Zhu, M.; Zhmoginov, A.; Chen, L.C. MobileNetV2: Inverted Residuals and Linear Bottlenecks. In Proceedings of the 2018 IEEE/CVF Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 4510–4520. [Google Scholar]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- Ganaie, M.A.; Hu, M.; Malik, A.K.; Tanveer, M.; Suganthan, P.N. Ensemble deep learning: A review. Eng. Appl. Artif. Intell. 2022, 115, 105151. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, J.; Chen, K.; Chen, Q.; Zheng, Q.; Liu, X.; Weng, H.; Wu, S.; Chen, W. Preventing corneal blindness caused by keratitis using artificial intelligence. Nat. Commun. 2021, 12, 3738. [Google Scholar] [CrossRef]
- Hu, S.; Sun, Y.; Li, J.; Xu, P.; Xu, M.; Zhou, Y.; Wang, Y.; Wang, S.; Ye, J. Automatic diagnosis of infectious keratitis based on slit lamp images analysis. J. Pers. Med. 2023, 13, 519. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, Y.; Li, Y.; Xiao, X.; Qiu, Q.; Yang, M.; Zhao, Y.; Cui, L. Automatic diagnosis of fungal keratitis using data augmentation and image fusion with deep convolutional neural network. Comput. Methods Programs Biomed. 2020, 187, 105019. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, K.; Chen, Q.; Chen, Q.; Huang, W.; Cui, L.; Li, M.; Li, J.; Chen, L.; Shen, C.; et al. Deep learning-based automated diagnosis of fungal keratitis with in vivo confocal microscopy images. Ann. Transl. Med. 2020, 8, 706. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Yoo, T.K. New era after ChatGPT in ophthalmology: Advances from data-based decision support to patient-centered generative artificial intelligence. Ann. Transl. Med. 2023, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.K.; Choi, J.Y.; Kim, H.K.; Ryu, I.H.; Kim, J.K. Adopting low-shot deep learning for the detection of conjunctival melanoma using ocular surface images. Comput. Methods Programs Biomed. 2021, 205, 106086. [Google Scholar] [CrossRef]
- Jadon, S. An overview of deep learning architectures in few-shot learning domain. arXiv 2020, arXiv:2008.06365. [Google Scholar]
- Quellec, G.; Lamard, M.; Conze, P.-H.; Massin, P.; Cochener, B. Automatic detection of rare pathologies in fundus photographs using few-shot learning. Med. Image Anal. 2020, 61, 101660. [Google Scholar] [CrossRef]
- Burlina, P.; Paul, W.; Mathew, P.; Joshi, N.; Pacheco, K.D.; Bressler, N.M. Low-shot deep learning of diabetic retinopathy with potential applications to address artificial intelligence bias in retinal diagnostics and rare ophthalmic diseases. JAMA Ophthalmol. 2020, 138, 1070–1077. [Google Scholar] [CrossRef]
- Delsoz, M.; Madadi, Y.; Munir, W.M.; Tamm, B.; Mehravaran, S.; Soleimani, M.; Djalilian, A.; Yousefi, S. Performance of chatgpt in diagnosis of corneal eye diseases. medRxiv 2023. [Google Scholar] [CrossRef]
- Lee, P.; Bubeck, S.; Petro, J. Benefits, limits, and risks of GPT-4 as an AI Chatbot for medicine. N. Engl. J. Med. 2023, 388, 1233–1239. [Google Scholar] [CrossRef]
- Singh, S.; Watson, S. Chatgpt as a tool for conducting literature review for dry eye disease. Clin. Exp. Ophthalmol. 2023, 51, 731–732. [Google Scholar] [CrossRef]
- Bartimote, C.; Foster, J.; Watson, S.L. The spectrum of microbial keratitis: An updated review. Open Ophthalmol. J. 2019, 13, 100–130. [Google Scholar] [CrossRef]
- Karsten, E.; Watson, S.L.; Foster, L.J. Diversity of microbial species implicated in keratitis: A review. Open Ophthalmol. J. 2012, 6, 110–124. [Google Scholar] [CrossRef] [PubMed]
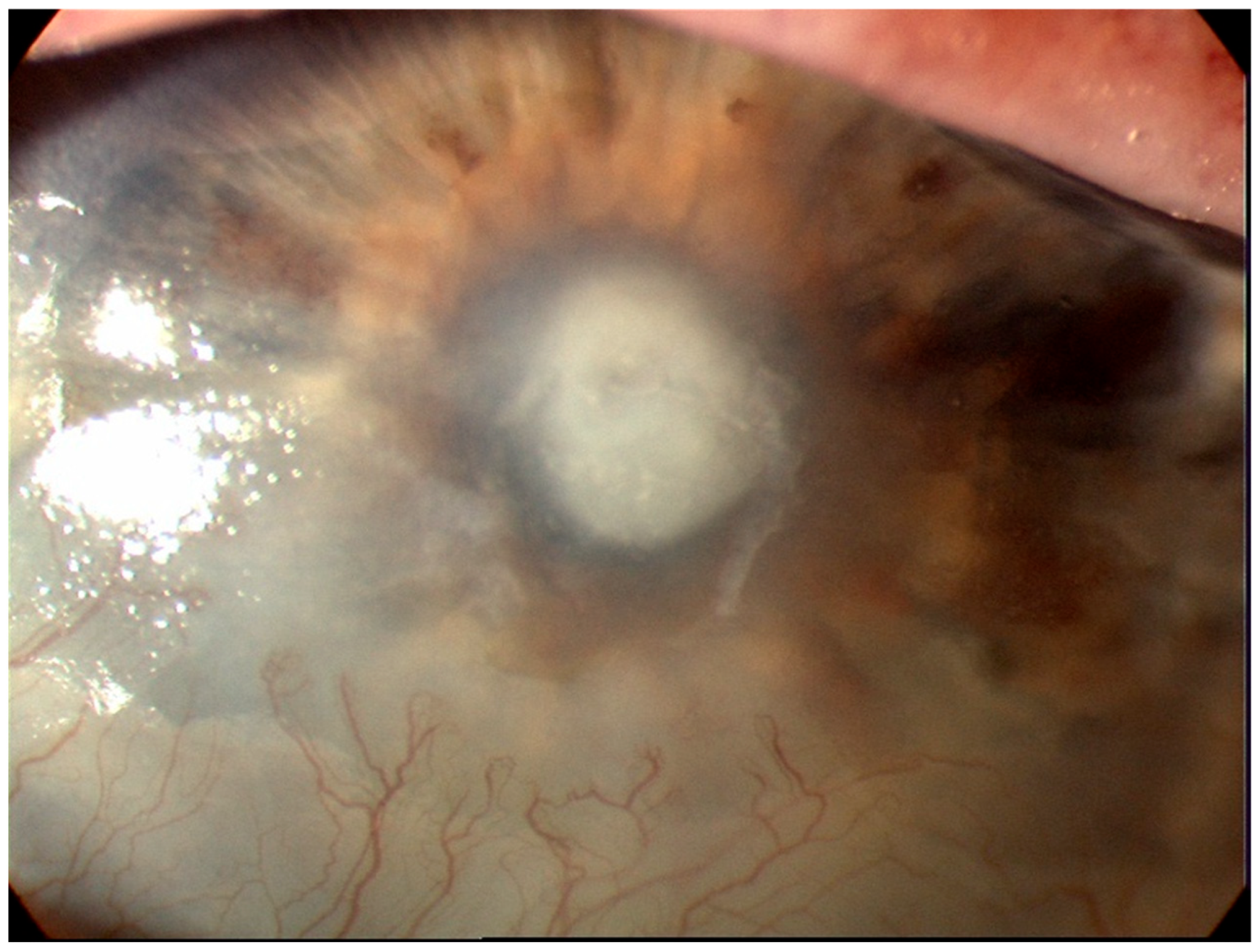







| BK | HSK | FK | AK | |
|---|---|---|---|---|
| Most causal organism |
| Herpes simplex virus |
| Acanthamoeba spp. |
| Clinical signs |
|
|
|
|
| Imaging Method | Clinical Features | Resolution of Images | Clinical Indications | Sensitivity | Specificity | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|
| SS OCT |
|
|
| ||||
| IVCM |
| HRT3 uses 670 nm red wavelength and high-resolution images with lateral resolution close to 1 µm, axial resolution of 7.6 µm and 400× magnification |
|
|
|
|
|
| Characteristic on AS-OCT | Points |
|---|---|
| Corneal thickness change 5–10% | 1 |
| Corneal thickness change 10–30% | 2 |
| Corneal thickness change 30–50% | 3 |
| Corneal thickness change >50% | 4 |
| Epithelial defect 0.1–1 × 0.1–1 | 1 |
| Epithelial defect 1–2 × 1–2 | 2 |
| Epithelial defect 2–3 × 2–3 | 3 |
| Epithelial defect >3x > 3 | 4 |
| Hypopyon | 1 |
| Infiltrates 0.1 mm–1 | 1 |
| Infiltrates >1 | 2 |
| Stromal thinning | 1 |
| Cysts | 1 |
| Scarring | 1 |
| Fibrin deposits | 1 |
| Total | 28 |
| (A) | ||
| Actual Outcome | ||
| Disease | No Disease | |
| Predicted outcome | Disease | TP |
| No Disease | FN | |
| (B) | ||
| Sensitivity | TP/(TP + FN) | Actual positive cases predicted correctly by the model |
| Specificity | TN/(TN + FP) | Actual negative cases predicted correctly by the model |
| PPV (Precision) | TP/(TP + FP) | Positively classified cases that were actually positive |
| NPV | TN/(TN + FN) | Negatively classified cases that were actually negative |
| Accuracy | (TP + TN)/(TP + TN + FP + FN) | Overall accuracy in predicting both positive and negative cases. |
| Authors | Year | Study Population | Image Modality | Image Size | AI Algorithm | Outcome Measures | AUROC (95% CI) | AUPRC (95% CI) | F1 Score | Accuracy % (95% CI) | Sensitivity % (95% CI) | Specificity % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li et al. [70] | 2021 | Healthy eyes | Smart phone (Huawei P30) | 1030 | DN121, IC-v3, RN50 | Detect of keratitis, cornea with other abnormalities and normal cornea |
|
|
|
| ||
| Redd et al. [55] | 2022 | Culture-proven BK or FK | Handheld Nikon D-series digital single-lens reflex camera | 980 | MNV2, DN201, RN152V2, VGG19, Xception | Different BK and FK | Multicentre set of 80 images:
| CNN:
| ||||
| Wang et al. [56] | 2021 | Normal eyes and with IK | Slit lamp-mounted camera and smart phone |
| IC-v3, RN50, DN121 | Different normal cornea, BK, FK and VK | Slit lamp Global images:
Images:
| |||||
| Hung et al. [57] | 2021 | Culture-proven IK | Slit lamp mounted camera | 1330 | RN50, RN101, DN121, DN 161, DN 169, DN201, IC-v3, ENB3 | Identify BK and FK |
| BK vs. FK:
|
|
| ||
| Kuo et al. [54] | 2021 | Culture-proven IK or three specialists have a consensus impression of one type of IK | Slit lamp mounted camera | 1512 | RN50, RNXt50, SE-RN50, DN121, ENB0, ENB1, ENB2, ENB3, | Diagnose BK |
|
|
|
| ||
| Ghosh et al. [62] | 2022 | Culture-proven IK | Slit lamp-mounted camera | 2167 | Deep-Keratitis model: VGG19, RN50, DN121, | Classify BK and FK |
|
|
| |||
| Hu et al. [71] | 2023 | Culture-proven IK | Slit lamp-mounted camera | 2757 | VGG16, RN34, IC-v4, DN121, Vit-Base, ENV2-M | Different BK, FK and VK |
|
|
|
| ||
| Kuo et al. [61] | 2022 | Culture-proven BK | Slit lamp mounted camera | 929 | RN50, RNXt50, SE-RN50, DN121, ENB0, ENB1, ENB2, ENB3, | Diagnose Pseudomonas aeruginosa keratitis | RN50 = 70.2 (64.4–75.9) RNXt50 = 69.8 (65.8–73.7) SE-RN50 = 70 (66.2–73.7) DN121 = 70.9 (65.7–76.1) ENB0 = 67 (60.3–73.6) ENB1 = 68.5 (64.3 -72.6) ENB2 = 71.2 (68.5–73.8) ENB3 = 68.6 (64.6–72.5) | RN50 = 80.4 (73.5–87.3) RNXt50 = 81.2 (74.6–87.9) SE-RN50 = 82.4 (68.7–96.1) DN121 = 82.5 (78.5–86.6) ENB0 = 66.5 (55.7–77.4) ENB1 = 74.6 (64.4 -84.8) ENB2 = 81.1 (76.3–85.8) ENB3 = 68.8 (62–75.5) | RN50 = 49.9 (39–60.7) RNXt50 = 46.9 (32.2–61.7) SE-RN50 = 45.3 (20.54–70.1) DN121 = 47.9 (37–58.8) ENB0 = 67.9 (62.2–73.5) ENB1 = 56.3 (47–65.6) ENB2 = 51.5 (47.1–55.8) ENB3 = 68.2 (65–71.3) | |||
| Natarajan et al. [63] | 2022 | Active HSV stromal necrotising keratitis PCR-proven and culture-proven nonviral keratitis | Slit lamp-mounted camera | 307 | DN201, RN-50, Google-Net | Diagnose HSK stromal necrotising | DN201 = 0.73 (0.568–0.892) | DN201 = 72 RN-50 = 60 | DN201 = 69.6 | DN201 = 76.5 | ||
| Koyama et al. [58] | 2021 | Culture-proven IK | Slit lamp mounted camera | 4306 | RN-50, IC, RN-v2 | Determine the type of IK | IC+RN-v2:
| IC+RN-v2:
| IC+RNV2:
| |||
| Zhang et al. [59] | 2022 |
| Slit lamp mounted camera | 4830 | RN18, RN50, DN121, DN169, EN-b0, EN-b5, EN-b7, RestNext 101_32 × 8 d, ResNext101_32 × 16 d | Differentiate BK, FK, HSK, AK |
|
FK = 77.71, AK = 83.81, HSK = 79.31 | ||||
| Liu et al. [72] | 2020 | Healthy eyes and FK | IVCM | 1213 | AlexNet VGGNet | Detect FK | AlexNet = 99.35 VGGNet = 99.14 | 99.9 | 100 | |||
| Lv et al. [73] | 2020 | Culture-proven FK | IVCM | 2088 | RN | Detect FK | 0.9769 | 0.9364 | 0.8256 | 0.9889 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera-Aguas, M.; Watson, S.L. Updates in Diagnostic Imaging for Infectious Keratitis: A Review. Diagnostics 2023, 13, 3358. https://doi.org/10.3390/diagnostics13213358
Cabrera-Aguas M, Watson SL. Updates in Diagnostic Imaging for Infectious Keratitis: A Review. Diagnostics. 2023; 13(21):3358. https://doi.org/10.3390/diagnostics13213358
Chicago/Turabian StyleCabrera-Aguas, Maria, and Stephanie L Watson. 2023. "Updates in Diagnostic Imaging for Infectious Keratitis: A Review" Diagnostics 13, no. 21: 3358. https://doi.org/10.3390/diagnostics13213358
APA StyleCabrera-Aguas, M., & Watson, S. L. (2023). Updates in Diagnostic Imaging for Infectious Keratitis: A Review. Diagnostics, 13(21), 3358. https://doi.org/10.3390/diagnostics13213358





