Phenotypically Discordant Anomalies in Conjoined Twins: Quirks of Nature Governed by Molecular Pathways?
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Discordance in Laterally United Twins
3.2. Discordance in Ventrally United Twins
3.3. Discordance in Caudally United Twins
4. Discussion
4.1. The Twinning Dogma and the Occurrence of Structural (Discordant) Defects
4.2. Unilateral Birth Defects in Singletons: Discordances in Left–Right Patterning?
4.3. The Molecular Basis for Asymmetry
4.4. Early Patterning in Vertebrate Development: Axis Formation
4.5. Morphogenetic Fields and Morphogens
4.6. Effects of Hypoxia and Hemodynamics on Embryological Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mutchinick, O.M.; Luna-Muñoz, L.; Amar, E.; Bakker, M.K.; Clementi, M.; Cocchi, G.; da Graça Dutra, M.; Feldkamp, M.L.; Landau, D.; Leoncini, E.; et al. Conjoined twins: A worldwide collaborative epidemiological study of the International Clearinghouse for Birth Defects Surveillance and Research. Am. J. Med. Genet. Part C Semin. Med. Genet. 2011, 157, 274–287. [Google Scholar] [CrossRef]
- Hall, J.G. Twinning. Lancet 2003, 362, 735–743. [Google Scholar] [CrossRef]
- Boer, L.L.; Schepens-Franke, A.N.; Winter, E.; Oostra, R.J. Characterizing the coalescence area of conjoined twins to elucidate congenital disorders in singletons. Clin. Anat. 2021, 34, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Oostra, R.J.; Schepens-Franke, A.N.; Magno, G.; Zanatta, A.; Boer, L.L. Conjoined twins and conjoined triplets: At the heart of the matter. Birth Defects Res. 2022, 114, 596–610. [Google Scholar] [CrossRef] [PubMed]
- Boer, L.L.; Schepens-Franke, A.N.; Oostra, R.J. Two is a Crowd: Two is a Crowd: On the Enigmatic Etiopathogenesis of Conjoined Twinning. Clin. Anat. 2019, 32, 722–741. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Sharma, S.; Mittal, P.; Yadav, R.K.; Barolia, D. Heteropagus twins: Six cases with systematic review and embryological insights. Pediatr. Surg. Int. 2022, 38, 963–983. [Google Scholar] [CrossRef]
- Spencer, R. Parasitic conjoined twins: External, internal (fetuses in fetu and teratomas), and detached (acardiacs). Clin. Anat. 2001, 14, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R. Conjoined Twins: Developmental Malformations and Clinical Implications; Johns Hopkins University: Baltimore, MD, USA, 2003; pp. 1–476. [Google Scholar]
- Esenkaya, S.; Gürbüz, B.; Yalti, S. Asymmetric parasitic dicephalus conjoined twins. J. Clin. Ultrasound 2004, 32, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, B.D. Abnormal umbilical vessels and systemic circulatory reversal in thoracopagus twins. Acta Genet. Med. Gemellol. 1975, 24, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liu, B.; Tan, L.; Zuo, Y.X. Successful tracheal intubation using the Airtraq in thoraco-omphalopagus twins. Anesth. Analg. 2011, 113, 204–205. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, T.C.; Crombleholme, T.M.; Johnson, M.P.; Schnaufer, L.; Flake, A.W.; Hedrick, H.L.; Howell, L.J.; Adzick, N.S. The natural history of prenatally diagnosed conjoined twins. J. Pediatr. Surg. 2002, 37, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Ornoy, A.; Navot, D.; Menashi, M.; Laufer, N.; Chemke, J. Asymmetry and discordance for congenital anomalies in conjoined twins: A report of six cases. Teratology 1980, 22, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Bovendeert, J.F.M.; Nievelstein, R.A.J.; Bleys, R.L.A.W.; Cleypool, C.G.J. A parapagus dicephalus tripus tribrachius conjoined twin with a unique morphological pattern: A case report. J. Med. Case Rep. 2020, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Usang, U.E.; Olasode, B.J.; Archibong, A.E.; Udo, J.J.; Eduwem, D.-A.U. Dicephalus parapagus conjoined twins discordant for anencephaly: A case report. J. Med. Case Rep. 2010, 4, 38. [Google Scholar] [CrossRef]
- Edmonds, L.D.; Layde, P.M. Conjoined twins in the united states, 1970–1977. Teratology 1982, 25, 301–308. [Google Scholar] [CrossRef]
- Métneki, J.; Czeizel, A. Conjoined twins in Hungary, 1970–1986. Acta Genet. Med. Gemellol. 1989, 38, 285–299. [Google Scholar] [CrossRef]
- Chatkupt, S.; Chatkupt, S.; Kohut, G.; Chervenak, F.A. Antepartum diagnosis of discordant anencephaly in dicephalic conjoined twins. J. Clin. Ultrasound 1993, 21, 138–142. [Google Scholar] [CrossRef]
- Vogel, F.S. The association of vascular anomalies with anencephaly: A postmortem study of nine cases in one of which unilateral anencephaly was present in a conjoined double monster. Am. J. Pathol. 1958, 34, 169–183. [Google Scholar] [PubMed]
- Kumru, P. Diagnosis of male dicephalus parapagus dibrachıus conjoined twins discordant for anencephaly at early weeks of pregnancy. Case report. Gineco.eu 2014, 10, 38–39. [Google Scholar]
- Shrestha, T.; Baral, G.; Sedhain, N. Sirenomelia in Dicephalic parapagus twins discordant for anencephaly and spina bifida. Nepal. J. Obstet. Gynaecol. 2020, 15, 81–83. [Google Scholar] [CrossRef]
- Hamon, A.; Dinno, N. Dicephalus dipus tribrachius conjoined twins in a female infant. Birth Defects Orig. Artic. Ser. 1978, 14, 213–218. [Google Scholar]
- Boer, L.L.; Boek, P.L.J.; van Dam, A.J.; Oostra, R.J. History and highlights of the teratological collection in the Museum Anatomicum of Leiden University, The Netherlands. Am. J. Med. Genet. A 2018, 176, 618–637. [Google Scholar] [CrossRef] [PubMed]
- De Bils, L.; Buenius, G.; Leers, A. Generosissimi, Nobilissimi, Expertissimique Di. Di. Ludovici de Bils à Copensdamme, Bonem, &c. Di. urbis atque Territorii Ardenburgici Praetoris Specimina Anatomica Cum Clariss. Doctissimorumque Virorum Epistolis Aliquot & Testimoniis; Ex Officinâ Arnoldi Leers: Rotterdam, The Netherlands, 1661. [Google Scholar]
- Oostra, R.J.; Baljet, B.; Verbeeten, B.W.; Hennekam, R.C. Congenital anomalies in the teratological collection of Museum Vrolik in Amsterdam, The Netherlands. V: Conjoined and acardiac twins. Am. J. Med. Genet. 1998, 80, 74–89. [Google Scholar] [CrossRef]
- Al Muti Zaitoun, A.; Chang, J.; Booker, M. Diprosopus (partially duplicated head) associated with anencephaly: A case report. Pathol. Res. Pract. 1999, 195, 45–50. [Google Scholar] [CrossRef]
- Rehder, H.; Kircher, S.G.; Schoner, K.; Smogavec, M.; Behunova, J.; Ihm, U.; Plassmann, M.; Hofer, M.; Ringl, H.; Laccone, F. Brain malformations in diprosopia observed in clinical cases, museum specimens and artistic representations. Orphanet J. Rare Dis. 2023, 18, 57. [Google Scholar] [CrossRef]
- Onankpa, B.O.; Ukwu, A.E.; Singh, S.; Adoke, A.U.; Tahir, A. Fetal diprosopus (double-face): A case report. Int. J. Res. Health Sci. 2014, 3, 461–463. [Google Scholar] [CrossRef]
- Bidondo, M.P.; Groisman, B.; Tardivo, A.; Tomasoni, F.; Tejeiro, V.; Camacho, I.; Vilas, M.; Liascovich, R.; Barbero, P. Diprosopus: Systematic review and report of two cases. Birth Defects Res. A Clin. Mol. Teratol. 2016, 106, 993–1007. [Google Scholar] [CrossRef]
- Dhaifalah, I.; Curtisova, V.; Santavy, J. Prenatal diagnosis of monocephalic bifacial tetraophthalmic diprosopus (conjoined twin). Fetal Diagn. Ther. 2008, 23, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Masuzaki, H.; Miura, K.; Yoshiura, K.; Yoshimura, S.; Ishimaru, T. A monozygotic conjoined twin pregnancy discordant for laterality of cleft lip. Gynecol. Obstet. Investig. 2004, 57, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Barr, R.J.; Benirschke, K. Cytogenetic studies of conjoined twins. A case report. Obstet. Gynecol. 1971, 38, 877–881. [Google Scholar] [PubMed]
- Gilbert-Barness, E.; Debich-Spicer, D.; Opitz, J.M. Conjoined twins: Morphogenesis of the heart and a review. Am. J. Med. Genet. A 2003, 120, 568–582. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.M.; Ziessman, H.A. Cholescintigraphy for assessing the separation potential of thoracoomphalopagus twins. Clin. Nucl. Med. 1990, 15, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Offringa, P.J.; Wildschut, H.I.; Tutein Nolthenius-Puylaert, M.C.; Leon, S.; Boersma, E.R. Conjoined twins and abdominal pregnancy. Int. J. Gynaecol. Obstet. 1989, 30, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Seller, M.J. Conjoined twins discordant for cleft lip and palate. Am. J. Med. Genet. 1990, 37, 530–531. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G. Thoracopagus Conjoined Twins: A Case Report with Multiple Anomalies; Medwin Publishers: Troy, MI, USA, 2017. [Google Scholar]
- Lobe, T.E.; Oldham, K.T.; Richardson, C.J. Successful separation of a conjoined biliary tract in a set of omphalopagus twins. J. Pediatr. Surg. 1989, 24, 930–932. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.Y.; Joo, D.H.; Won, H.S.; Lee, P.R.; Kim, A. “Hugging sisters”: Thoracoomphalopagus with anencephaly confirmed by three-dimensional ultrasonography at 9 weeks of gestation. J. Clin. Ultrasound 2011, 39, 279–282. [Google Scholar] [CrossRef]
- Singh, M.; Jacob, R.; Naik, V.; Baines, D. Separation of thoraco-omphalopagus twins in a rural secondary hospital: Perioperative management. Indian. J. Anaesth. 2012, 56, 442–447. [Google Scholar] [CrossRef]
- Spencer, R.; Robichaux, W.H.; Superneau, D.W.; Lucas, V.W., Jr. Unusual cardiac malformations in conjoined twins: Thoracopagus twins with conjoined pentalogy of Cantrell and an omphalopagus twin with atretic ventricles. Pediatr. Cardiol. 2002, 23, 631–638. [Google Scholar] [CrossRef]
- Wittich, A.C. Conjoined twins: Report of a case and review of the literature. J. Am. Osteopath. Assoc. 1989, 89, 1175–1179. [Google Scholar] [CrossRef]
- Bankl, H. Thoracopagus with common malformed heart (author’s transl). Padiatr. Padol. 1971, 6, 309–312. [Google Scholar] [PubMed]
- Wenzl, R.; Schurz, B.; Amann, G.; Eppel, W.; Schön, H.J.; Reinold, E. Diagnosis of cephalothoracopagus—A case report. Ultraschall Med. 1992, 13, 199–201. [Google Scholar] [CrossRef]
- de Jong, G.; Kirby, P.A. Defects of blastogenesis: Counseling dilemmas in two families. Am. J. Med. Genet. 2000, 91, 175–179. [Google Scholar] [CrossRef]
- Delprado, W.J.; Baird, P.J. Cephalothoracopagus syncephalus: A case report with previously unreported anatomical abnormalities and chromosomal analysis. Teratology 1984, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bates, A.W.; Dodd, S.M. Anomalies in cephalothoracopagus synotus twins and their implications for morphogenesis in conjoined twins. Pediatr. Dev. Pathol. 1999, 2, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Jo, D.S.; Jang, K.Y.; Cho, S.C. Extremely rare case of cephalothoracopagus characterized by differences of external genitalia. Prenat. Diagn. 2007, 27, 1151–1153. [Google Scholar] [CrossRef] [PubMed]
- Votteler, T.P. Surgical separation of conjoined twins. AORN J. 1982, 35, 35–46. [Google Scholar] [CrossRef]
- Chen, W.J.; Chen, K.M.; Chen, M.T.; Liu, T.K.; Chu, S.H.; Tsai, T.C.; Hwang, F.Y. Emergency separation of omphaloischiopagus tetrapus conjoined twins in the newborn period. J. Pediatr. Surg. 1989, 24, 1221–1224. [Google Scholar] [CrossRef]
- Kate, B.R. Double monsters. Indian. J. Med. Sci. 1970, 24, 341–353. [Google Scholar]
- Chan, D.P.; Lee, M.M. A Report of a Case of Ischiopagus Tetrapus. Singap. Med. J. 1964, 4, 125–129. [Google Scholar]
- Lawson, G.W. Female pseudohermaphroditism in conjoined twins. Case report. Br. J. Obstet. Gynaecol. 1980, 87, 1166–1168. [Google Scholar] [CrossRef]
- Khan, Y.A. Ischiopagus tripus conjoined twins. APSP J. Case Rep. 2011, 2, 5. [Google Scholar] [PubMed]
- Chen, T.L.; Lin, C.J.; Lai, H.S.; Chen, W.J.; Chao, C.C.; Liu, C.C. Anaesthetic managements for conjoined twins with complex cardiac anomalies. Can. J. Anaesth. 1996, 43, 1161–1167. [Google Scholar] [CrossRef]
- Rating, B. Über eine ungewöhnliche Gesichtsmißbildung bei Anencephalie. Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 1933, 288, 223–242. [Google Scholar] [CrossRef]
- Winkler, N.; Kennedy, A.; Byrne, J.; Woodward, P. The imaging spectrum of conjoined twins. Ultrasound Q. 2008, 24, 249–255. [Google Scholar] [CrossRef]
- Blaas, H.G.; Eriksson, A.G.; Salvesen, K.A.; Isaksen, C.V.; Christensen, B.; Møllerløkken, G.; Eik-Nes, S.H. Brains and faces in holoprosencephaly: Pre- and postnatal description of 30 cases. Ultrasound Obstet. Gynecol. 2002, 19, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Austin, E.; Schifrin, B.S.; Pomerance, J.J.; Gans, S.L.; Komaiko, M.S. The antepartum diagnosis of conjoined twins. J. Pediatr. Surg. 1980, 15, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, S.A.; Goel, S.S.; Subhedar, V.S. A thoracophagus conjoined twins with myelomeningocele: An unusual case. Int. J. Reprod. Contracept. Obstet. Gynecol. 2012, 1, 61–63. [Google Scholar] [CrossRef]
- Mansour, A.M.; Mansour, N.; Rosenberg, H.S. Ocular findings in conjoined (Siamese) twins. J. Pediatr. Ophthalmol. Strabismus 1991, 28, 261–264. [Google Scholar] [CrossRef]
- Goo, H.W.; Park, J.J.; Kim, E.A.; Won, H.S. Cardiac fusion and complex congenital cardiac defects in thoracopagus twins: Diagnostic value of cardiac CT. Pediatr. Radiol. 2014, 44, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Basgül, A.; Kavak, Z.N.; Sezen, D.; Basgul, A.; Gokaslan, H. Thoraco-omphalopagus conjoined twins detected at as early as 9 weeks of gestation: Transvaginal two-dimensional ultrasound, color Doppler and fetoplacental Doppler velocity waveform findings. Fetal Diagn. Ther. 2006, 21, 477–480. [Google Scholar] [CrossRef]
- Politzer, G.; Portele, K. Die Zehen der Sirenen. Wilhelm. Roux’ Arch. Entwicklungsmech. Org. 1956, 148, 452–462. [Google Scholar] [CrossRef]
- Boklage, C.E. Embryogenesis of chimeras, twins and anterior midline asymmetries. Hum. Reprod. 2006, 21, 579–591. [Google Scholar] [CrossRef] [PubMed]
- McNamara, H.C.; Kane, S.C.; Craig, J.M.; Short, R.V.; Umstad, M.P. A review of the mechanisms and evidence for typical and atypical twinning. Am. J. Obstet. Gynecol. 2016, 214, 172–191. [Google Scholar] [CrossRef] [PubMed]
- Boklage, C.E. Traces of embryogenesis are the same in monozygotic and dizygotic twins: Not compatible with double ovulation. Hum. Reprod. 2009, 24, 1255–1266. [Google Scholar] [CrossRef]
- Dirican, E.K.; Olgan, S. On the origin of zygosity and chorionicity in twinning: Evidence from human in vitro fertilization. J. Assist. Reprod. Genet. 2021, 38, 2809–2816. [Google Scholar] [CrossRef]
- Trombetta, G.; Fabbro, D.; Demori, E.; Driul, L.; Damante, G.; Xodo, S. Rare spontaneous monochorionic dizygotic twins: A case report and a systematic review. BMC Pregnancy Childbirth 2022, 22, 564. [Google Scholar] [CrossRef] [PubMed]
- Peters, H.E.; König, T.E.; Verhoeven, M.O.; Schats, R.; Mijatovic, V.; Ket, J.C.; Lambalk, C.B. Unusual Twinning Resulting in Chimerism: A Systematic Review on Monochorionic Dizygotic Twins. Twin Res. Hum. Genet. 2017, 20, 161–168. [Google Scholar] [CrossRef]
- Lee, H.J.; Yoon, S.C.; Ko, J.M.; Seong, M.W.; Park, S.S.; Choi, J.S.; Oh, S.K. Monochorionic dizygotic twins with discordant sex and confined blood chimerism. Eur. J. Pediatr. 2014, 173, 1249–1252. [Google Scholar] [CrossRef]
- Smeets, D.; van Vugt, J.M.; Gomes, I.; van den Heuvel, S.; van Heijst, A.; Reuss, A.; Claahsen-van der Grinten, H.L. Monochorionic dizygous twins presenting with blood chimerism and discordant sex. Twin Res. Hum. Genet. 2013, 16, 799–801. [Google Scholar] [CrossRef]
- Tong, S.; Vollenhoven, B.; Meagher, S. Determining zygosity in early pregnancy by ultrasound. Ultrasound Obstet. Gynecol. 2004, 23, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Glinianaia, S.V.; Rankin, J.; Wright, C. Congenital anomalies in twins: A register-based study. Hum. Reprod. 2008, 23, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Khairudin, D.; Khalil, A. Monochorionic monoamniotic twin pregnancies. Best. Pract. Res. Clin. Obstet. Gynaecol. 2022, 84, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Fernandez, J.E.; Spector, T.D.; Bell, J.T. Epigenetics of discordant monozygotic twins: Implications for disease. Genome Med. 2014, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Schinzel, A.A.; Smith, D.W.; Miller, J.R. Monozygotic twinning and structural defects. J. Pediatr. 1979, 95, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cozen, W.; Hwang, A.E.; Cockburn, M.G.; Zadnick, J.; Hamilton, A.S.; Mack, T.; Figueiredo, J.C. Birth Anomalies in Monozygotic and Dizygotic Twins: Results From the California Twin Registry. J. Epidemiol. 2019, 29, 18–25. [Google Scholar] [CrossRef]
- Weber, M.A.; Sebire, N.J. Genetics and developmental pathology of twinning. Semin. Fetal Neonatal Med. 2010, 15, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Hubinont, C.; Lewi, L.; Bernard, P.; Marbaix, E.; Debiève, F.; Jauniaux, E. Anomalies of the placenta and umbilical cord in twin gestations. Am. J. Obstet. Gynecol. 2015, 213, S91–S102. [Google Scholar] [CrossRef]
- Charles, E.B. Human Embryogenesis. In Embryogenesis; Ken-ichi, S., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Machin, G. Non-identical monozygotic twins, intermediate twin types, zygosity testing, and the non-random nature of monozygotic twinning: A review. Am. J. Med. Genet. C Semin. Med. Genet. 2009, 151, 110–127. [Google Scholar] [CrossRef]
- Gringras, P.; Chen, W. Mechanisms for differences in monozygous twins. Early Hum. Dev. 2001, 64, 105–117. [Google Scholar] [CrossRef]
- Czyz, W.; Morahan, J.M.; Ebers, G.C.; Ramagopalan, S.V. Genetic, environmental and stochastic factors in monozygotic twin discordance with a focus on epigenetic differences. BMC Med. 2012, 10, 93. [Google Scholar] [CrossRef]
- Acuna-Hidalgo, R.; Bo, T.; Kwint, M.P.; van de Vorst, M.; Pinelli, M.; Veltman, J.A.; Hoischen, A.; Vissers, L.E.; Gilissen, C. Post-zygotic Point Mutations Are an Underrecognized Source of De Novo Genomic Variation. Am. J. Hum. Genet. 2015, 97, 67–74. [Google Scholar] [CrossRef]
- Kaplowitz, P.B.; Bodurtha, J.; Brown, J.; Spence, J.E. Monozygotic twins discordant for Ullrich-Turner syndrome. Am. J. Med. Genet. 1991, 41, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Martins, Y.; Matias, A.; Blickstein, I. Why are monozygotic twins different? J. Perinat. Med. 2011, 39, 195–202. [Google Scholar] [CrossRef]
- Petronis, A.; Gottesman, I.I.; Kan, P.; Kennedy, J.L.; Basile, V.S.; Paterson, A.D.; Popendikyte, V. Monozygotic twins exhibit numerous epigenetic differences: Clues to twin discordance? Schizophr. Bull. 2003, 29, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Bruder, C.E.; Piotrowski, A.; Gijsbers, A.A.; Andersson, R.; Erickson, S.; Diaz de Ståhl, T.; Menzel, U.; Sandgren, J.; von Tell, D.; Poplawski, A.; et al. Phenotypically concordant and discordant monozygotic twins display different DNA copy-number-variation profiles. Am. J. Hum. Genet. 2008, 82, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Corroenne, R.; Al Ibrahim, A.; Stirnemann, J.; Zayed, L.H.; Essaoui, M.; Russell, N.E.; Chalouhi, G.E.; Salomon, L.J.; Ville, Y. Management of monochorionic twins discordant for structural fetal anomalies. Prenat. Diagn. 2020, 40, 1375–1382. [Google Scholar] [CrossRef]
- Rustico, M.A.; Lanna, M.; Faiola, S.; Casati, D.; Spaccini, L.; Righini, A.; Parazzini, C.; Napolitano, M.; Scelsa, B.; Lista, G.; et al. Major Discordant Structural Anomalies in Monochorionic Twins: Spectrum and Outcomes. Twin Res. Hum. Genet. 2018, 21, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Bourthoumieu, S.; Yardin, C.; Terro, F.; Gilbert, B.; Laroche, C.; Saura, R.; Vincent, M.C.; Esclaire, F. Monozygotic twins concordant for blood karyotype, but phenotypically discordant: A case of “mosaic chimerism”. Am. J. Med. Genet. A 2005, 135, 190–194. [Google Scholar] [CrossRef]
- Gilbert, B.; Yardin, C.; Briault, S.; Belin, V.; Lienhardt, A.; Aubard, Y.; Battin, J.; Servaud, M.; Philippe, H.J.; Lacombe, D. Prenatal diagnosis of female monozygotic twins discordant for Turner syndrome: Implications for prenatal genetic counselling. Prenat. Diagn. 2002, 22, 697–702. [Google Scholar] [CrossRef]
- Haque, F.N.; Gottesman, I.I.; Wong, A.H. Not really identical: Epigenetic differences in monozygotic twins and implications for twin studies in psychiatry. Am. J. Med. Genet. C Semin. Med. Genet. 2009, 151, 136–141. [Google Scholar] [CrossRef]
- Shur, N. The genetics of twinning: From splitting eggs to breaking paradigms. Am. J. Med. Genet. C Semin. Med. Genet. 2009, 151, 105–109. [Google Scholar] [CrossRef]
- Jones, K.L.; Jones, M.C.; Campo, M. Smith’s Recognizable Patterns of Human Malformation: Expert Consult—Online and Print; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Stevenson, R.E. Human Malformations and Related Anomalies; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Guo, Y.; Sun, Y.; Yang, H. Growth discordance of monoamniotic twin because of difference of cords diameter in forked umbilical cord: Case report. Medicine 2017, 96, e8042. [Google Scholar] [CrossRef] [PubMed]
- Kutuk, M.S.; Sahin, N.; Takmaz, T. Prenatal Ultrasound Detection of Mirror Twins With a Fused Proximal Umbilical Cord. J. Ultrasound Med. 2020, 39, 833–835. [Google Scholar] [CrossRef] [PubMed]
- Paulozzi, L.J.; Lary, J.M. Laterality patterns in infants with external birth defects. Teratology 1999, 60, 265–271. [Google Scholar] [CrossRef]
- Bargiela, D.; Burr, S.P.; Chinnery, P.F. Mitochondria and Hypoxia: Metabolic Crosstalk in Cell-Fate Decisions. Trends Endocrinol. Metab. 2018, 29, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Zheng, Y.; Qi, B.; Zheng, W.; Zhao, C.; Zhai, H.; Wang, G.; Luo, Z.; Li, T. Case report: Novel TBX5-related pathogenic mechanism of Holt-Oram syndrome. Front. Genet. 2023, 14, 1063202. [Google Scholar] [CrossRef]
- Lewin, S.O.; Opitz, J.M. Fibular a/hypoplasia: Review and documentation of the fibular developmental field. Am. J. Med. Genet. Suppl. 1986, 2, 215–238. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Nakamura, R.; Imamura, T. Polydactyly of the hands and feet. J. Hand Surg. Am. 1987, 12, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Orioli, I.M.; Castilla, E.E. Thumb/hallux duplication and preaxial polydactyly type I. Am. J. Med. Genet. 1999, 82, 219–224. [Google Scholar] [CrossRef]
- Cohen, M.M., Jr. Asymmetry: Molecular, biologic, embryopathic, and clinical perspectives. Am. J. Med. Genet. 2001, 101, 292–314. [Google Scholar] [CrossRef]
- Little, R.B.; Norris, D.P. Right, left and cilia: How asymmetry is established. Semin. Cell Dev. Biol. 2021, 110, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Schweickert, A.; Vick, P.; Wright, C.V.; Danilchik, M.V. Symmetry breakage in the vertebrate embryo: When does it happen and how does it work? Dev. Biol. 2014, 393, 109–123. [Google Scholar] [CrossRef]
- Schier, A.F. Nodal morphogens. Cold Spring Harb. Perspect. Biol. 2009, 1, a003459. [Google Scholar] [CrossRef]
- Levin, M. Left-right asymmetry in embryonic development: A comprehensive review. Mech. Dev. 2005, 122, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.A.; Wolpert, L. The development of handedness in left/right asymmetry. Development 1990, 109, 1–9. [Google Scholar] [CrossRef]
- Oostra, R.J.; Keulen, N.; Jansen, T.; van Rijn, R.R. Absence of the spleen(s) in conjoined twins: A diagnostic clue of laterality defects? Radiological study of historical specimens. Pediatr. Radiol. 2012, 42, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.B. Left-right asymmetry in animal development. Annu. Rev. Cell Dev. Biol. 1997, 13, 53–82. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B. Molecular Biology of the Cell; W.W. Norton: New York, NY, USA, 2017. [Google Scholar]
- Ambros, V.; Moss, E.G. Heterochronic genes and the temporal control of C. elegans development. Trends Genet. 1994, 10, 123–127. [Google Scholar] [CrossRef]
- McMenamin, M.A.S. Cambrian Chordates and Vetulicolians. Geosciences 2019, 9, 354. [Google Scholar] [CrossRef]
- Feinberg, T.E.; Mallatt, J. The evolutionary and genetic origins of consciousness in the Cambrian Period over 500 million years ago. Front. Psychol. 2013, 4, 667. [Google Scholar] [CrossRef]
- Xu, P.F.; Houssin, N.; Ferri-Lagneau, K.F.; Thisse, B.; Thisse, C. Construction of a vertebrate embryo from two opposing morphogen gradients. Science 2014, 344, 87–89. [Google Scholar] [CrossRef]
- Thisse, B.; Thisse, C. Formation of the vertebrate embryo: Moving beyond the Spemann organizer. Semin. Cell Dev. Biol. 2015, 42, 94–102. [Google Scholar] [CrossRef]
- Little, S.C.; Mullins, M.C. Extracellular modulation of BMP activity in patterning the dorsoventral axis. Birth Defects Res. C Embryo Today 2006, 78, 224–242. [Google Scholar] [CrossRef] [PubMed]
- Thisse, B.; Wright, C.V.; Thisse, C. Activin- and Nodal-related factors control antero-posterior patterning of the zebrafish embryo. Nature 2000, 403, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, W.; Wang, Y.; Wei, Y.; Huang, J. Integrated multiomics reveal the molecular characteristics of conjoined twin fetuses. Reprod. Biomed. Online 2023, 47, 26–34. [Google Scholar] [CrossRef]
- Nascone, N.; Mercola, M. Organizer induction determines left-right asymmetry in Xenopus. Dev. Biol. 1997, 189, 68–78. [Google Scholar] [CrossRef]
- Darras, S.; Fritzenwanker, J.H.; Uhlinger, K.R.; Farrelly, E.; Pani, A.M.; Hurley, I.A.; Norris, R.P.; Osovitz, M.; Terasaki, M.; Wu, M.; et al. Anteroposterior axis patterning by early canonical Wnt signaling during hemichordate development. PLoS Biol. 2018, 16, e2003698. [Google Scholar] [CrossRef]
- Shi, D.L. Wnt/planar cell polarity signaling controls morphogenetic movements of gastrulation and neural tube closure. Cell Mol. Life Sci. 2022, 79, 586. [Google Scholar] [CrossRef]
- Ulloa, F.; Martí, E. Wnt won the war: Antagonistic role of Wnt over Shh controls dorso-ventral patterning of the vertebrate neural tube. Dev. Dyn. 2010, 239, 69–76. [Google Scholar] [CrossRef]
- Martyn, I.; Kanno, T.Y.; Ruzo, A.; Siggia, E.D.; Brivanlou, A.H. Self-organization of a human organizer by combined Wnt and Nodal signalling. Nature 2018, 558, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Levin, M. Morphogenetic fields in embryogenesis, regeneration, and cancer: Non-local control of complex patterning. Biosystems 2012, 109, 243–261. [Google Scholar] [CrossRef] [PubMed]
- Oligny, L.L. Human molecular embryogenesis: An overview. Pediatr. Dev. Pathol. 2001, 4, 324–343. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.W.; Schier, A.F. Morphogen gradients: From generation to interpretation. Annu. Rev. Cell Dev. Biol. 2011, 27, 377–407. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Markson, J.S.; Wang, S.; Chen, S.; Vachharajani, V.; Elowitz, M.B. Morphogen gradient reconstitution reveals Hedgehog pathway design principles. Science 2018, 360, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Spemann, H.; Mangold, H. Induction of embryonic primordia by implantation of organizers from a different species. 1923. Int. J. Dev. Biol. 2001, 45, 13–38. [Google Scholar]
- Tisler, M.; Schweickert, A.; Blum, M. Xenopus, an ideal model organism to study laterality in conjoined twins. Genesis 2017, 55, e22993. [Google Scholar] [CrossRef]
- McCaig, C.D.; Rajnicek, A.M.; Song, B.; Zhao, M. Controlling cell behavior electrically: Current views and future potential. Physiol. Rev. 2005, 85, 943–978. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, K.; Donnet, C.; Rejtar, T.; Karger, B.L.; Barisone, G.A.; Díaz, E.; Kortagere, S.; Lemire, J.M.; Levin, M. Histone deacetylase activity is necessary for left-right patterning during vertebrate development. BMC Dev. Biol. 2011, 11, 29. [Google Scholar] [CrossRef]
- Fukumoto, T.; Kema, I.P.; Levin, M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr. Biol. 2005, 15, 794–803. [Google Scholar] [CrossRef]
- Levin, M.; Buznikov, G.A.; Lauder, J.M. Of minds and embryos: Left-right asymmetry and the serotonergic controls of pre-neural morphogenesis. Dev. Neurosci. 2006, 28, 171–185. [Google Scholar] [CrossRef]
- Tabata, T.; Takei, Y. Morphogens, their identification and regulation. Development 2004, 131, 703–712. [Google Scholar] [CrossRef]
- Haron, A.; Ruzal, M.; Shinder, D.; Druyan, S. Hypoxia during incubation and its effects on broiler’s embryonic development. Poult. Sci. 2021, 100, 100951. [Google Scholar] [CrossRef] [PubMed]
- Webster, W.S.; Abela, D. The effect of hypoxia in development. Birth Defects Res. C Embryo Today 2007, 81, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Bonkowsky, J.L.; Son, J.-H. Hypoxia and connectivity in the developing vertebrate nervous system. Dis. Models Mech. 2018, 11, dmm037127. [Google Scholar] [CrossRef] [PubMed]
- Dunwoodie, S.L. The Role of Hypoxia in Development of the Mammalian Embryo. Dev. Cell 2009, 17, 755–773. [Google Scholar] [CrossRef]
- Scully, D.; Keane, E.; Batt, E.; Karunakaran, P.; Higgins, D.F.; Itasaki, N. Hypoxia promotes production of neural crest cells in the embryonic head. Development 2016, 143, 1742–1752. [Google Scholar] [CrossRef]
- Vieira, A.R.; Dattilo, S. Oxygen, Left/Right Asymmetry, and Cleft Lip and Palate. J. Craniofacial Surg. 2018, 29, 396–399. [Google Scholar] [CrossRef]
- Hoog, T.G.; Fredrickson, S.J.; Hsu, C.W.; Senger, S.M.; Dickinson, M.E.; Udan, R.S. The effects of reduced hemodynamic loading on morphogenesis of the mouse embryonic heart. Dev. Biol. 2018, 442, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Reece, E.A.; Lin, X.; Wu, Y.; AriasVillela, N.; Yang, P. New development of the yolk sac theory in diabetic embryopathy: Molecular mechanism and link to structural birth defects. Am. J. Obstet. Gynecol. 2016, 214, 192–202. [Google Scholar] [CrossRef]
- Chalouhi, G.E.; Stirnemann, J.J.; Salomon, L.J.; Essaoui, M.; Quibel, T.; Ville, Y. Specific complications of monochorionic twin pregnancies: Twin-twin transfusion syndrome and twin reversed arterial perfusion sequence. Semin. Fetal Neonatal Med. 2010, 15, 349–356. [Google Scholar] [CrossRef]
- Saxena, R.; Sinha, A.; Pathak, M.; Rathod, K.J. Conjoined thoracopagus twins: A systematic review of the anomalies and outcome of surgical separation. Afr. J. Paediatr. Surg. 2023, 20, 157–165. [Google Scholar] [CrossRef]
- McMahon, C.J.; Spencer, R. Congenital heart defects in conjoined twins: Outcome after surgical separation of thoracopagus. Pediatr. Cardiol. 2006, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Boer, L.L.; Schepens-Franke, A.N.; van Asten, J.J.A.; Bosboom, D.G.H.; Kamphuis-van Ulzen, K.; Kozicz, T.L.; Ruiter, D.J.; Oostra, R.J.; Klein, W.M. Radiological imaging of teratological fetuses: What can we learn? Insights Imaging 2017, 8, 301–310. [Google Scholar] [CrossRef]
- Lin, A.E. Congenital heart defects in malformation syndromes. Clin. Perinatol. 1990, 17, 641–673. [Google Scholar] [CrossRef] [PubMed]
- Brizot, M.L.; Liao, A.W.; Lopes, L.M.; Okumura, M.; Marques, M.S.; Krebs, V.; Schultz, R.; Zugaib, M. Conjoined twins pregnancies: Experience with 36 cases from a single center. Prenat. Diagn. 2011, 31, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Roberts, D.J.; Holmes, L.B.; Tabin, C. Laterality defects in conjoined twins. Nature 1996, 384, 321. [Google Scholar] [CrossRef] [PubMed]



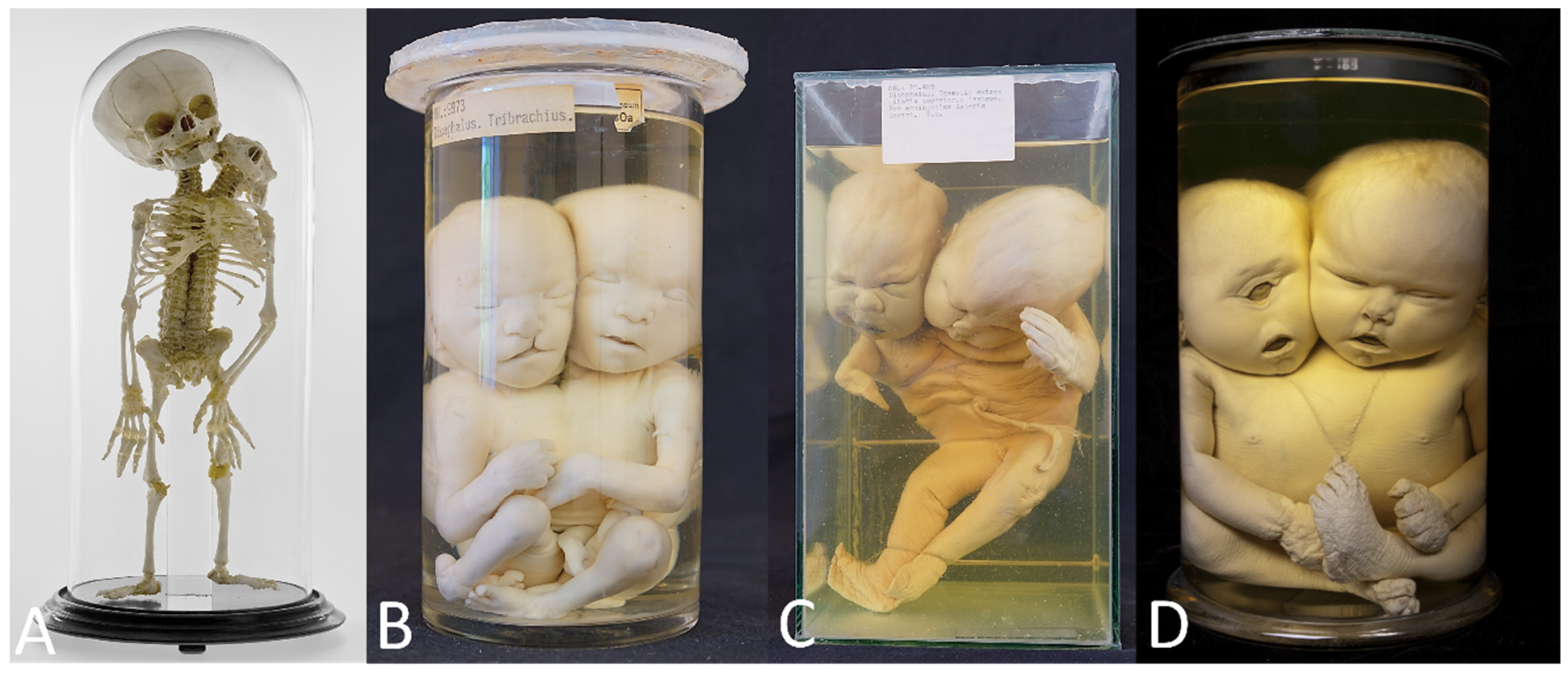
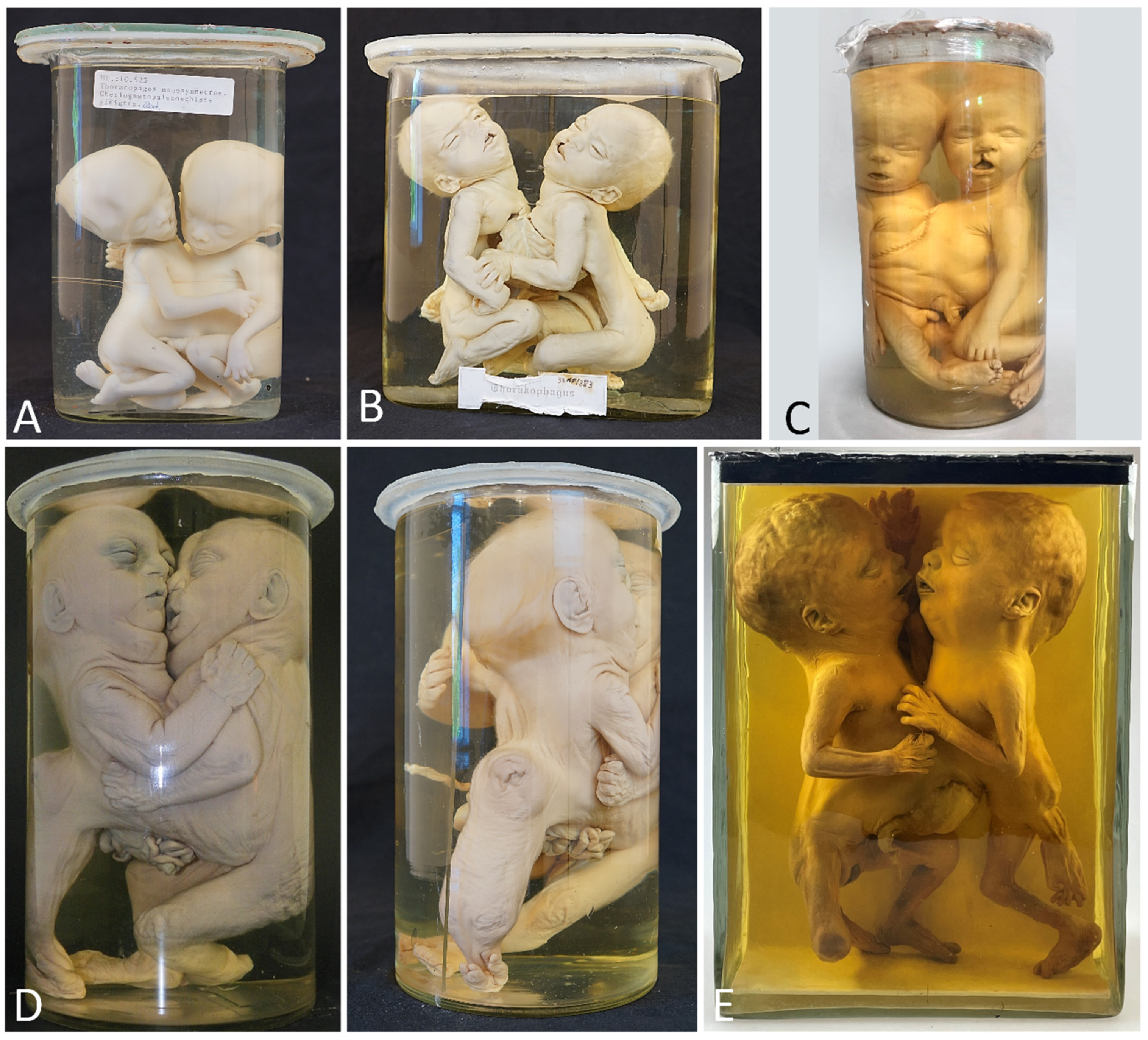
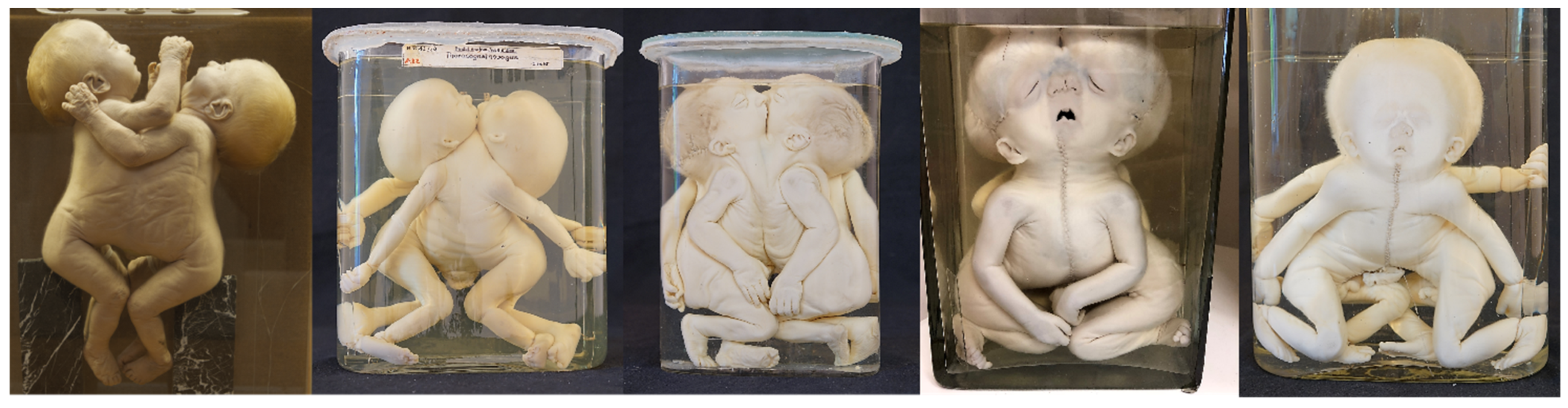


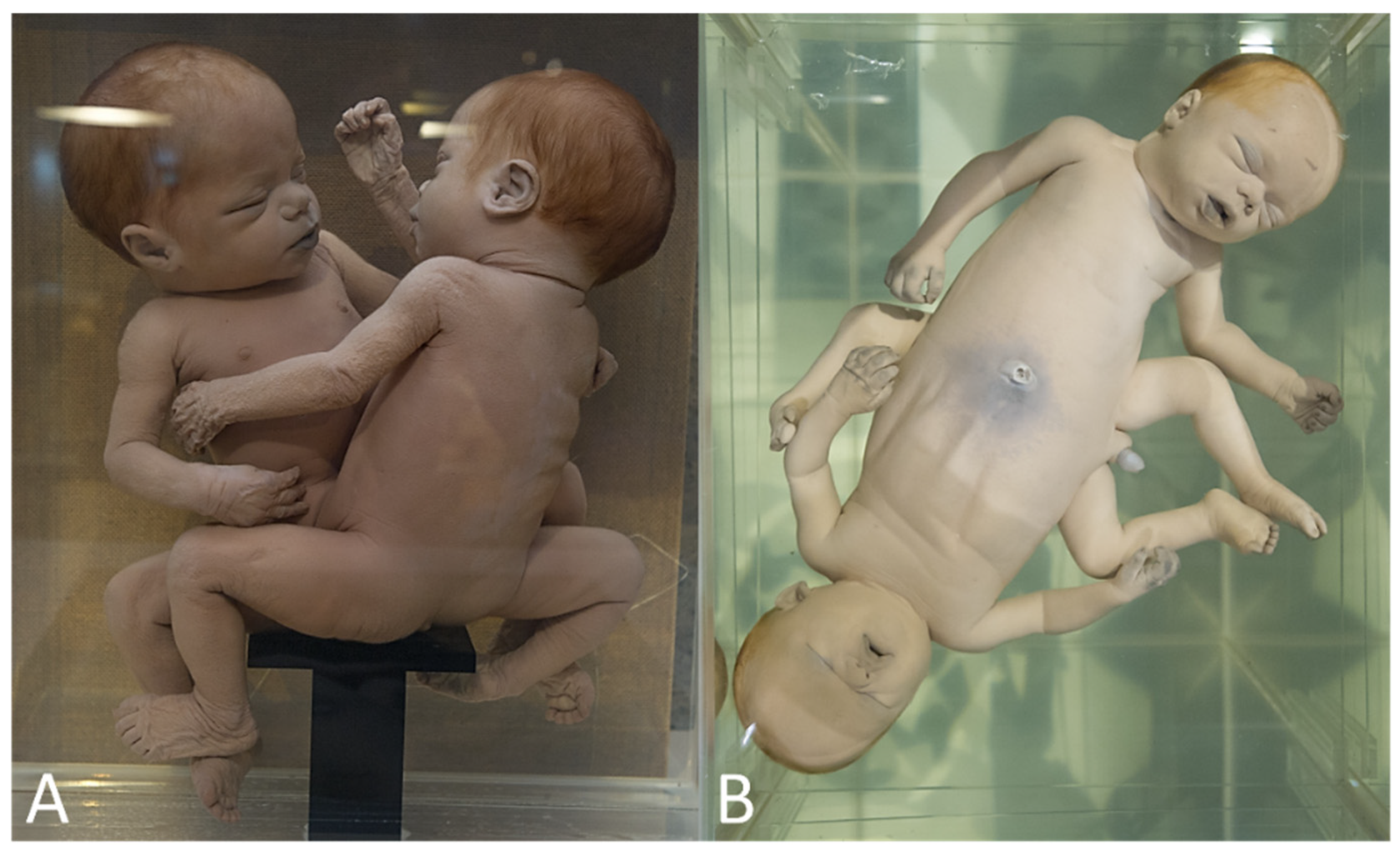
| Type of Twins with Discordances Retrieved from the Literature | Amount | Literature |
|---|---|---|
| Laterally united twins | ||
| Parapagus dicephalus with discordant anencephaly | 7 | [9,14,15,18,19,20,21] |
| Parapagus dicephalus with discordant cleft lip/palate | 1 | [22] |
| Parapagus dicephalus with discordant holoprosencephaly | 2 | [23,24] Figure 4D |
| Parapagus dicephalus with discordant polydactyly | 1 | [25] |
| Parapagus diprosopus with discordant synophthalmia/proboscis | 1 | [26] |
| Parapagus diprosopus with discordant cebocephaly | 1 | [25] |
| Parapagus diprosopus with discordant anophthalmia and cleft lip palate | 1 | [27] |
| Parapagus diprosopus with discordant cyclopia | 1 | [28] |
| Parapagus diprosopus with discordant cleft lip | 1 | [29] |
| Parapagus diprosopus with discordant polydactyly | 1 | [30] |
| Ventrally united twins | ||
| Thoracoileopagus with discordant cleft lip/palate | 9 | [8,13,31,32,33,34,35,36,37] |
| Thoracoileopagus with discordant scoliosis and vertebral anomalies | 1 | [38] |
| Thoracoileopagus with discordant anencephaly | 1 | [39] |
| Thoracoileopagus with multiple discordances in both twins * | 1 | [10] |
| Thoracoileopagus with multiple discordances in both twins * | 1 | [40] |
| Thoracoileopagus with discordant Treacher Collins-like features | 1 | [41] |
| Thoracoileopagus with multiple discordances * | 1 | [25] |
| Thoracoileopagus with discordant sirenomelia | 1 | [23] |
| Thoracoileopagus with discordant anal/cloacal malformations | 3 | [35,42,43] |
| Thoracoileopagus with discordant cloacal exstrophy | 1 | [25] |
| Cephalothoracoileopagus with discordant OEIS-like malformations | 7 | [3,8,25,44,45,46,47] (Figure 7) |
| Cephalothoracoileopagus with discordant polydactyly/syndactyly | 3 | [25,45,46] |
| Cephalothoracoileopagus with phenotypically discordant sex but genotypically female | 1 | [48] |
| Caudally united twins | ||
| Ileoischiopagus with discordant hydrocephaly | 1 | [49] |
| Ileoischiopagus with discordant microcephaly | 1 | [50] |
| Ileoischiopagus with discordant anencephaly | 1 | [51] |
| Ileoischiopagus with discordant meningocele | 1 | [52] |
| Ileoischiopagus with discordant cleft lip and palate | 2 | [49,50] |
| Ileoischiopagus with discordant pseudohermaphroditism, gastroschisis and anencephaly | 1 | [53] |
| Ileoischiopagus with multiple discordances * | 1 | [54] |
| Ileoischiopagus with discordant hygroma colli and Down syndrome-like features | 1 | [25] |
| Ileoischiopagus with discordant Pierre Robin syndrome-like features | 1 | [55] |
| Total found twins with discordances from literature | 58 | |
| Novel cases of Twins with Discordances Retrieved from Museal Surveys | Amount | Location |
| Laterally united twins | ||
| Parapagus dicephalus with discordant anencephaly | 2 | 1 from Berliner Medizinhistorisches Museum der Charité (Figure 4A) and 1 from Narrenturm |
| Parapagus dicephalus with discordant cleft lip/palate | 1 | Narrenturm (Figure 4B) |
| Parapagus dicephalus with discordant transverse limb defects | 1 | Narrenturm (Figure 4C) |
| Ventrally united twins | ||
| Thoracoileiopagus with discordant celft lip/palate | 3 | 2 from Narrenturm (Figure 5A,B) and 1 from Collections d’anatomie pathologique Dupuytren (Figure 5C) |
| Thoracoileiopagus with discordant sirenomelia | 1 | Narrenturm (Figure 5D) |
| Thoracoileopagus with discordant anal atresia, micromelia and polydactyly | 1 | Berliner Medizinhistorisches Museum der Charité (Figure 5E) |
| Prosopothoracoileopagus with discordant cleft lip/palate | 1 | Narrenturm (Figure 8A) |
| Cephalothoracoileopagus discordant for longitudinal limb deficiency | 1 | Collections d’anatomie pathologique Dupuytren (Figure 8B) |
| Caudally united twins | ||
| None | ||
| Total found twins with discordances from museal collections | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boer, L.L.; Winter, E.; Gorissen, B.; Oostra, R.-J. Phenotypically Discordant Anomalies in Conjoined Twins: Quirks of Nature Governed by Molecular Pathways? Diagnostics 2023, 13, 3427. https://doi.org/10.3390/diagnostics13223427
Boer LL, Winter E, Gorissen B, Oostra R-J. Phenotypically Discordant Anomalies in Conjoined Twins: Quirks of Nature Governed by Molecular Pathways? Diagnostics. 2023; 13(22):3427. https://doi.org/10.3390/diagnostics13223427
Chicago/Turabian StyleBoer, Lucas L., Eduard Winter, Ben Gorissen, and Roelof-Jan Oostra. 2023. "Phenotypically Discordant Anomalies in Conjoined Twins: Quirks of Nature Governed by Molecular Pathways?" Diagnostics 13, no. 22: 3427. https://doi.org/10.3390/diagnostics13223427
APA StyleBoer, L. L., Winter, E., Gorissen, B., & Oostra, R.-J. (2023). Phenotypically Discordant Anomalies in Conjoined Twins: Quirks of Nature Governed by Molecular Pathways? Diagnostics, 13(22), 3427. https://doi.org/10.3390/diagnostics13223427






