Hepatocellular Carcinoma in Non-Fibrotic Liver: A Narrative Review
Abstract
1. Introduction
2. Incidence of HCC in Non-Fibrotic Liver (F0)
3. Materials and Methods (Literature Search and Study Selection)
4. Results
4.1. Patient Demographics and Clinical Data
4.2. Tumor Characteristics and Medical Imaging Findings
4.3. Treatment and Outcome
5. Reported Possible Causal Factors of HCC in Non-Fibrotic Liver (F0)
5.1. Family History and Genetic Factors
5.2. Aging and Sex
5.3. Alcohol Consumption
5.4. Obesity and Diabetes Mellitus (DM)
5.5. Viral Infection
5.6. Autoimmune Hepatitis
5.7. Exposure to Chemicals
5.8. Multiple Primary Malignancies (MPMs)
5.9. Hepatocellular Adenoma (HCA)
5.10. The Other Less Common Risk Factors
6. Clinical Manifestation and Diagnosis
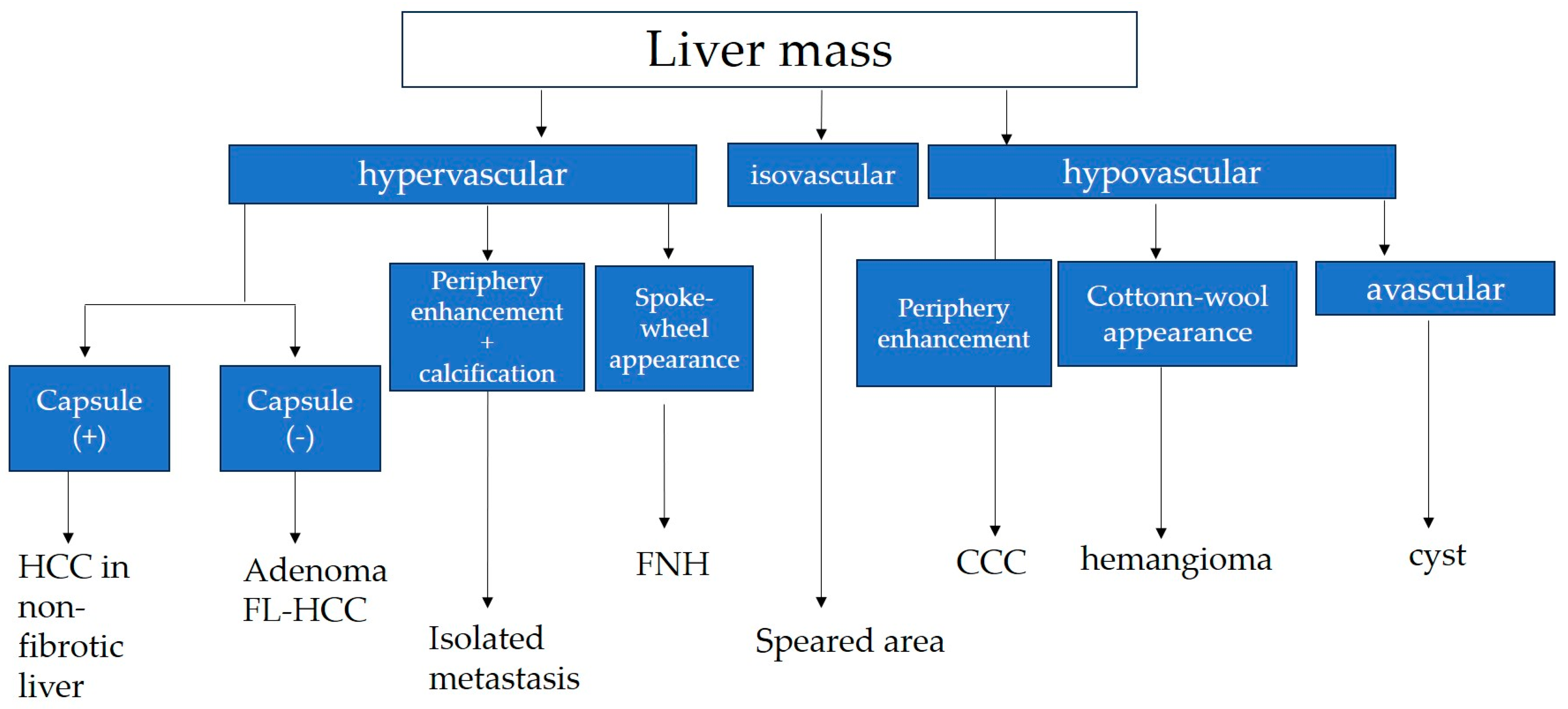
7. Differential Diagnosis
7.1. Fibrolamellar Hepatocellular Carcinoma (FL-HCC)
7.2. Hepatocellular Adenoma
7.3. Focal Nodular Hyperplasia (FNH)
7.4. Hypervascular Hemangioma (Hy-Heman)
7.5. Solitary Metastasis
8. Treatment and Outcome
9. Screening System
10. Discussion
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mcglynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemilogy of hepatocellular carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef]
- Samant, H.; Amiri, H.S.; Zibari, G.B. Addressing the worldwide hepatocellular carcinoma: Epidemiology, prevention and management. J. Gastrointest. Oncol. 2021, 12, S361–S373. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, Y.H.; Wu, W.; Zhang, W.J.; Yang, J.; Chen, Z. Association between occult hepatitis B infection and the risk of hepatocellular carcinoma: A meta-analysis. Liver Int. 2012, 32, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Donato, F.; Tagger, A.; Gelatti, U.; Parrinello, G.; Boffetta, P.; Albertini, A.; Decarli, A.; Trevisi, P.; Ribero, M.L.; Martelli, C.; et al. Alcohol and hepatocellular carcinoma: The effect of lifetime intake and hepatitis virus infection and women. Am. J. Epidemiol. 2002, 155, 323–331. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Harrison, S.A.; Ratziu, V.; Abdelmalek, M.F.; Diehl, A.M.; Caldwell, S.; Shiffman, M.L.; Schall, R.A.; Jia, C.; McColgan, B.; et al. The natural history of advanced fibrosis due to nonalcoholic steatohepatitis: Data from the simtuzumab trials. Hepatology 2019, 70, 1913–1927. [Google Scholar] [CrossRef]
- Fazel, Y.; Koenig, A.B.; Sayiner, M.; Goodman, Z.D.; Younossi, Z.M. Epidemiology and natural history of non-alcoholic liver disease. Metabolism 2016, 65, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zheng, F.; Li, Z.; Zhou, R.; Deng, L.; Xiao, W.; Chen, W.; Zhao, R.; Chen, Y.; Tan, Y.; et al. Association between environmental and socioeconomic risk factors and hepatocellular carcinoma: A meta-analysis. Front. Public. Health 2022, 10, 741490. [Google Scholar] [CrossRef] [PubMed]
- Bralet, M.P.; Réginbeau, J.M.; Pineau, P.; Dubois, S.; Loas, G.; Degos, F.; Valla, D.; Belghiti, J.; Degott, C.; Terris, B.; et al. Hepatocellular carcinoma occurring in nonfibrotic liver: Epidemiologic and histopathologic analysis of 80 french cases. Hepatology 2000, 32, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Roey, G.V.; Fevery, J.; Van Steenbergen, W. Hepatocellular carcinoma in Belgium: Clinical and virological characteristics of 154 consecutive cirrhotic and non-cirrhotic patients. Eur. J. Gastroenterol. Hepatol. 2000, 12, 61–66. [Google Scholar] [CrossRef]
- Okuda, K.; Nakashima, T.; Kojiro, M.; Kondo, Y.; Wada, K. Hepatocellular carcinoma without cirrhosis in Japanese patients. Gastroenterology 1989, 97, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Sandhu, S.; Lai, J.P.; Sandhu, D.S. Hepatocellular carcinoma in non-cirrhotic liver: A comprehensive review. World J. Hepatol. 2019, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pinyopornpanish, K.; Al-Yaman, W.; Dasarathy, S.; Romero-Marrero, C.; McCullough, A. Hepatocellular carcinoma in patients without cirrhosis: The fibrosis stage distribution, characteristics and survival. Dig. Dis. Sci. 2022, 67, 2677–2687. [Google Scholar] [CrossRef]
- Yip, V.S.; Gomez, D.; Tan, C.Y.; Staettner, S.; Terlizzo, M.; Fenwick, S.; Malik, H.Z.; Ghaneh, P.; Poston, G. Tumor size and differentiation predict survival after liver resection for hepatocellular carcinoma arising from non-cirrhotic and non-fibrotic liver: A case-controlled study. Int. J. Surg. 2013, 11, 1078–1082. [Google Scholar] [CrossRef]
- Bedossa, P.; Poynard, T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996, 24, 289–293. [Google Scholar] [CrossRef]
- Chopinet, S.; Cauchy, F.; Hobeika, C.; Beaufrère, A.; Poté, N.; Farges, O.; Dokmak, S.; Bouattour, M.; Ronot, M.; Vilgrain, V.; et al. Long-term outcomes following resection of hepatocellular adenomas with small foci of malignant transformation or malignant adenomas. JHEP Rep. 2021, 3, 100326. [Google Scholar] [CrossRef] [PubMed]
- Bismuth, H.; Chiche, L.; Castaing, D. Surgical treatment of hepatocellular carcinoma in noncirrhotic liver: Experience with 68 liver resections. World J. Surg. 1995, 19, 35–41. [Google Scholar] [CrossRef]
- Lubrano, J.; Huet, E.; Tsillividis, B.; François, A.; Goria, O.; Ghassan Riachi, G.; Scotté, M. Long-term outcomes of liver resection for hepatocellular carcinoma in noncirrhotic liver with no viral hepatitis or alcohol abuse. World J. Surg. 2008, 32, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K. Liver Cancer Study Group of Japan: Primary liver cancers in Japan. Cancer 1980, 45, 2663–2669. [Google Scholar] [CrossRef]
- Anonymous. Liver Cancer Study Group of Japan: Primary liver cancer in Japan. Cancer 1984, 54, 747–755. [Google Scholar]
- Anonymous. Liver Cancer Study Group of Japan: Primary liver cancer in Japan. Sixth report. Cancer 1987, 60, 1400–1411. [Google Scholar] [CrossRef]
- Liver Cancer Study Group of Japan. Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann. Surg. 1990, 211, 277–287. [Google Scholar]
- Tobe, T.; Kameda, H.; Okudaira, M.; Ohto, M.; Endo, Y.; Mito, M.; Okamoto, E.; Tanikawa, K.; Kojiro, M. (Eds.) Primary Liver Cancer in Japan; Springer: Tokyo, Japan, 1992. [Google Scholar]
- Ikai, I.; Itai, Y.; Okita, K.; Omata, M.; Kojiro, M.; Kobayashi, K.; Nakanuma, Y.; Futagawa, S.; Makuuchi, M.; Yamaoka, Y. Report of the 15th follow-up survey of primary liver cancer. Hepatol. Res. 2004, 28, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ikai, I.; Arii, S.; Ichida, T.; Okita, K.; Omata, M.; Kojiro, M.; Takayasu, K.; Nakanuma, Y.; Makuuchi, M.; Matsuyama, Y.; et al. Report of the 16th follow-up survey of primary liver cancer. Hepatol. Res. 2005, 32, 163–172. [Google Scholar] [CrossRef]
- Ikai, I.; Arii, S.; Okazaki, M.; Okita, K.; Omata, M.; Kojiro, M.; Takayasu, K.; Nakanuma, Y.; Makuuchi, M.; Matsuyama, Y.; et al. Report of the 17th Nationwide Follow-up Survey of Primary Liver Cancer in Japan. Hepatol. Res. 2007, 37, 676–691. [Google Scholar] [CrossRef]
- Ikai, I.; Kudo, M.; Arii, S.; Omata, M.; Kojiro, M.; Sakamoto, M.; Takayasu, K.; Hayashi, N.; Makuuchi, M.; Matsuyama, Y.; et al. Report of the 18th follow-up survey of primary liver cancer in Japan. Hepatol. Res. 2010, 40, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Izumi, N.; Ichida, T.; Ku, Y.; Kokudo, N.; Sakamoto, M.; Takayama, T.; Nakashima, O.; Matsui, O.; Matsuyama, Y. Report of the 19th follow-up survey of primary liver cancer in Japan. Hepatol. Res. 2016, 46, 372–390. [Google Scholar] [CrossRef]
- Kudo, M.; Izumi, N.; Kubo, S.; Kokudo, N.; Sakamoto, M.; Shiina, S.; Tateishi, R.; Nakashima, O.; Murakami, T.; Matsuyama, Y.; et al. Report of the 20th Nationwide follow-up survey of primary liver cancer in Japan. Hepatol. Res. 2020, 50, 15–46. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Izumi, N.; Kokudo, N.; Sakamoto, M.; Shiina, S.; Takayama, T.; Tateishi, R.; Nakashima, O.; Murakami, T.; Matsuyama, Y.; et al. Report of the 21st Nationwide Follow-up Survey of Primary Liver Cancer in Japan (2010–2011). Hepatol. Res. 2021, 51, 355–405. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Izumi, N.; Kokudo, N.; Sakamoto, M.; Shiina, S.; Takayama, T.; Tateishi, R.; Nakashima, O.; Murakami, T.; Matsuyama, Y.; et al. Report of the 22nd nationwide follow-up Survey of Primary Liver Cancer in Japan (2012–2013). Hepatol. Res. 2022, 52, 5–66. [Google Scholar] [CrossRef]
- Stroffolini, T.; Andreone, P.; Andriulli, A.; Ascione, A.; Craxi, A.; Chiaramonte, M.; Galante, D.; Manghisi, O.G.; Mazzanti, R.; Medaglia, C.; et al. Characteristics of hepatocellular carcinoma in Italy. J. Hepatol. 1998, 29, 944–952. [Google Scholar] [CrossRef]
- Shrestha, A. Liver Cancer in Nepal. Euro. J. Hepatogastroenterol. 2018, 8, 63–65. [Google Scholar] [CrossRef]
- Kar, P. Risk factors for hepatocellular carcinoma in India. J. Clin. Exp. Hepatol. 2014, 4, S34–S42. [Google Scholar] [CrossRef]
- Global Burden of Disease Liver Cancer Collaboration; Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Ale Mayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the global burden of disease study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Florio, A.A.; Znaor, A.; Ruggieri, D.; Laversanne, M.; Alvarez, C.S.; Ferlay, J.; Valery, P.C.; Bray, F.; McGlynn, K.A. International trends in hepatocellular carcinoma incidence, 1978–2012. Int. J. Cancer 2020, 147, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Pembroke, T.P.I.; John, G.; Puyk, B.; Howkins, K.; Clarke, R.; Yousuf, F.; Czajkowski, M.; Godkin, A.; Salmon, J.; Yeoman, A. Rising incidence, progression and changing patterns of liver disease in Wales 1999–2019. World J. Hepatol. 2023, 15, 89–106. [Google Scholar] [CrossRef]
- Eguchi, S.; Ijtsma, A.J.; Slooff, M.J.; Porte, R.J.; de Jong, K.P.; Peeters, P.M.; Gouw, A.S.; Kanematsu, T. Outcome and pattern of recurrence after curative resection for hepatocellular carcinoma in patients with a normal liver compared to patients with a diseased liver. Hepatogastroenterology 2006, 53, 592–596. [Google Scholar] [PubMed]
- Bège, T.; LeTreut, Y.P.; Hardwigsen, J.; Hardwigsen, J.; Ananian, P.; Richa, H.; Campan, P.; Garcia, S. Prognostic factors after resection for hepatocellular carcinoma in nonfibrotic or moderately fibrotic liver. A 116-case European series. J. Gastrointest. Surg. 2007, 11, 619–625. [Google Scholar] [CrossRef]
- Guzman, G.; Brunt, E.M.; Petrovic, L.M.; Chejfec, G.; Layden, T.J.; Costler, S.J. Does nonalcoholic fatty liver disease predispose patints to hepatocellular carcinoma in the absence of cirrhosis? Arch. Pathol. Lab. Med. 2008, 132, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Saito, H.; Kanno, Y.; Abe, K.; Yokokawa, J.; Irisawa, A.; Kenjo, A.; Saito, T.; Gotoh, M.; Ohira, H. Case of clear-cell hepatocellular carcinoma that developed in the normal liver of a middle-aged woman. World J. Gastroenterol. 2008, 14, 129–131. [Google Scholar] [CrossRef]
- Lewis, S.; Roayaie, S.; Ward, S.C.; Shyknevsky, I.; Jibara, G.; Taouli, B. Hepatocellular carcinoma in chronic hepatitis C in the absence of advanced fibrosis or cirrhosis. Am. J. Roentgenol. 2013, 200, W610–W616. [Google Scholar] [CrossRef]
- Komiyama, S.; Okazaki, H.; Nakao, S.; Nishigori, S.; Terada, M.; Hamanaka, J.; Miura, Y.; Oka, H.; Suzaki, F.; Tanaka, K. Diffuse fatty metamorphosis of a large, well-differentiated hepatocellular carcinoma originating in the normal liver: A case report and literature review. Clin. J. Gastroenterol. 2015, 8, 345–350. [Google Scholar] [CrossRef]
- Sheng, R.F.; Zeng, M.S.; Ji, Y.; Yang, L.; Chen, C.Z.; Rao, S.X. MR features of small hepatocellular carcinoma in normal, fibrotic, and cirrhotic livers: A comparative study. Abdom. Imaging 2015, 40, 3062–3069. [Google Scholar] [CrossRef]
- Naganuma, H.; Ishida, H.; Ogawa, M.; Sato, T.; Sageshima, M.; Suzuki, K.; Ohyama, Y. Hetaocellular carcinoma in otherwise sonographically normal liver. J. Clin. Ultrasound 2019, 47, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, N.; Saitoh, S.; Denpou, H.; Kinowaki, K.; Akuta, N.; Suzuki, F.; Hashimoto, M.; Fujiyama, S.; Kawamura, Y.; Sezaki, H.; et al. Poorly differentiated hepatocellular carcinoma in a low-risk patient with an otherwise normal liver. Intern. Med. 2020, 59, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Onishi, S.; Tajika, M.; Tanaka, T.; Yamada, K.; Kamiya, T.; Natsume, S.; Shimizu, Y.; Niwa, Y. Juvenile hepatocellular carcinoma in a healthy liver. Intern. Med. 2022, 61, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, L.; Zhou, S.; Jiang, Q.; Chen, S.; Gao, Y.; Chen, Y. Familial correlations of onset age of hepatocellular carcinomas: A population-based case-control family study. PLoS ONE 2014, 9, e108391. [Google Scholar] [CrossRef]
- An, J.; Chang, S.; Kim, H.I.; Song, G.W.; Shim, J.H. The clinical behavior and survival of patients with hepatocellular carcinoma and a family history of the disease. Cancer Med. 2019, 8, 6624–6633. [Google Scholar] [CrossRef]
- Villanuev, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Tajiri, K.; Shimizu, Y. Liver physiology and liver diseases in the elderly. World J. Gastroenterol. 2013, 19, 8459–8467. [Google Scholar] [CrossRef]
- Hashim, D.; Carioli, G.; Malvezzi, M.; Bertuccio, P.; Waxman, S.; Negri, E.; La Vecchia, C.; Boffetta, P. Cancer mortality in the oldest old: A global overview. Aging 2020, 12, 16744–16758. [Google Scholar] [CrossRef] [PubMed]
- Fane, M.; Weeraratna, A. How the aging microenvironment influences tumour progression. Nat. Rev. Cancer 2020, 20, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Macias, R.I.R.; Monte, M.J.; Serrano, M.A.; Macias, R.I.R.; Monte, M.J.; Serrano, M.A.; González-Santiago, J.M.; Martín-Arribas, I.; Simão, A.L.; Castro, R.E.; et al. Impact of aging on primary liver cancer: Epidemiology, pathogenesis and therapeutics. Aging 2021, 13, 23416–23434. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, L.; Hong, L.; Su, Z.; Zhong, X.; Zhou, H.; Zhang, X.; Wu, J.; Shao, L. Identification of aging-related genes associated with clinical and prognostic features of hepatocellular carcinoma. Front. Genet. 2021, 12, 661988. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Mellinger, J.; Arab, J.P.; Shah, V.H. Reducing the global burden alcohol-associated liver disease: A blueprint for action. Hepatology 2021, 73, 2039–2050. [Google Scholar] [CrossRef]
- Yi, S.W.; Cjoi, J.S.; Yi, J.J.; Lee, Y.H.; Han, K.J. Risk factors for hepatocellular carcinoma by age, sex, and liver disorder status. A prospective cohort study in Korea. Cancer 2018, 24, 2748–2757. [Google Scholar] [CrossRef]
- Myers, S.; Neyroud-Caspar, I.; Spahr, L.; Gkouvatsos, K.; Fournier, E.; Giostra, E.; Magini, G.; Frossard, J.L.; Bascaron, M.E.; Vernaz, N.; et al. NAFLD and MAFLD as emerging causes of HCC: A populational study. JHEP Rep. 2021, 3, 100231. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Otogonsuren, M.; Henry, L.; Venkatesan, C.; Mishra, A.; Erario, M.; Hunt, S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the united states from 2004 to 20009. Hepatology 2015, 62, 1723–1730. [Google Scholar] [CrossRef]
- Fujiwara, N.; Friedman, S.L.; Goossens, N.; Hoshida, Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J. Hepatol. 2018, 68, 526–549. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 77–491.e1. [Google Scholar] [CrossRef] [PubMed]
- Kemp, L.; Clare, K.E.; Brennan, P.N.; Dillon, J.F. New horizons in hepatitis B and C in the older adult. Age Ageing 2019, 48, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Van Gerven, N.M.; de Boer, Y.S.; Mulder, C.J.; van Nieuwkerk, C.M.; Bouma, G. Autoimmune hepatitis. World J. Gastroenterol. 2016, 22, 4651–4661. [Google Scholar] [CrossRef]
- Rigopoulou, E.I.; Dalekos, G.N. Current trends and characteristics of hepatocellular carcinoma in patients with autoimmune liver diseases. Cancers 2021, 13, 1023. [Google Scholar] [CrossRef]
- Bolt, H.M. Vinyl chloride-a classical industrial toxicant of new interest. Crit. Rev. Toxicol. 2005, 35, 307–323. [Google Scholar] [CrossRef]
- Zeng, Q.A.; Qiu, J.; Zou, R.; Li, Y.; Li, S.; Li, B.; Huang, P.; Hong, J.; Zheng, Y.; Lao, X.; et al. Clinical features and outcome of multiple primary malignancies involving hepatocellular carcinoma: A long-term follow-up study. BMC Cancer 2012, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liao, W.; Ge, P.; Ren, J.; Xu, H.; Yang, H.; Sang, X.; Lu, X.; Mao, Y. Multiple primary malignancies in patients with hepatocellular carcinoma: A largest series with 26-year follow-up. Medicine 2016, 95, e3491. [Google Scholar] [CrossRef]
- Sung, H.; Hyun, N.; Learch, C.R.; Yabroff, K.R.; Jemal, A. Association of first primary cancer with risk of subsequent primary cancer among survivors of adult-onset cancers in the United States. JAMA 2020, 324, 2521–2535. [Google Scholar] [CrossRef] [PubMed]
- Thevathasan, T.; Colbatzky, T.; Schmelzle, M.; Pratschke, J.; Krenzien, F. Risk factors for malignant transformation of hepatocellular adenoma to hepat ocellular carcinoma: Protocol for systematic review and meta-analysis. BMJ Open 2021, 11, e045733. [Google Scholar] [CrossRef]
- Sempoux, C.; Balabaud, C.; Bioulac-Sage, P. Pictures of focal nodular hyperplasia and hepatocellular adenomas. World J. Hepatol. 2014, 6, 580–595. [Google Scholar] [CrossRef]
- van Rosmalen, B.V.; Furumaya, A.; Klompenhouwer, A.J.; Tushuizen, M.E.; Braat, A.E.; Reinten, R.J.; Ligthart, M.A.P.; Haring, M.P.D.; de Meijer, V.E.; van Voorthuizen, T.; et al. Hepatocellular adenoma in men: A nationwide assessment of pathology and correlation with clinical course. Liver Int. 2021, 41, 2474–2484. [Google Scholar] [CrossRef]
- Khanna, R.; Verma, S.K. Pediatric hepatocellular carcinoma. World J. Gastroenterol. 2018, 24, 3980–3999. [Google Scholar] [CrossRef] [PubMed]
- Berben, L.; Floris, G.; Wildiers, H.; Sigrid Hatse, S. Cancer and Aging: Two Tightly Interconnected Biological Processes. Cancers 2021, 13, 1400. [Google Scholar] [CrossRef]
- Patel, J.; Baptiste, B.A.; Kim, E.; Hussain, M.; Croteau, D.L.; Bohr, V.A. DNA damage and mitochondria in cancer and aging. Carcinogenesis 2020, 41, 1625–1634. [Google Scholar] [CrossRef]
- Cho, E.; Cho, H.A.; Jun, C.H.; Kim, H.J.; Cho, S.B.; Choi, S.K. A review of hepatocellular carcinoma in elderly patients focused on management and outcomes. Vivo 2019, 33, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Kimura, T.; Kita, R.; Osaki, Y. Treatment for hepatocellular carcinoma in elderly patients: A literature review. J. Cancer 2013, 4, 635–643. [Google Scholar] [CrossRef]
- Centonze, L.; Di Sandro, S.; Lauterio, A.; De Carlis, R.; Frassoni, S.; Rampoldi, A.; Tuscano, B.; Bagnardi, V.; Vanzulli, A.; De Carlis, L. Surgical resection vs. percutaneous ablation for single hepatocellular carcinoma: Exploring the impact of Li-RADS classification on oncological outcomes. Cancers 2021, 13, 1671. [Google Scholar] [CrossRef]
- Strobel, D. Using contrast-enhanced ultrasound in the diagnosis of hepatocellular carcinoma—What we have and have not achieved. Ultraschall Med. 2021, 42, 120–124. [Google Scholar] [CrossRef]
- Mayo, S.C.; Mavros, M.N.; Nathan, H.; Cosgrove, D.; Herman, J.M.; Kamel, I.; Anders, R.A.; Pawlik, T.M. Treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma: A national perspective. J. Am. Coll. Surg. 2014, 218, 196–205. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X. Hepatocellular adenoma: Where are we now? World J. Gastroenterol. 2022, 28, 1384–1393. [Google Scholar] [CrossRef]
- Trillaud, H.; Bruel, J.M.; Valette, P.J.; Vilgrain, V.; Schmutz, G.; Oyen, R.; Jakubowski, W.; Danes, J.; Valek, V.; Greis, C. Characterization of focal liver lesions with SonoVue-enhanced sonography: International multicenter-study in comparison to CT and MRI. World J. Gastroenterol. 2009, 15, 3748–3756. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, H.; Ishida, H.; Ogawa, M.; Watanabe, Y.; Watanabe, D.; Ohyama, Y.; Watanabe, T. Focal nodular hyperplasia: Our experience of 53 Japanese cases. J. Med. Ultrason. 2017, 44, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Roche, V.; Pigneur, F.; Tselikas, L.; Roux, M.; Baranes, L.; Djabbari, M.; Costentin, C.; Calderaro, J.; Laurent, A.; Rahmouni, A.; et al. Differentiation of focal nodular hyperplasia from hepatocellular adenomas with low-mechanical-index contrast-enhanced sonography (CEUS): Effect of size on diagnostic confidence. Eur Radiol. 2015, 25, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, H.; Ishida, H.; Konno, K.; Hamashima, Y.; Komatsuda, T.; Ishida, J.; Masamune, O. Hepatic hemangioma with arterioportal shunts. Abdom. Imaging 1999, 24, 42–46. [Google Scholar] [CrossRef]
- Xian, M.F.; Li, W.; Lan, W.; Zeng, D.; Xie, W.; Lu, M.; Huang, Y.; Wang, W. Strategy for accurate diagnosis by contrast-enhanced ultrasound of focal liver lesions in patients not at high risk for hepatocellular carcinoma. J. Ultrasound Med. 2023, 42, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Song, M.J.; Jung, Y.; Lee, W.S.; Jang, H.H. Proteomic Analysis of Primary Colon Cancer and Synchronous Solitary Liver Metastasis. Cancer Genom. Proteom. 2019, 16, 583–592. [Google Scholar] [CrossRef]
- Aloia, T.A.; Vauthey, J.N.; Loyer, E.M.; Ribero, D.; Pawlik, T.M.; Wei, S.H.; Curley, S.A.; Zorzi, D.; Abdalla, E.K. Solitary colorectal liver metastasis: Resection determines outcome. Arch. Surg. 2006, 141, 460–466, discussion 466–467. [Google Scholar] [CrossRef]
- Naganuma, H.; Hideaki Ishida, H.; Uno, A.; Nagai, H.; Ogawa, M.; Kamiyama, N. Refraction artifact on abdominal sonogram. J. Med. Ultrason. 2021, 48, 273–283. [Google Scholar] [CrossRef]
- Borzio, M.; Dionigi, E.; Parisi, G.; Raguzzi, I.; Sacco, R. Management of hepatocellular carcinoma in the erderly. World J. Hepatol. 2015, 7, 1521–1529. [Google Scholar] [CrossRef][Green Version]
- Radosevic, A.; Quesada, R.; Serlavos, C.; Sánchez, J.; Zugazaga, A.; Sierra, A.; Coll, S.; Busto, M.; Aguilar, G.; Daniel Flores, D.; et al. Microwave versus radiofrequency ablation for the treatment of liver malignancies: A randomized controlled phase 2 trial. Sci. Rep. 2022, 12, 316. [Google Scholar] [CrossRef]
- Chang, Y.; Jeong, S.W.; Jang, J.Y.; Kim, Y.J. Recent updates of transarterial chemoembolilzation in hepatocellular carcinoma. Int. J. Mol. Sci. 2020, 21, 8165. [Google Scholar] [CrossRef]
- Lazzaro, A.; Hartshorn, K.L. A comprehensive narrative review on the history, current landscape, and future directions of hepatocellular carcinoma (HCC) systemic therapy. Cancers 2023, 15, 2506. [Google Scholar] [CrossRef]
- Kimura, T.; Fujiwara, T.; Kameoka, T.; Adachi, Y.; Kariya, S. The current role of stereotactic body radiation therapy (SBRT) in hepatocellular carcinoma (HCC). Cancers 2022, 14, 4383. [Google Scholar] [CrossRef]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Heimbach, J.K. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ 2020, 371, m3544. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef]
- Ahn, J.C.; Lee, Y.T.; Agopian, V.G.; Zhu, Y.; You, S.; Tseng, H.R.; Yang, J.D. Hepatocellular carcinoma surveillance: Current practice and future directions. Hepatoma Res. 2022, 8, 10. [Google Scholar] [CrossRef]
- Teng, Y.X.; Xie, S.; Guo, P.P.; Deng, Z.J.; Zhang, Z.Y.; Gao, W.; Zhang, W.G.; Zhong, J.H. Hepatocellular carcinoma in non-alcoholic fatty liver disease: Current progresses and challenges. J. Clin. Transl. Hepatol. 2022, 10, 955–964. [Google Scholar] [CrossRef]
- Nguyen, X.-V.K.; Zhang, J.; Chin, K.L.; Bloom, S.; Nicoll, A.J. Is hepatocellular carcinoma in fatty liver different to non-fatty liver? Nutrients 2022, 14, 3875. [Google Scholar] [CrossRef]
- Orci, L.A.; Sanduzzi-Zamparelli, M.; Caballol, B.; Sapena, V.; Colucci, N.; Torres, F.; Bruix, J.; Reig, M.; Toso, C. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: A systematic review, meta-analysis, and meta-regression. Clin. Gastroenterol. Hepatol. 2022, 20, 283–292.e10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Pu, Y.; Bao, Y.; He, S. Investigation of Potential Molecular Biomarkers for Diagnosis and Prognosis of AFP-Negative HCC. Int. J. Gen. Med. 2021, 14, 4369–4380. [Google Scholar] [CrossRef] [PubMed]
- Nia, A.; Dhanasekaran, R. Genomic Landscape of HCC. Curr. Hepatol. Rep. 2020, 19, 448–461. [Google Scholar] [CrossRef]



| Year | Author [Reference] | Patient No. | Sex (M/F) | Age, Mean Age (Range) | HBV Infection | HCV Infection | AFP Normal/Elevated | Liver Enzyme Normal/Elevated | Metabolic Disease |
|---|---|---|---|---|---|---|---|---|---|
| 2006 | Eguchi [38] | 27 | 16/11 | 59 (26–76) | 0 | 0 | ND | ND | ND |
| 2007 | Bège [39] | 67 | 46/21 | 57 | 12 | 3 | 35/32 | ND | ND |
| 2008 | Guzman [40] | 3 | 1/2 | 57 (45–70) | 0 | 0 | 2/1 (1533 ng/mL) | 2/1 | 2/3 |
| 2008 | Takahashi [41] | 1 | F | 36 | − | − | Normal | Normal | − |
| 2013 | Lewis [42] | 1 | F | 75 | − | + | Normal | ND | ND |
| 2015 | Komiyama [43] | 1 | M | 76 | − | − | Normal | Normal | + |
| 2015 | Sheng [44] | 10 | 9/1 | 65 (4–8) | ND | ND | 5/5 | ND | ND |
| 2019 | Naganuma [45] | 12 | 8/4 | 70.7 (50–86) | 1 | 0 | 11/1 (1306 ng/mL) | 7/5 | 3/12 |
| 2020 | Ogasawara [46] | 1 | F | 48 | − | − | Elevated (224 ng/mL) | Normal | − |
| 2022 | Onishi [47] | 1 | F | 23 | − | − | Elevated (941.8 ng/mL) | Normal | − |
| Year | Author [Reference] | Patient No. | Tumor | Tumor Vascularity | Resection (Cases) | |||
|---|---|---|---|---|---|---|---|---|
| Size (Range) (mm) | Histology wel/mod/por | Capsule (Cases) | Arterial Enhancement | Wash-Out | ||||
| 2006 | Eguchi [38] | 27 | 90 (17–250) | 19 (wel-mod)/2 (por) (6 unknown) | 20/27 | ND | ND | 27 |
| 2007 | Bège [39] | 67 | 115 | non-WHO | 54/67 | ND | ND | 67 |
| 2008 | Guzman [40] | 3 | 71 (68–100) | 1/2/0 | ND | ND | ND | 3 |
| 2008 | Takahashi [41] | 1 | 60 | mod | + | hyper | + | 1 |
| 2013 | Lewis [42] | 1 | 38 | mod | ND | hyper | + | 1 |
| 2015 | Komiyama [43] | 1 | 58 | wel | + | hypo | ND | 1 |
| 2015 | Sheng [44] | 10 | <20 | 0/9/1 | 8/10 | hyper 10/10 | 4/10 | 10 |
| 2019 | Naganuma [45] | 12 | 78.6 (23–200) | 6/6/0 | 11/12 | hyper 12/12 | 12/12 | 12 |
| 2020 | Ogasawara [46] | 1 | 25 | por | − | hyper | + | 1 |
| 2022 | Onishi [47] | 1 | 50 | por | + | hyper | + | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naganuma, H.; Ishida, H. Hepatocellular Carcinoma in Non-Fibrotic Liver: A Narrative Review. Diagnostics 2023, 13, 3426. https://doi.org/10.3390/diagnostics13223426
Naganuma H, Ishida H. Hepatocellular Carcinoma in Non-Fibrotic Liver: A Narrative Review. Diagnostics. 2023; 13(22):3426. https://doi.org/10.3390/diagnostics13223426
Chicago/Turabian StyleNaganuma, Hiroko, and Hideaki Ishida. 2023. "Hepatocellular Carcinoma in Non-Fibrotic Liver: A Narrative Review" Diagnostics 13, no. 22: 3426. https://doi.org/10.3390/diagnostics13223426
APA StyleNaganuma, H., & Ishida, H. (2023). Hepatocellular Carcinoma in Non-Fibrotic Liver: A Narrative Review. Diagnostics, 13(22), 3426. https://doi.org/10.3390/diagnostics13223426





