Abstract
This study was conducted to identify the risk causes and predictive models based on the clinical features of patients with breast cancer classified as triple-negative breast cancer (TNBC) and non-triple-negative breast cancer (non-TNBCs) using Korean cancer statistics. A total of 2045 cases that underwent three types of hormone receptor tests were obtained from Korean cancer data in 2016. Research data were analyzed with the software SPSS Ver. 26.0. TNBC and non-TNBCs accounted for 12.4% and 87.6% of the data, respectively. Tubular and lobular tumors occurred most frequently in the external quadrant of the breast (C50.4–C50.5; 43.1%). Compared to non-TNBCs, the incidence of TNBC was the most common in patients under the age of 39 (19.5%), followed by those over the age of 70 (17.3%). Tumors larger than 2 cm accounted for 16.0%, which was higher than the number of tumors smaller than 2 cm. Cases in stage IV cancer represented 21.7% of the data. Additionally, 21.0% of the patients were in the SEER stage of distant metastasis, which was the most prevalent SEER (surveillance, epidemiology, and end outcomes) stage. Neoadjuvant therapy was administered more frequently, with a rate of 24.1%. In the logistic regression and decision-making tree model, the variables that affected TNBC were age, differentiation grade, and neoadjuvant therapy. The predictive accuracies of logistic regression and decision-making tree models were 87.8% and 87.6%, respectively. In a decision-marking tree model, the differentiation grades of TNBC affect neoadjuvant therapy, and neoadjuvant therapy affects the cancer stage. Therefore, in order to promote the health of breast cancer patients, it is urgent to apply an intensive early health check-up program for those in their 40s and 50s with a high prevalence of TNBC. For patients with breast cancer, in TNBC cases, except for poorly differentiated cases, neoadjuvant therapy must be applied first at all differentiation grades. The establishment of a policy system is necessary for the success of this process.
1. Introduction
In 2021, the Korean Central Cancer Registry (KCCR) reported that approximately 36% of women would develop cancer during their lifetime. Breast cancer has the greatest incidence rate among female cancers, making up 20.6% of all malignancies [1]. It remarkably influences hormone receptors. Immunohistochemistry analysis reveals that cancerous breast tissues contain estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2), which are receptors for female hormones. Thus, it is largely classified into breast cancer with female hormone receptors (ER, PR) and HER-2 and breast cancer without them, according to cancer that expresses these proteins [2,3,4]. On the basis of receptor status, hormone treatment, chemotherapy, and targeted treatment before and after surgery are customized [2,3,4].
Breast cancer that is positive for female hormone receptors (ER, PR) progresses more slowly than breast cancer that is not [4]. Cases positive for the ER and PR benefit significantly more from hormonal therapy than cases with only one positive aspect (ER+, PR− and ER−, PR+). If both are negative (ER−, PR−), the effect of hormone therapy cannot be expected [5]. When detected early, these cancers are often cured by anti-estrogen therapy and undergoing surgery or chemotherapy according to the stage [5]. However, since cancer cells may remain hidden in the bones, lungs, and liver for extended periods, patients must undergo long-term follow-ups to prevent recurrences. Premenopausal patients with large tumors, patients with rapidly growing cancer cells, and young patients are at high risk. Conversely, HER2-positive breast cancer, where the HER2 gene is amplified and/or overexpressed, is characterized by faster and more aggressive progression than other breast cancers. If targeted therapy is used in the early stages of cancer, the prognosis is good. It also lowers the risk of recurrence by more than 40% at stages I, II, and III [6].
As previously mentioned, TNBC does not express ER, PR, and HER2. It is the most hostile of all cancers; it has a reduced prognosis and various pathological features; it also progresses rapidly and often affects young patients [7]. Therefore, the treatment method for breast cancer should depend on the presence or absence of hormones. TNBC is difficult to treat because neither hormone therapy nor targeted anticancer drugs are effective. The chemotherapy process is employed to attack and destroy cancer cells using various strategies to carry out its cytotoxic effects. Chemotherapeutic drugs mostly stop cancer cells from proliferating quickly by interfering with vital biological functions, including DNA replication and mitosis. Furthermore, these substances cause cancer cells to undergo programmed cell death or apoptosis. Chemotherapy targets fast-dividing cells specifically in an effort to stop cancer’s unstoppable spread and eventually eliminate the cancerous cell population. But this treatment also kills normal cells; consequently, it causes serious side effects, such as vomiting, diarrhea, hair loss, bone marrow dysfunction, and lethargy. Currently, the routine treatment for TNBC patients is chemotherapy, but in addition to serious toxicity effects (vomiting, diarrhea, hair loss, bone marrow dysfunction, and lethargy), the malignant cells may also develop resistance to anticancer drugs and evolve into highly drug-resistant cells [8,9]. Hence, targeted therapy, which specifically attacks cancer cells, and immunotherapy have been widely used, but their effects and applications are limited; furthermore, resistance problems occur during long-term treatment.
Recently, the World Health Organization (WHO) announced that more than 2.3 million cases of breast cancer per year, which is the most common disease among women, are reported. The death rate of women due to breast cancer is increasing day by day and become a remarkable cause in almost 95% of countries. In 2020, a study conducted by the International Agency for Research on Cancer reported that nearly 4.4 million women died in 2020 because of cancer, of which 25% of women died due to breast cancer [10,11].
The research studies pointed out that significant contributions In the progression of breast cancer might be implicated by epigenetic controls and non-coding RNAs and impactfully affect the variety and spread of the disease, especially in the context of triple-negative breast cancer (TNBC). It can originate from molecular, cellular, and genetic abnormalities or dysregulation [12,13].
Therefore, this study is conducted to recognize risk factors and predictive models based on the clinical characteristics of the cancer subjects by classifying them into TNBCs (triple-negative breast cancer) and non-TNBCs (one or more positive receptors). Based on this, the health promotion plan for breast cancer patients is to conduct early screening as the primary prevention. This is because early screening can detect potential health problems. If breast cancer is diagnosed through early screening, active cancer treatment, and secondary prevention should be performed by blocking the risk factors identified in studies based on epidemiological characteristics. This may block further disease progression or complications. And it is because you can secure the opportunity to apply various treatment methods.
Accordingly, the specific research goals are as follows: First, the clinical characteristics of TNBCs and non-TNBCs were compared and analyzed. Second, the influencing factors were analyzed through regression and tree models, focusing on statistically significant variables among the clinical characteristics of TNBCs and non-TNBCs.
The Literature Review
Breast cancer is classified according to gene expression and immunohistochemical staining. Breast cancer is classified into the type A group (estrogen receptor+, HER2−), the type B group (estrogen receptor+, HER2+), the HER2 overexpression group (estrogen receptor−, HER2+), and the estrogen by RNA microarray method. The receptor- and HER2-negative subjects are classified into the basal type group and the normal type group [14,15]. The classification method, according to immunohistochemical staining, evaluates breast cancer by the presence or absence of positive immunohistochemical staining. According to immunohistochemical staining, breast cancer can be divided into TNBCs without estrogen and progesterone receptors, HER2 and non-TNBCs with estrogen and progesterone receptors, and HER2 [4,16].
The gene expression pattern-based classification method is difficult to apply clinically, and currently, a method of distinguishing breast cancer by the presence or nonexistence of hormone receptors and HER2 expression through immunohistochemical staining is used [16]. In gene expression-based breast cancer classification, it is known that the prognosis of the HER2 overexpression group (estrogen receptor−, HER2+) can improve with targeted therapy, and the basal type group (estrogen receptor−, HER2−) has many early recurrences and poor prognoses [16]. In the immunohistochemical staining-based classification, when both ER and PR are positive, as opposed to cases where only one receptor is positive (estrogen receptor+, progesterone receptor− or estrogen receptor−, progesterone receptor+), hormone treatment is known to have a good prognosis [5].
The five-year and ten-year survival rates were better when both hormone receptors were positive rather than negative, and survival rates were better when at least one hormone receptor was positive. Even when they were analyzed by stage, the survival rate was better in the hormone receptor-positive cases [17].
In the classification based on immunohistochemical staining, it is known that ER- and PR-positive breast cancer accounts for 75–80%, and HER2-positive breast cancer accounts for 15–20%. TNBC lacking ER, PR, and HER2 expression accounts for 10–15% [7,18]. TNBC is particularly common in those under the age of 40, often called early breast cancer. In addition, because of its aggressive characteristics, it has a rapid rate of cancer progression and a high risk of metastasis and recurrence [19]. In fact, one in three patients with TNBCs experience distant metastasis, where cancer has spread to sites distant from the breast, and overall survival after metastasis averages only from 1 to 1.5 years [19]. Moreover, non-TNBCs are characterized by metastasis to the bone, but TNBCs often metastasize to the brain and lungs, which can have a more fatal effect [19].
ER and PR are intracellular receptors that can be directly measured in tumor tissue [20] and are expressed in about 75–80% of all breast cancer patients [20,21]. Although TNBC does not exactly match the basal type of gene expression, it is sometimes used interchangeably because it shows a similar clinical course [22].
It is known that TNBC patients have a relatively common clinical recurrence, distant metastasis, and poor prognosis compared to non-TNBC patients. In addition, distant metastasis and mortality are high, and a high recurrence is reported within four years after diagnosis [22]. The poor prognosis of TNBC is thought to be related to histopathologic characteristics different from those of non-TNBCs and differences in recurrence and metastasis [4].
Therefore, this research was conducted to recognize the risk factors and analytical models based on the clinical characteristics of breast cancer subjects classified as TNBCs and non-TNBCs.
2. Research Method
2.1. Research Subjects
The research subjects were 2300 breast cancer patients registered in South Korea in 2016. This study was conducted on 2045 patients who had cancer and underwent all three types of ER, PR, and HER2 tests, and 255 circumstances were excluded because ER, PR, and HER2 were not performed.
Statistics were attained from collaborative stage data from the National Cancer Incidence Database. The American Joint Committee on Cancer (AJCC) surveillance and stage partner organization developed a standardized and integrated staging system, which is called the collaborative stage data collection system (CS) [23]. TNM (tumor, node, and metastasis) staging differs from SEER (surveillance, epidemiology, and end outcomes program) staging, which is used in cancer registration in terms of its goals and intentions [14]. Collaborative Stage Data aim to solve resource waste and inconsistency issues between the two staging systems [23].
Since 1980, the KCCR has served as a countrywide hospital-based program for collecting data from 47 training hospitals across the country [24]. As of 2021, yearly reports of cancer data in Korea from 2008 to 2019 were published [24]. The last follow-up date was 31 December 2018 [24]. These data were approved by the National Statistical Office (Approval No. 117044). The basis for approval is in accordance with Article 14 of the Cancer Control Act and Article 18 of the Statistical Act [25].
2.2. Research Tools
The tools of this study set included clinical characteristics, such as age, as independent variables and TNBCs and non-TNBCs as dependent variables.
2.3. Clinical Characteristics
Ages included in this analysis were divided into the following groups: ≤39 years; 40–49 years; 50–59 years; 60–69 years; and ≥70 years. Topographic code (T-code), morphologic code (M-code), tumor size, differentiation grade, cancer stage, SEER stage, and neoadjuvant therapy were the clinical parameters considered in this analysis. T-codes were classified from C50.0 to C50.1 (nipple, areola, and central area of the breast), from C50.2 to C50.3 (inner quadrants), from C50.4 to C50.5 (outer quadrants), C50.8 (overlapping lesion), C50.6, and C50.9 (other areas). M-codes were classified as M850–M854 (ductal and lobular neoplasm) and other tissue forms. Tumor size was classified as less than 2 cm and more than 2 cm. The differentiation grade was defined as moderate, poor, and unknown. The four stages of cancer are I, II, III, and IV. Regional, localized, and distant metastases were divided into SEER stages. Neoadjuvant therapy was classified as present, absent, and unknown.
2.4. TNBC and Non-TNBCs
The dependent variables included in this analysis, the categories TNBCs and non-TNBCs, are either helpful or harmful for hormone receptors and HER2. Cases that were harmful for estrogen, progesterone, and HER2 were defined as TNBCs, and cases that were positive for any of these three receptors were classified as non-TNBCs. The classification of breast cancer by hormone type is shown in Table 1.

Table 1.
Classification of breast cancer by hormone type.
2.5. Data Analysis
The clinical characteristics and prognosis of TNBCs and non-TNBCs were compared. All data were evaluated using IBM SPSS 26.0 statistics, Korean edition. The analysis packages were performed in Microsoft Windows 10. An X2 test was used to compare TNBC and non-TNBC. Furthermore, the influencing factors of the two groups were analyzed through regression and tree models. First, the numerical results of the influencing factors of TNBCs and non-TNBCs were confirmed by binary logistic regression. Next, the TNBC influencing factors identified in logistic regression were classified and predicted into a decision-making tree using classification CART (regression tree) and CHAID (chi-squared automatic interaction detection) methods. CART, developed by Leo Brineman and Associates in 1984, and CHAID, developed by J. A. Hartigan in 1975, are the most widely used decision-making tree algorithms [26].
CART [27] uses the Gini index as the separation criterion for categorical target variables. The Gini index is one of the measures used to measure the impurity or diversity of each node. It is expressed as
Here, n is the number of observations included in the node. ni is the number of observations in the i-th category of the target variable.
CHAID [28] is an algorithm that completes a multiway split by an X2 test (definite target variables) or F-test (constant target variables). This algorithm uses Pearson’s X2 test or likelihood-ratio X2 test as the separation criterion when the target variable is categorical. Here, the likelihood ratio X2 test is used when a target variable is ordinal or grouped continuously. The Pearson’s X2 test and likelihood-ratio X2 test statistics are expressed as
Here, fij is the observation frequency, and eij is the expected frequency.
A decision-making tree is a knowledge discovery technique used for classification purposes. It is a classified tree, and it is differentiated into branches and leaves from the root node [29]. The decision-making tree model is composed of the structure of “If A, then B. Otherwise, B2”. This means that if A is the case, go to B; otherwise, go to B2 [30].
3. Results
3.1. Distribution of ER, PR, and HER2
According to Table 2, there were 253 (12.4%) TNBC subjects and 1792 non-TNBC subjects, accounting for 87.6% of the total. Among non-TNBCs, ER, PR helpful, and HER2 harmful were the highest in 1047 (51.2%) patients. Triple-positive breast cancer (TPBC) was 261 (12.8%), and hormone receptor-negative and HER2-positive were 205 (10.0%).

Table 2.
Distribution of ER, PR, and HER2.
3.2. Cross-Analysis between T-Code and M-Code
Ductal and lobular tissue breast cancer occurred most frequently in the outer quadrant (C50.4–C50.5) in 833 patients (43.1%). In other tissue breast cancers, overlapping lesions (C50.8) were 390 (20.2%), and the inner quadrant (C50.2–C50.3) was 375 (19.4%). A cross-analysis between T-code and M-code is shown in Table 3.

Table 3.
Cross-analysis of T-code and M-code in breast cancer.
3.3. Clinical Characteristics Comparison of TNBCs and Non-TNBCs
According to Table 4, TNBC was found in 38 patients (19.5%) under the age of 39, followed by 33 patients (17.3%) over the age of 70. Non-TNBCs were the most frequent in the 40–49-year group, with 603 patients (90.7%), followed by 581 patients (88.3%) in the 50–59 year group. There was a statistically significant age change among the two groups (p = 0.001).

Table 4.
Comparison of clinic characteristics between TNBCs and non-TNBCs.
For TNBC, the T-code of C50.4–C50.5 had the highest frequency in 125 cases (14.4%), followed by C50.8 in 52 cases (12.6%). For non-TNBCs, C50.6 and C50.9 had the highest frequency in 222 cases (91.0%), followed by C50.0–C50.1 in 108 cases (90.8%). There was no statistically significant change in the T-codes among the two groups (p = 0.104).
Likewise, the M-code of TNBC showed the highest frequency in 20 cases (18.0%) in tumors of other tissue forms. There were 233 cases (12.0%) of ductal and lobular tumors (M850–M854). For non-TNBCs, 1701 (88.0%) ductal and lobular tumors (M850–M854) were more frequent than other forms of tissue tumors 91 (82.0%). Among the two groups, there was no statistically significant change in M-code (p = 0.074).
Regarding tumor size, TNBC was more than 2 cm in 159 cases (16.0%) and less than 2 cm in 94 cases (9.0%). Non-TNBCs were less than 2 cm in 956 cases (91.0%) and more than 2 cm in 836 cases (84.0%). There was a statistically significant change in tumor size among the two groups (p = 0.001).
As for the differentiation grade in the TNBC, poorly differentiated had the highest incidence at 45 cases (24.7%), followed by an unknown differentiated with 189 cases (12.3%). Non-TNBCs were well-differentiated in 83 cases (98.8%) and moderately differentiated in 225 cases (92.6%). The distinction between the two groups’ differentiation grades was statistically important (p = 0.001).
Among the TNBC cases, stage IV was the most common in 15 cases (21.7%), followed by stage II in 127 cases (15.7%). In non-TNBC cases, stage I was the most common in 839 cases (90.8%), followed by stage II in 212 cases (89.1%). Among the two groups, there was a statistically significant change in the cancer stage (p = 0.001).
For the SEER stage, TNBC had 17 cases (21.0%) with distant metastasis (code 7) and 156 cases (12.3%) with localized metastasis (code 1). On the other hand, non-TNBCs had 614 cases (88.5%) with regional metastasis (code 2–4), which was the most frequent, followed by 1114 cases (87.7%) with localized metastasis (code 1). A statistically significant change in the SEER stage existed among the two groups (p = 0.049).
Finally, neoadjuvant therapy for TNBC was received in 26 cases (24.1%), whereas 204 cases (11.3%) did not receive treatment. For non-TNBCs, 1609 cases (88.7%) did not receive treatment, and 82 patients (75.9%) received neoadjuvant therapy. It was statistically significant (p 0.001) that the two groups differed in their use of neoadjuvant therapy.
3.4. Clinical Characteristics as Factors Associated with TNBC
A study using logistic regression was conducted to determine the clinical traits that were factors influencing the differences between the TNBC and non-TNBC groups (Table 5). Age, T-code, differentiation grade, SEER stage, and neoadjuvant therapy were identified as factors associated with TNBC. M-code is also associated with the TNBC. It might be possible that the association of the T-code is more prominent than that of the M-code.

Table 5.
Influencing factors on clinical characteristics according to TNBC.
Standard in the age of 39 years or younger, TNBC was 2.12 times (p = 0.001) more prevalent in those aged 40–49 and 1.78 times (p = 0.012) more likely to occur in people aged 50–59. Standard in the ductal and lobular tumors (M850-M854), TNBC was 0.55 times (p = 0.025) less likely in other forms of tissue. Standard on the well-differentiation scale, TNBC is 0.04 times (p = 0.002) less likely to be poorly differentiated. Standard in the SEER code localized, TNBC was 1.61 times (p = 0.020) more likely to be regional in SEER. Standard in the neoadjuvant therapy, TNBC was 2.66 times (p < 0.001) more likely to be a non-treated group. Incidentally, T-code, tumor size, and cancer stage were not statistically significant factors.
3.5. Prediction Model According to the Presence or Absence of TNBC
The decision-making tree methods (CART and CHAID), also known as decision-making trees, have been used to predict factors in TNBC, showing the nodes of the decision-making tree, from the main node to the fatal node, and the separation criteria. Through CART method analysis, TNBC predictors were found for differentiation grade, neoadjuvant therapy, age, and cancer stage (Figure 1). On the other hand, the CHAID method analysis found TNBC predictors for differentiation grade, neoadjuvant therapy, and age.
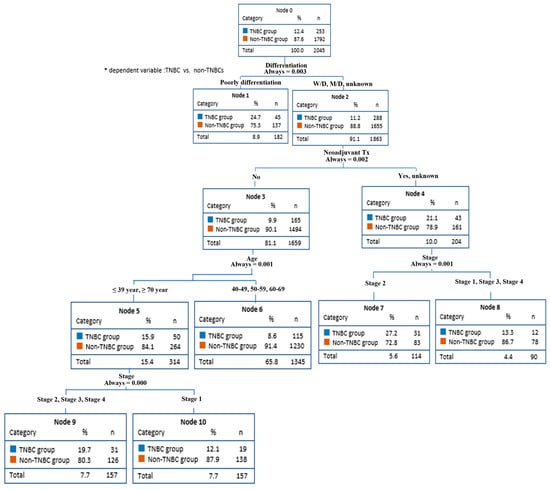
Figure 1.
Breast cancer prediction model (CART).
This model predicted that the non-TNBCs had an 88.8% probability of all differentiation grades (node 2) except for poorly differentiated. The differentiation grade affected neoadjuvant therapy. The non-TNBC group had a 90.1% chance of not receiving neoadjuvant therapy (node 3). In addition, because neoadjuvant therapy affects age, patients aged <39 years and >70 years (node 5) had an 84.1% chance of being included in the non-TNBCs group. Finally, age influences the stage of cancer. Stages II, III, and IV (node 9) had an 80.3% probability of being in the non-TNBC group.
On the other hand, the non-TNBC group had a 78.9% probability of undergoing neoadjuvant therapy or unknown (node 4). Since neoadjuvant therapy affected the stage, there was a 72.8% probability of stage II being in the non-TNBC group (node 2).
Figure 2 shows that the decision-making tree method by CHAID was used to predict factors in TNBC. This analysis found TNBC predictors for differentiation grade, neoadjuvant therapy, and age. Specifically, this model predicted that the non-TNBCs had an 87.7% probability of unknown differentiation (node 1). Differentiation grade influences neoadjuvant therapy. The non-TNBCs group had an 89.0% chance of not receiving neoadjuvant therapy (node 5). In addition, because neoadjuvant therapy affects age, patients aged 40–60 years (node7) had a 90.4% chance of being included in the non-TNBCs group.
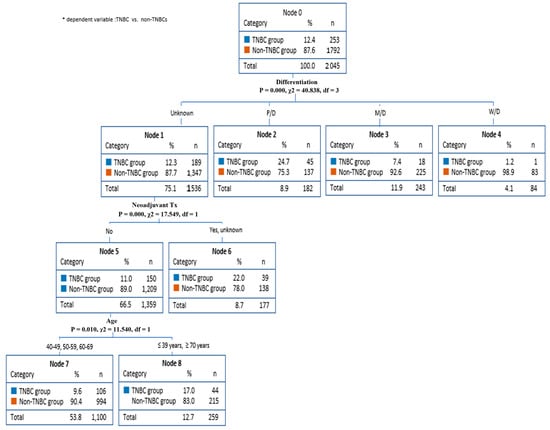
Figure 2.
Breast cancer prediction model (CHAID).
Table 6 is a risk chart for evaluating the breast cancer prediction model according to the presence or absence of TNBC. A risk estimate indicates the risk of being misclassified or predicted, and the smaller this value, the more successful the model building is [31].

Table 6.
The prediction rate of regression and decision-making tree models.
The possibility estimation of this model was 1.954 in the logistic regression study; the standard error (SE of risk estimate) was 0.067, and the classification correctness was 87.8%. The decision-making tree classification accuracy was 87.6%; the standard error was 0.007, and the risk estimate was 0.124.
4. Discussion
The TNBC is multifaceted, and breast cancer subtypes lack the expression of ER, PR, and HER2, thereby causing difficulty in therapeutic targeting. It generally has a high malignancy rate and a poorer prognosis [32]. TNBC accounts for 12.4% of breast cancer cases, and the relative incidence is higher in individuals younger than 39 and older than 70. The following variables were also relatively higher in TNBCs than in non-TNBCs. The tumor size was more than 2 cm; the stage was IV, and the SEER was distant metastasis (code 7).
Among patients with cancer, the percentage of TNBC patients was 24.6–31.6% for African American women, 10.8–23% for Caucasian women, and 15% for Japanese women, showing slightly different patterns, depending on race [33,34,35,36,37]. In the present study, 12.4% of patients were determined to be TNBC, and this value was lower than the overseas incidence rate of TNBC. ER (+)/PR (+) and HER2 (−) showed the largest distribution with 51.2%. The anatomical sites of tumor development most frequently occurred in the outer quadrant (C50.4–C50.5) and in ductal and lobular areas (M850–M854). The breast is an anatomically tubular region with a high incidence in the axillary proximal lateral quadrant, which is rich in lymph nodes.
As for the frequency of occurrence by age, TNBC was more common in those aged under 39 and over 70, and non-TNBCs were more common in those in their 40s and 50s. Similar to these study results, previous findings showed that the occurrence of TNBC is high among individuals aged <35 years [38]. The occurrence of TNBC is high in the age group with low incidence, not in the 40–50 age group, where breast cancer is common. As for the size of the tumor in this study, 62.8% of TNBC larger than 2 cm were reported. Dent et al. [22] reported that approximately 70% of the TNBC patient group had tumors larger than 2 cm, and Ahn et al. [38] reported 55.5%. TNBC has a higher proportion of tumors of >2 cm than those of <2 cm. This finding suggests that TNBC tumors are more likely to develop when tumors are larger than 2 cm in size. Histological differentiation is worse in TNBC [22,39,40], and histological and nuclear differentiation in TNBC is worse than that in non-TNBCs [22,40]. In the present study, TNBC showed a high rate of poor differentiation.
The TNBC group has a relatively poorer prognosis than the non-TNBC group, likely because of the lack of a known therapeutic target such as hormone receptor or HER2 expression, but no specific treatment method, other than nonspecific chemotherapy, is available [6]. The prognosis was poor as the ratio of stage IV to distant metastasis was high even in the stage of cancer or SEER of cancer. Similar to previous findings [38,41], the present study result showed that the TNBC group received more neoadjuvant therapy than the non-TNBC group.
As a result of the CART and CHAID methods of decision-making trees for predicting risk factors of TNBC, differentiation degree, neoadjuvant therapy, and age were predicted to be common factors, and the high prediction rate was 87.6%. In this study, breast cancer subjects were retrospectively analyzed. The prospective approach of TNBC, which targets women under 40 and over 70 with a high incidence of TNBC, requires regular self-examinations, imaging education through multiple channels, and national cancer screening.
Therefore, preventive interventions are very necessary. In addition, if cancer has occurred, the differentiation status must be evaluated, and a treatment approach that prioritizes neoadjuvant therapy is necessary. On the other hand, TNBC cannot be treated with hormone therapy. Since targeted therapeutics such as Herceptin® (trastuzumab) cannot be used, only chemotherapy is used for neoadjuvant therapy [37]. The main anticancer agents used as neoadjuvant therapy before surgery and postoperative adjuvant therapy are anthracycline and taxane systems [38].
This retrospective study was conducted to analyze the incidence of TNBC and non-TNBC breast cancer in Korean women and to examine its clinical characteristics and risk factors. In future studies, a prospective approach based on the risk factors identified in this study or a cohort study through continuous observation should be performed.
5. Conclusions
This research was conducted to identify the risk factors and predictive models based on the clinical characteristics of breast cancer subjects classified as TNBCs and non-TNBCs using Korean cancer statistics. The main research results and conclusions are as follows:
The occurrence of TNBC is the most common in patients aged ≤39, followed by those aged ≥70. Tumors larger than 2 cm had more frequent occurrence than tumors smaller than 2 cm. Poor differentiation was the highest. Additionally, stage IV was the most common, and SEER was in distant metastasis (code 7). The performance of neoadjuvant therapy was higher than that of non-treatment.
Logistic regression analysis revealed that age, M-code, differentiation grade, SEER stage, and neoadjuvant therapy were factors associated with TNBC. Conversely, decision-making tree analysis showed that differentiation grade, neoadjuvant therapy, and age were factors associated with TNBC. Both predictive models identified age, differentiation grade, and neoadjuvant therapy as influencing factors.
Therefore, the therapeutic approach for patients with TNBC should consider differentiation grade, neoadjuvant therapy, age, and staging. In addition, patients with TNBC should be carefully observed and actively treated because of their poor differentiation, high staging, and the chance of distant metastases is considerable. To promote the health of breast cancer patients, it is urgent to apply an intensive early health check-up program for those in their 40s and 50s with a high prevalence of TNBC. This allows for the early discovery of breast cancer and screening for TNBC-positive patients. The establishment of a policy system is necessary for the success of this process.
Since this study applied clinical characteristics and risk factors of breast cancer patients to Koreans, there is a limitation in generalizing it globally. However, it is meaningful to study pathological characteristics and risk factors for health promotion in Korean breast cancer patients using a retrospective method. Additional variables that may lead to breast cancer in the future, mortality, and their relationship to other factors should be analyzed in detail. In addition, it is necessary to understand the characteristics of each country, race, and continent through comparative studies between Korea and other countries. Therefore, these studies are extremely pertinent for research purposes and can offer guidance for analyzing the statistics of patients with breast cancer in diverse communities from various regions.
Funding
The funding for this paper was provided by Namseoul University, Republic of Korea.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the National Statistical Office (protocol code 117044 and on 31 December 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The author declares that all works are original, and this manuscript has not been published in any other journal.
References
- Korea Central Cancer Registry. 2019 National Cancer Registry Statistics; Korea Central Cancer Registry: Goyang, Republic of Korea, 2021. [Google Scholar]
- Ambs, S. Prognostic significance of subtype classification for short and long-term survival in breast cancer: Survival time holds the key. PLoS Med. 2010, 7, 1000281. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.; Figueroa, J.; Ali, H.R.; Blows, F.; Lissowska, J.; Caldas, C. Combined quantitative measures of ER, PR, HER2, and KI67 provide more prognostic information than categorical combinations in luminal breast cancer. Mod. Pathol. 2019, 32, 1244–1256. [Google Scholar] [CrossRef]
- Lee, K.K.; Kim, J.Y.; Jung, J.H.; Park, J.Y.; Park, H.Y. Clinicopathological feature and recurrence pattern of triple negative breast cancer. J. Korean Surg. Soc. 2010, 79, 14–19. [Google Scholar] [CrossRef]
- Hong, Y.S. Medical treatment of breast cancer. J. Korean Med. Assoc. 2003, 46, 512–520. [Google Scholar] [CrossRef]
- Breast Cancer Is a Treatable Cancer. Available online: https://health.chosun.com/site/data/html_dir/2019/10/04/2019100401006.html (accessed on 27 May 2022).
- Konecny, G.; Pauletti, G.; Pegram, M.; Untch, M.; Dandekar, S.; Aguilar, Z. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J. Natl. Cancer Inst. 2003, 95, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Returns Incurable Triple-Negative Breast Cancer Cells to a Treatable State. Available online: https://www.doctorsnews.co.kr/news/articleView.html?idxno=142174 (accessed on 27 May 2022).
- Female Hormone Positive, HER2 Positive, Triple Negative Breast Cancer…What Are the Differences? Available online: http://www.canceranswer.co.kr/news/articleView.html?idxno=3435 (accessed on 27 May 2022).
- Breast Cancer Factsheet. Global Cancer Observatory. International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/20-Breast-fact-sheet.pdf (accessed on 31 March 2021).
- Mehrotra, R.; Yadav, K. Breast Cancer in India: Present Scenario and the Challenges Ahead. World J. Clin. Oncol. 2022, 13, 209–218. [Google Scholar] [CrossRef]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast Cancer Development and Progression: Risk Factors, Cancer Stem Cells, Signaling Pathways, Genomics, and Molecular Pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Shlyakhtina, Y.; Katherine, L.M.; Maximiliano, M.P. Genetic and Non-Genetic Mechanisms Underlying Cancer Evolution. Cancers 2021, 13, 181380. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef]
- Nielsen, T.O.; Hsu, F.D.; Jensen, K.; Cheang, M.; Karaca, G.; Hu, Z.; Hernandez-Boussard, T.; Livasy, C.; Cowan, D.; Dressler, L.; et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin. Cancer Res. 2004, 10, 5367–5374. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Cheung, K.Y.; Kim, Y.S. Correlation between hormonal receptor status and clinicopathologic factors with prognostic assessment in breast cancer. J. Korean Surg. Soc. 2003, 65, 198–204. [Google Scholar]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A.; et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef]
- Health Trends. Available online: https://www.k-health.com/news/articleView.html?idxno=50725 (accessed on 7 February 2023).
- Robert, D.B.; Marc, E.L. Cancer of the breast. In Cancer, Principle and Practice of Oncology, 6th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2001; pp. 1633–1651. [Google Scholar]
- Sue, A.J. Hormone receptors in breast cancer: Racial difference in distribution and survival. Breast Cancer Res. Treat. 2002, 73, 45–59. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Korea Central Cancer Registry. National Cancer Center, Collaborative Stage Data User Guide; Korea Central Cancer Registry: Goyang, Republic of Korea, 2018. [Google Scholar]
- Korea Central Cancer Registry; National Cancer Center. Annual Report of Cancer Statistics in Korea in 2019; Ministry of Health and Welfare: Taipei City, Taiwan, 2021; p. 15. Available online: http://ncc.re.kr/cancerStatsList.ncc?searchKey=total&searchValue=&pageNum=1 (accessed on 27 May 2022).
- Korea Central Cancer Registry. Annual Report of Cancer Statistics in Korea in 2016; Korea Central Cancer Registry: Goyang, Republic of Korea, 2018. [Google Scholar]
- Jung, M.K.; Lee, S.K. A study on decision tree using logistic regression coefficients. J. Korean Data Anal. Soc. 2008, 10, 1517–1526. [Google Scholar]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Wadsworth: Belmont, Australia, 1984. [Google Scholar]
- Kass, G.V. An exploratory technique for investigating large quantities of categorical data. Appl. Stat. 1980, 29, 119–129. [Google Scholar] [CrossRef]
- Choi, J.S.; Seo, D.S. Decision trees and its applications. Stat. Korea Stat. Anal. Res. 1999, 4, 61–83. [Google Scholar]
- Huh, M.H.; Lee, Y.G. Data Mining Modeling and Case, 2nd ed.; Hannarae: Seoul, Republic of Korea, 2008; pp. 171–250. [Google Scholar]
- Choi, J.H.; Seo, D.S. Data mining prediction and utilization. Stat. Anal. Res. 2002, 4, 61–83. [Google Scholar]
- Lu, C.; Yun, N. Triple negative breast cancer: Special histological types and emerging therapeutic methods. Cancer Biol. Med. 2020, 17, 293–306. [Google Scholar] [CrossRef]
- Yuli, C.; Shao, N.; Rao, R.; Aysola, P.; Reddy, V.; Oprea-llies, G.; Lee, L.; Okoli, J.; Partridge, E.; Reddy, E.S.P.; et al. BRCA1a has antitumor activity in TN breast, ovarian and prostate cancers. Oncogene 2007, 26, 6031–6037. [Google Scholar] [CrossRef][Green Version]
- Carey, L.A.; Perou, C.M.; Livasy, C.A.; Dressler, L.G.; Cowan, D.; Conway, K.; Karaca, G.; Troester, M.A.; Tse, C.K.; Edmiston, S.; et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006, 295, 2492–2502. [Google Scholar] [CrossRef]
- Bauer, K.R.; Brown, M.; Cress, R.D.; Parise, C.A.; Caggiano, V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California cancer Registry. Cancer 2007, 109, 1721–1728. [Google Scholar] [CrossRef]
- Rakha, E.A.; El-Sayed, M.E.; Green, A.R.; Lee, A.H.; Robertson, J.F.; Ellis, I.O. Prognostic markers in triple-negative breast cancer. Cancer 2007, 109, 25–32. [Google Scholar] [CrossRef]
- Sasa, M.; Bando, Y.; Takahashi, M.; Hirose, T.; Nagao, T. Screening for basal marker expression is necessary for decision of therapeutic strategy for triple-negative breast cancer. J. Surg. Oncol. 2008, 97, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.S.; Cho, J.H.; Kwon, S.Y.; Kang, S.H. Clinicopathologic characteristics and prognosis of early-stage triple negative breast cancer: Comparison with non-triple negative group. J. Korean Surg. Soc. 2009, 77, 37–42. [Google Scholar] [CrossRef]
- Haffty, B.G.; Yang, Q.; Reiss, M.; Kearney, T.; Higgins, S.A.; Weidhaas, J.; Harris, L.; Hait, W.; Toppmeyer, D. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J. Clin. Oncol. 2006, 24, 5652–5657. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.P.; Martel, M.; Harris, L.N. Triple negative breast cancer: Current understanding of biology and treatment options. Curr. Opin. Obstet. Gynecol. 2008, 20, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, Y.J.; Choi, Y.J. Does she advance her development in the face of cancer? A structural equation model of posttraumatic growth after diagnosed with cancer. Int. J. Adv. Nurs. Educ. Res. 2018, 3, 1–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).