Racioethnic Disparities in Endometrial Cancer Outcomes
Abstract
1. Introduction
2. Risk Factors for Endometrial Cancer Development
3. Disparities in the Pre-Diagnostic Patient Pathway
3.1. Access to Healthcare
3.2. Patient-Related Delays
3.3. Care Provider Factors
4. Disparities in Endometrial Cancer Diagnosis
5. Histological and Molecular Differences
5.1. Histological Differences
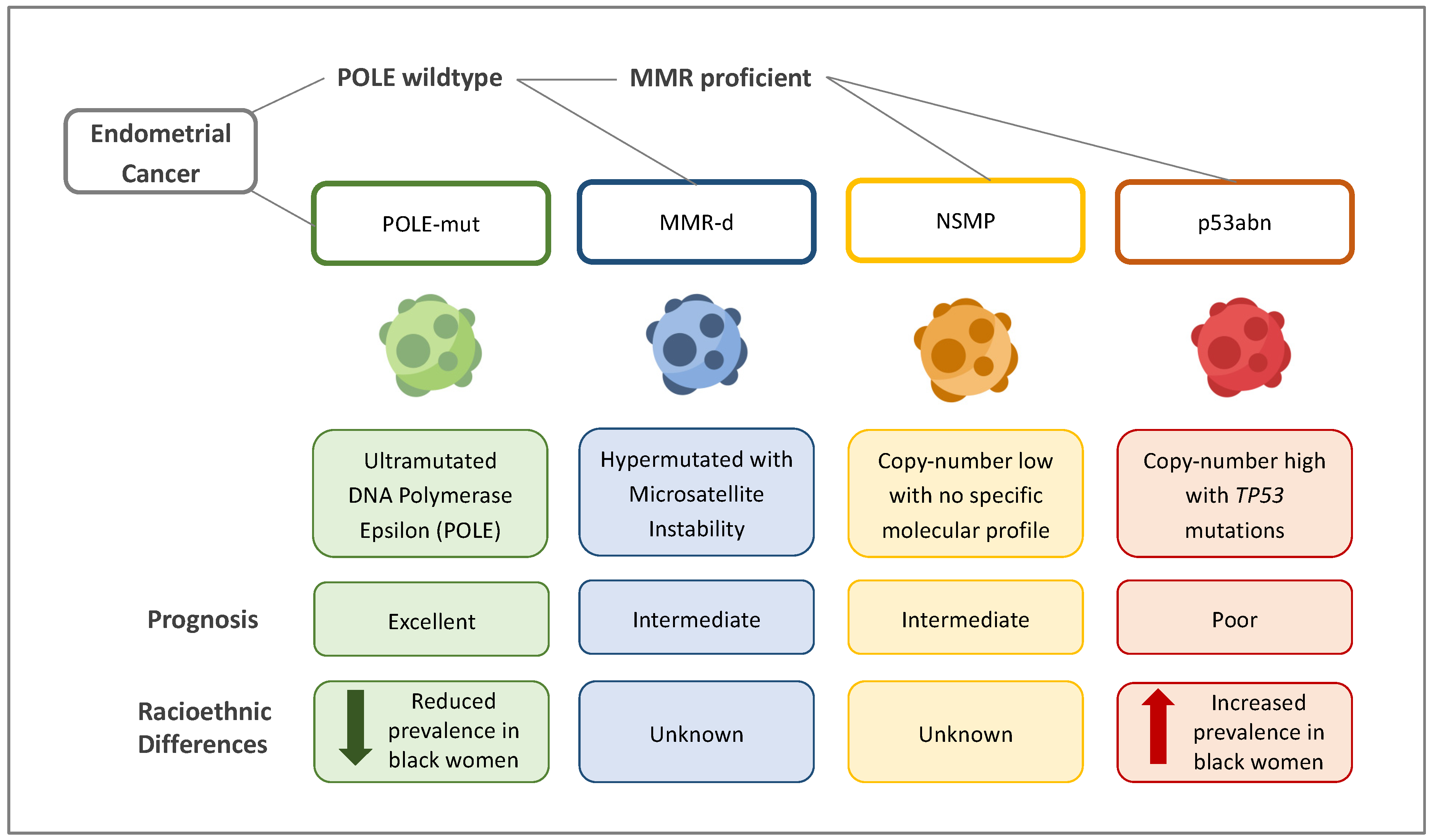
5.2. Molecular and Genetic Differences
- (i)
- Ultramutated/DNA polymerase epsilon (POLE)-mutated (POLE-mut) ECs, characterised by mutations in the POLE region, which result in a high transversion mutation frequency. These affect younger women and have good clinical outcomes.
- (ii)
- Hypermutated ECs with microsatellite instability (MSI)/mismatch-repair-deficient (MMRd) ECs, characterised by a ten-fold higher mutation frequency compared with MMR-proficient tumours. Ten per cent of these mutations are due to germline defects in the MMR gene (Lynch syndrome). The remainder are due to somatic defects. ECs in this group have an intermediate prognosis.
- (iii)
- Copy-number-high/p53-abnormal (p53abn) ECs, characterised by mutations in TP53. Most serous ECs and carcinosarcomas belong to this group, which is associated with the worst clinical outcomes.
- (iv)
- Copy-number-low/no specific molecular profile group (NSMP) ECs, which largely consist of endometrioid ECs and have stage-dependent intermediate–excellent clinical outcomes.
5.3. Updated FIGO Staging
6. Disparities in Endometrial Cancer Treatment
6.1. Evidence-Based Care
6.2. Delays in Treatment
6.3. Surgical Management
6.4. Treatment Refusal
7. Gaps in Knowledge and Potential Solutions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Today. Available online: http://gco.iarc.fr/today/home (accessed on 11 November 2023).
- Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow/en (accessed on 18 November 2023).
- World Health Organization Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 17 December 2023).
- Gottwald, L.; Pluta, P.; Piekarski, J.; Spych, M.; Hendzel, K.; Topczewska-Tylinska, K.; Nejc, D.; Bibik, R.; Korczyński, J.; Ciałkowska-Rysz, A. Long-Term Survival of Endometrioid Endometrial Cancer Patients. Arch. Med. Sci. AMS 2010, 6, 937–944. [Google Scholar] [CrossRef]
- SEER*Explorer Application. Available online: https://seer.cancer.gov/statistics-network/explorer/application.html (accessed on 18 November 2023).
- Whetstone, S.; Burke, W.; Sheth, S.S.; Brooks, R.; Cavens, A.; Huber-Keener, K.; Scott, D.M.; Worly, B.; Chelmow, D. Health Disparities in Uterine Cancer: Report from the Uterine Cancer Evidence Review Conference. Obstet. Gynecol. 2022, 139, 645. [Google Scholar] [CrossRef]
- Office for National Statistics Age-Standardised Mortality Rates for Uterine and Cervical Cancer by Ethnic Group, Females Aged 10 and above, Deaths Registered in England and Wales: 2012 and 2019. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/adhocs/15495agestandardisedmortalityratesforuterineandcervicalcancerbyethnicgroupfemalesaged10andabovedeathsregisteredinenglandandwales2012and2019 (accessed on 30 August 2023).
- Moss, E.L.; Teece, L.; Darko, N. Uterine Cancer Mortality and Black Women: Time to Act. Lancet Oncol. 2023, 24, 586–588. [Google Scholar] [CrossRef]
- Lillard, J.W.; Moses, K.A.; Mahal, B.A.; George, D.J. Racial Disparities in Black Men with Prostate Cancer: A Literature Review. Cancer 2022, 128, 3787–3795. [Google Scholar] [CrossRef] [PubMed]
- Nnorom, S.O.; Wilson, L.L. Breast Cancer in Black Women: Racial/Ethnic Disparities Affecting Survival. J. Womens Health 2022, 31, 1255–1261. [Google Scholar] [CrossRef]
- Clarke, M.A.; Devesa, S.S.; Harvey, S.V.; Wentzensen, N. Hysterectomy-Corrected Uterine Corpus Cancer Incidence Trends and Differences in Relative Survival Reveal Racial Disparities and Rising Rates of Nonendometrioid Cancers. J. Clin. Oncol. 2019, 37, 1895–1908. [Google Scholar] [CrossRef] [PubMed]
- Towner, M.; Kim, J.J.; Simon, M.A.; Matei, D.; Roque, D. Disparities in Gynecologic Cancer Incidence, Treatment, and Survival: A Narrative Review of Outcomes among Black and White Women in the United States. Int. J. Gynecol. Cancer 2022, 32, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Limb, M. Black Women in England Are at Greater Risk of Late Cancer Diagnosis than White Women. BMJ 2023, 380, 211. [Google Scholar] [CrossRef] [PubMed]
- Terzic, M.; Aimagambetova, G.; Kunz, J.; Bapayeva, G.; Aitbayeva, B.; Terzic, S.; Laganà, A.S. Molecular Basis of Endometriosis and Endometrial Cancer: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 9274. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial Cancer. Lancet Lond. Engl. 2022, 399, 1412–1428. [Google Scholar] [CrossRef]
- Huang, M.; Hunter, T.; Fein, L.A.; Galli, J.; George, S.; Schlumbrecht, M.; McCarter, K.; Sinno, A.K.; Guido, L.P.; Pinto, A. Lost Opportunities for Mismatch Repair (MMR) Screening among Minority Women with Endometrial Cancer. Sci. Rep. 2021, 11, 11712. [Google Scholar] [CrossRef]
- Kim, J.J.; Kurita, T.; Bulun, S.E. Progesterone Action in Endometrial Cancer, Endometriosis, Uterine Fibroids, and Breast Cancer. Endocr. Rev. 2013, 34, 130–162. [Google Scholar] [CrossRef]
- Onstad, M.A.; Schmandt, R.E.; Lu, K.H. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. J. Clin. Oncol. 2016, 34, 4225–4230. [Google Scholar] [CrossRef]
- MacKintosh, M.L.; Derbyshire, A.E.; McVey, R.J.; Bolton, J.; Nickkho-Amiry, M.; Higgins, C.L.; Kamieniorz, M.; Pemberton, P.W.; Kirmani, B.H.; Ahmed, B.; et al. The Impact of Obesity and Bariatric Surgery on Circulating and Tissue Biomarkers of Endometrial Cancer Risk. Int. J. Cancer 2019, 144, 641–650. [Google Scholar] [CrossRef]
- Overweight Adults. Available online: https://www.ethnicity-facts-figures.service.gov.uk/health/diet-and-exercise/overweight-adults/latest#data-sources (accessed on 18 November 2023).
- Lofton, H.; Ard, J.D.; Hunt, R.R.; Knight, M.G. Obesity among African American People in the United States: A Review. Obesity 2023, 31, 306–315. [Google Scholar] [CrossRef]
- Sponholtz, T.R.; Palmer, J.R.; Rosenberg, L.; Chen, C.; Chen, Y.; Clarke, M.A.; Clendenen, T.; Du, M.; Johnson, L.; Liao, L.M.; et al. Risk Factors for Endometrial Cancer in Black Women. Cancer Causes Control 2023, 34, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Gulland, A. UK Has Best Health System in Developed World, US Analysis Concludes. BMJ 2017, 358, j3442. [Google Scholar] [CrossRef]
- Park, A.B.; Darcy, K.M.; Tian, C.; Casablanca, Y.; Schinkel, J.K.; Enewold, L.; McGlynn, K.A.; Shriver, C.D.; Zhu, K. Racial Disparities in Survival among Women with Endometrial Cancer in an Equal Access System. Gynecol. Oncol. 2021, 163, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Gentry-Maharaj, A.; Karpinskyj, C. Current and Future Approaches to Screening for Endometrial Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 65, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.A.; Long, B.J.; Del Mar Morillo, A.; Arbyn, M.; Bakkum-Gamez, J.N.; Wentzensen, N. Association of Endometrial Cancer Risk with Postmenopausal Bleeding in Women. JAMA Intern. Med. 2018, 178, 1210–1222. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.R. African American Race and Low Income Neighborhoods Decrease Cause Specific Survival of Endometrial Cancer: A SEER Analysis. Asian Pac. J. Cancer Prev. 2013, 14, 2567–2570. [Google Scholar] [CrossRef]
- Donkers, H.; Bekkers, R.; Massuger, L.; Galaal, K. Systematic Review on Socioeconomic Deprivation and Survival in Endometrial Cancer. Cancer Causes Control 2019, 30, 1013–1022. [Google Scholar] [CrossRef]
- Fedewa, S.A.; Lerro, C.; Chase, D.; Ward, E.M. Insurance Status and Racial Differences in Uterine Cancer Survival: A Study of Patients in the National Cancer Database. Gynecol. Oncol. 2011, 122, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.E.L.; Maben, J.; Lucas, G.; Davies, E.A.; Jack, R.H.; Ream, E. Barriers to Early Diagnosis of Symptomatic Breast Cancer: A Qualitative Study of Black African, Black Caribbean and White British Women Living in the UK. BMJ Open 2015, 5, e006944. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.; Abel, G.; Ukoumunne, O.C.; Price, S.; Lyratzopoulos, G.; Chinegwundoh, F.; Hamilton, W. Assessing Ethnic Inequalities in Diagnostic Interval of Common Cancers: A Population-Based UK Cohort Study. Cancers 2022, 14, 3085. [Google Scholar] [CrossRef] [PubMed]
- Coates, R.J.; Click, L.A.; Harlan, L.C.; Robboy, S.; Barrett, R.J.; Eley, J.W.; Reynolds, P.; Chen, V.W.; Darity, W.A.; Blacklow, R.S.; et al. Differences between Black and White Patients with Cancer of the Uterine Corpus in Interval from Symptom Recognition to Initial Medical Consultation (United States). Cancer Causes Control 1996, 7, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.D.; Whitaker, K.L.; Piano, M.; Marlow, L.A.V. Ethnic Differences in Barriers to Symptomatic Presentation in Primary Care: A Survey of Women in England. Psychooncology 2019, 28, 2336–2343. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.; White, B.; Nagarwalla, D.; Shelton, J.; Jack, R.H. Relationship between Ethnicity and Stage at Diagnosis in England: A National Analysis of Six Cancer Sites. BMJ Open 2023, 13, e062079. [Google Scholar] [CrossRef] [PubMed]
- Waller, J.; Robb, K.; Stubbings, S.; Ramirez, A.; Macleod, U.; Austoker, J.; Hiom, S.; Wardle, J. Awareness of Cancer Symptoms and Anticipated Help Seeking among Ethnic Minority Groups in England. Br. J. Cancer 2009, 101, S24–S30. [Google Scholar] [CrossRef] [PubMed]
- Niksic, M.; Rachet, B.; Warburton, F.G.; Forbes, L.J.L. Ethnic Differences in Cancer Symptom Awareness and Barriers to Seeking Medical Help in England. Br. J. Cancer 2016, 115, 136–144. [Google Scholar] [CrossRef]
- Licqurish, S.; Phillipson, L.; Chiang, P.; Walker, J.; Walter, F.; Emery, J. Cancer Beliefs in Ethnic Minority Populations: A Review and Meta-Synthesis of Qualitative Studies. Eur. J. Cancer Care 2017, 26, e12556. [Google Scholar] [CrossRef]
- Doll, K.M.; Hempstead, B.; Alson, J.; Sage, L.; Lavallee, D. Assessment of Prediagnostic Experiences of Black Women With Endometrial Cancer in the United States. JAMA Netw. Open 2020, 3, e204954. [Google Scholar] [CrossRef]
- Ekechi, C.; Olaitan, A.; Ellis, R.; Koris, J.; Amajuoyi, A.; Marlow, L.A. Knowledge of Cervical Cancer and Attendance at Cervical Cancer Screening: A Survey of Black Women in London. BMC Public Health 2014, 14, 1096. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.L.; He, J.; Edmonds, M.C.; Sheppard, V.B. Medical Mistrust in Black Breast Cancer Patients: Acknowledging the Roles of the Trustor and the Trustee. J. Cancer Educ. 2019, 34, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Munoz, R.; Ewy, J.; O’Donnell, J.; Zhang, X.; Tan, X.; Bae-Jump, V. Evaluation of Racial Disparities in Time to Presentation, Diagnosis, and Gynecologic Oncology Referral in Women with Serous Endometrial Cancer (431). Gynecol. Oncol. 2022, 166, S217. [Google Scholar] [CrossRef]
- Doll, K.M.; Khor, S.; Odem-Davis, K.; He, H.; Wolff, E.M.; Flum, D.R.; Ramsey, S.D.; Goff, B.A. Role of Bleeding Recognition and Evaluation in Black-White Disparities in Endometrial Cancer. Am. J. Obstet. Gynecol. 2018, 219, 593.e1–593.e14. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.; Abel, G.; Ukoumunne, O.C.; Mounce, L.T.A.; Price, S.; Lyratzopoulos, G.; Chinegwundoh, F.; Hamilton, W. Ethnic Inequalities in Routes to Diagnosis of Cancer: A Population-Based UK Cohort Study. Br. J. Cancer 2022, 127, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Lyratzopoulos, G.; Neal, R.D.; Barbiere, J.M.; Rubin, G.P.; Abel, G.A. Variation in Number of General Practitioner Consultations before Hospital Referral for Cancer: Findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol. 2012, 13, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, L.; Nunez-Smith, M.; Clark, M.; Wright, J.D. Racial Disparities in Diagnostic Evaluation of Uterine Cancer among Medicaid Beneficiaries. J. Natl. Cancer Inst. 2023, 115, 636–643. [Google Scholar] [CrossRef]
- NICE Guideline Suspected Cancer: Recognition and Referral. Available online: https://www.nice.org.uk/guidance/ng12 (accessed on 15 November 2023).
- NICE Guideline Heavy Menstrual Bleeding: Assessment and Management. Available online: https://www.nice.org.uk/guidance/ng88 (accessed on 18 November 2023).
- Morrison, J.; Balega, J.; Buckley, L.; Clamp, A.; Crosbie, E.; Drew, Y.; Durrant, L.; Forrest, J.; Fotopoulou, C.; Gajjar, K.; et al. British Gynaecological Cancer Society (BGCS) Uterine Cancer Guidelines: Recommendations for Practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 270, 50–89. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 734: The Role of Transvaginal Ultrasonography in Evaluating the Endometrium of Women with Postmenopausal Bleeding. Obstet. Gynecol. 2018, 131, e124–e129. [Google Scholar] [CrossRef]
- Berman, J.M.; Bradley, L.; Hawkins, S.M.; Levy, B. Uterine Fibroids in Black Women: A Race-Stratified Subgroup Analysis of Treatment Outcomes After Laparoscopic Radiofrequency Ablation. J. Womens Health 2022, 31, 593–599. [Google Scholar] [CrossRef]
- Rotenberg, O.; Escobar, P.; Fridman, D.; Dar, P. P14.10: Factors Associated with Inconsistency in Visibility of the Endometrial Echo on Transvaginal Ultrasound of Postmenopausal Women. Ultrasound Obstet. Gynecol. 2017, 50, 199–200. [Google Scholar] [CrossRef]
- Doll, K.M.; Romano, S.S.; Marsh, E.E.; Robinson, W.R. Estimated Performance of Transvaginal Ultrasonography for Evaluation of Postmenopausal Bleeding in a Simulated Cohort of Black and White Women in the US. JAMA Oncol. 2021, 7, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wieslander, C.; Hansen, G.; Cass, I.; Vasilev, S.; Holschneider, C.H. Thin Endometrial Echo Complex on Ultrasound Does Not Reliably Exclude Type 2 Endometrial Cancers. Gynecol. Oncol. 2006, 101, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, C.C.; Kenne, K.A.; Cansino, C.D.; Backes, F.J.; Cohn, D.E.; O’Malley, D.M.; Copeland, L.J.; Fowler, J.M.; Salani, R. The Use of Transvaginal Ultrasound in Type II Endometrial Cancer. Int. J. Gynecol. 2015, 25, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Kiff, J.M.; Williams-Weisenberger, M.; Spellacy, D.; Garg, B.; Munro, E.G.; Bruegl, A.S. Ultrasonographic Evaluation of Endometrial Stripe Thickness Is Insufficient to Rule out Uterine Serous Carcinoma. Cancer Causes Control 2023, 34, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Terzic, M.M.; Aimagambetova, G.; Terzic, S.; Norton, M.; Bapayeva, G.; Garzon, S. Current Role of Pipelle Endometrial Sampling in Early Diagnosis of Endometrial Cancer. Transl. Cancer Res. 2020, 9, 7716–7724. [Google Scholar] [CrossRef] [PubMed]
- Tanko, N.M.; Linkov, F.; Bapayeva, G.; Ukybassova, T.; Kaiyrlykyzy, A.; Aimagambetova, G.; Kenbayeva, K.; Ibrayimov, B.; Lyasova, A.; Terzic, M. Pipelle Endometrial Biopsy for Abnormal Uterine Bleeding in Daily Clinical Practice: Why the Approach to Patients Should Be Personalized? J. Pers. Med. 2021, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Den Helder, R.V.; Wever, B.M.; Van Trommel, J.A.; Ket, J.C.; Bleeker, M.C.; Steenbergen, R.D.; Van Trommel, N.E. DNA Methylation Markers for Endometrial Cancer Detection in Minimally Invasive Samples: A Systematic Review. Epigenomics 2020, 12, 1661–1672. [Google Scholar] [CrossRef]
- Evans, I.; Reisel, D.; Jones, A.; Bajrami, A.; Nijjar, S.; Solangon, S.A.; Arora, R.; Redl, E.; Schreiberhuber, L.; Ishaq-Parveen, I.; et al. Performance of the WID-qEC Test versus Sonography to Detect Uterine Cancers in Women with Abnormal Uterine Bleeding (EPI-SURE): A Prospective, Consecutive Observational Cohort Study in the UK. Lancet Oncol. 2023, 24, 1375–1386. [Google Scholar] [CrossRef]
- Bokhman, J.V. Two Pathogenetic Types of Endometrial Carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Masood, M.; Singh, N. Endometrial Carcinoma: Changes to Classification (WHO 2020). Diagn. Histopathol. 2021, 27, 493–499. [Google Scholar] [CrossRef]
- Miller, A.; Gordon, J.; Curtis, J.; Ajayakumar, J.; Schumacher, F.; Avril, S. The Geographic Context of Racial Disparities in Aggressive Endometrial Cancer Subtypes: Integrating Social and Environmental Aspects to Discern Biological Outcomes. Int. J. Environ. Res. Public. Health 2022, 19, 8613. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Fader, A.N.; Santin, A.D.; Liu, J.F. Uterine Serous Carcinoma: Molecular Features, Clinical Management, and New and Future Therapies. Gynecol. Oncol. 2021, 160, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Cote, M.L.; Ruterbusch, J.J.; Olson, S.H.; Lu, K.; Ali-Fehmi, R. The Growing Burden of Endometrial Cancer: A Major Racial Disparity Affecting Black Women. Cancer Epidemiol. Biomarkers Prev. 2015, 24, 1407–1415. [Google Scholar] [CrossRef]
- Clarke, M.A.; Devesa, S.S.; Hammer, A.; Wentzensen, N. Racial and Ethnic Differences in Hysterectomy-Corrected Uterine Corpus Cancer Mortality by Stage and Histologic Subtype. JAMA Oncol. 2022, 8, 895–903. [Google Scholar] [CrossRef]
- Wilhite, A.M.; Baca, Y.; Xiu, J.; Paladugu, R.; ElNaggar, A.C.; Brown, J.; Winer, I.S.; Morris, R.; Erickson, B.K.; Olawaiye, A.B.; et al. Molecular Profiles of Endometrial Cancer Tumors among Black Patients. Gynecol. Oncol. 2022, 166, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Yang, H.; Yu, X.; Lu, L.; Wang, Y.; Ji, J.; Xu, M.; Wei, W.; Li, B.; Zeng, H. Patterns and Trends in the Cause of Death for Patients with Endometrial Cancer. JNCI Cancer Spectr. 2023, 7, pkac082. [Google Scholar] [CrossRef] [PubMed]
- Kucera, C.W.; Tian, C.; Tarney, C.M.; Presti, C.; Jokajtys, S.; Winkler, S.S.; Casablanca, Y.; Bateman, N.W.; Mhawech-Fauceglia, P.; Wenzel, L.; et al. Factors Associated with Survival Disparities Between Non-Hispanic Black and White Patients with Uterine Cancer. JAMA Netw. Open 2023, 6, e238437. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated Genomic Characterization of Endometrial Carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, S.F.; Bao, W. Molecular Subtypes of Endometrial Cancer: Implications for Adjuvant Treatment Strategies. Int. J. Gynecol. Obstet. 2023, 164, 436–459. [Google Scholar] [CrossRef]
- Bell, D.W.; Ellenson, L.H. Molecular Genetics of Endometrial Carcinoma. Annu. Rev. Pathol. 2019, 14, 339–367. [Google Scholar] [CrossRef]
- Whelan, K.; Dillon, M.; Strickland, K.C.; Pothuri, B.; Bae-Jump, V.; Borden, L.E.; Thaker, P.H.; Haight, P.; Arend, R.C.; Ko, E.; et al. TP53 Mutation and Abnormal P53 Expression in Endometrial Cancer: Associations with Race and Outcomes. Gynecol. Oncol. 2023, 178, 44–53. [Google Scholar] [CrossRef]
- Kohler, M.F.; Berchuck, A.; Davidoff, A.M.; Humphrey, P.A.; Dodge, R.K.; Iglehart, J.D.; Soper, J.T.; Clarke-Pearson, D.L.; Bast, R.C.; Marks, J.R. Overexpression and Mutation of P53 in Endometrial Carcinoma. Cancer Res. 1992, 52, 1622–1627. [Google Scholar]
- Javadian, P.; Washington, C.; Mukasa, S.; Benbrook, D.M. Histopathologic, Genetic and Molecular Characterization of Endometrial Cancer Racial Disparity. Cancers 2021, 13, 1900. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Marra, A.; Selenica, P.; Rios-Doria, E.; Momeni-Boroujeni, A.; Berger, M.F.; Arora, K.; Nemirovsky, D.; Iasonos, A.; Chakravarty, D.; et al. Molecular Characterization of Endometrial Carcinomas in Black and White Patients Reveals Disparate Drivers with Therapeutic Implications. Cancer Discov. 2023, 13, 2356–2369. [Google Scholar] [CrossRef] [PubMed]
- Berera, S.; Koru-Sengul, T.; Miao, F.; Carrasquillo, O.; Nadji, M.; Zhang, Y.; Hosein, P.J.; McCauley, J.L.; Abreu, M.T.; Sussman, D.A. Colorectal Tumors from Different Racial and Ethnic Minorities Have Similar Rates of Mismatch Repair Deficiency. Clin. Gastroenterol. Hepatol. 2016, 14, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Javadian, P.; Xu, C.; Sjoelund, V.; Borden, L.E.; Garland, J.; Benbrook, D.M. Identification of Candidate Biomarker and Drug Targets for Improving Endometrial Cancer Racial Disparities. Int. J. Mol. Sci. 2022, 23, 7779. [Google Scholar] [CrossRef]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N.; Endometrial Cancer Staging Subcommittee; et al. FIGO Staging of Endometrial Cancer: 2023. Int. J. Gynecol. Obstet. 2023, 162, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, H.; Chelariu-Raicu, A.; Slomovitz, B.M. Immunotherapy in Endometrial Cancer. Int. J. Gynecol. Cancer 2023, 33, 351–357. [Google Scholar] [CrossRef]
- Huang, A.B.; Huang, Y.; Hur, C.; Tergas, A.I.; Khoury-Collado, F.; Melamed, A.; St Clair, C.M.; Hou, J.Y.; Ananth, C.V.; Neugut, A.I.; et al. Impact of Quality of Care on Racial Disparities in Survival for Endometrial Cancer. Am. J. Obstet. Gynecol. 2020, 223, 396.e1–396.e13. [Google Scholar] [CrossRef]
- Rodriguez, V.E.; LeBrón, A.M.W.; Chang, J.; Bristow, R.E. Racial–Ethnic and Socioeconomic Disparities in Guideline-Adherent Treatment for Endometrial Cancer. Obstet. Gynecol. 2021, 138, 21–31. [Google Scholar] [CrossRef]
- Kaspers, M.; Llamocca, E.; Quick, A.; Dholakia, J.; Salani, R.; Felix, A.S. Black and Hispanic Women Are Less Likely than White Women to Receive Guideline-Concordant Endometrial Cancer Treatment. Am. J. Obstet. Gynecol. 2020, 223, 398.e1–398.e18. [Google Scholar] [CrossRef]
- Joshi, T.; Hamilton, K.; Yi, M.; Scott, K.; Buchanan, T.; Burton, E.; Edelson, M.; Sorosky, J.; Shahin, M.; Chatterjee-Paer, S. Racial Determinants of Treatment Delays in Gynecologic Malignancies. Gynecol. Oncol. 2021, 162, S60. [Google Scholar] [CrossRef]
- AlHilli, M.M.; Tullio, K.; Elson, P.; Maggiotto, A.; Khorana, A.; Rose, P.G. Delay in Time to Surgery and Survival in Patients with Type i vs Type II Endometrial Cancer: A National Cancer Data Base Analysis. Gynecol. Oncol. 2017, 145, 56–57. [Google Scholar] [CrossRef]
- Randall, T.C.; Armstrong, K. Differences in Treatment and Outcome between African-American and White Women with Endometrial Cancer. J. Clin. Oncol. 2003, 21, 4200–4206. [Google Scholar] [CrossRef] [PubMed]
- Collins, Y.; Holcomb, K.; Chapman-Davis, E.; Khabele, D.; Farley, J.H. Gynecologic Cancer Disparities: A Report from the Health Disparities Taskforce of the Society of Gynecologic Oncology. Gynecol. Oncol. 2014, 133, 353–361. [Google Scholar] [CrossRef]
- Taylor, K.N.; Li, A.; Manuel, M.; Rimel, B.J.; Kim, K.H. The Association of Black Race with Receipt of Hysterectomy and Survival in Low-Risk Endometrial Cancer. Gynecol. Oncol. 2023, 175, 156–162. [Google Scholar] [CrossRef]
- Rauh-Hain, J.A.; Buskwofie, A.; Clemmer, J.; Boruta, D.M.; Schorge, J.O.; Del Carmen, M.G. Racial Disparities in Treatment of High-Grade Endometrial Cancer in the Medicare Population. Obstet. Gynecol. 2015, 125, 843–851. [Google Scholar] [CrossRef]
- Sud, S.; Holmes, J.; Eblan, M.; Chen, R.; Jones, E. Clinical Characteristics Associated with Racial Disparities in Endometrial Cancer Outcomes: A Surveillance, Epidemiology and End Results Analysis. Gynecol. Oncol. 2018, 148, 349–356. [Google Scholar] [CrossRef]
- Haddad, S.; Ghadimi, K.; Abrishamkar, R.; Asl, N.S.M. Comparing Laparoscopy and Laparotomy Procedures in the Radical Hysterectomy Surgery for Endometrial Cancer: A Basic Review. Am. J. Transl. Res. 2021, 13, 2456–2461. [Google Scholar]
- Ptacek, I.; Aref-Adib, M.; Mallick, R.; Odejinmi, F. Each Uterus Counts: A Narrative Review of Health Disparities in Benign Gynaecology and Minimal Access Surgery. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 265, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Moss, E.L.; Morgan, G.; Martin, A.P.; Sarhanis, P.; Ind, T. Surgical Trends, Outcomes and Disparities in Minimal Invasive Surgery for Patients with Endometrial Cancer in England: A Retrospective Cohort Study. BMJ Open 2020, 10, e036222. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Gerber, D.; Aphinyanaphongs, Y.; Curtin, J.P.; Boyd, L.R. Laparoscopy Decreases the Disparity in Postoperative Complications between Black and White Women after Hysterectomy for Endometrial Cancer. Gynecol. Oncol. 2018, 149, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Barrington, D.A.; Meade, C.E.; Cosgrove, C.M.; Cohn, D.E.; Felix, A.S. Racial and Ethnic Disparities in Readmission Risk Following the Surgical Management of Endometrial Cancer. Gynecol. Oncol. 2022, 166, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Felix, A.S.; Nafiu, T.; Cosgrove, C.M.; Ewing, A.P.; Mpody, C. Racial Disparities in Surgical Outcomes among Women with Endometrial Cancer. Ann. Surg. Oncol. 2022, 29, 8338–8344. [Google Scholar] [CrossRef] [PubMed]
- Barrington, D.A.; Sinnott, J.A.; Nixon, D.; Padamsee, T.J.; Cohn, D.E.; Doll, K.M.; Donneyong, M.M.; Felix, A.S. More than Treatment Refusal: A National Cancer Database Analysis of Adjuvant Treatment Refusal and Racial Survival Disparities among Women with Endometrial Cancer. Am. J. Obstet. Gynecol. 2022, 227, 244.e1–244.e17. [Google Scholar] [CrossRef] [PubMed]
- Aizer, A.A.; Chen, M.-H.; Parekh, A.; Choueiri, T.K.; Hoffman, K.E.; Kim, S.P.; Martin, N.E.; Hu, J.C.; Trinh, Q.-D.; Nguyen, P.L. Refusal of Curative Radiation Therapy and Surgery among Patients with Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 756–764. [Google Scholar] [CrossRef]
- Rapp, J.; Tuminello, S.; Alpert, N.; Flores, R.M.; Taioli, E. Disparities in Surgery for Early-Stage Cancer: The Impact of Refusal. Cancer Causes Control 2019, 30, 1389–1397. [Google Scholar] [CrossRef]
- Straubhar, A.M.; Parsons, M.W.; Francis, S.; Gaffney, D.; Maurer, K.A. Refusal of Surgery and Survival Outcomes in Endometrial Cancer. Int. J. Gynecol. Cancer 2021, 31, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.W.; Francis, S.; Maurer, K.A.; Grant, J.; Gaffney, D.K. Refusal of Radiation Results in Inferior Survival in Endometrial Cancer. Am. J. Clin. Oncol. 2020, 43, 399–410. [Google Scholar] [CrossRef] [PubMed]
- The College’s Ambition for Race Equality. Available online: https://www.rcog.org.uk/about-us/the-college-s-ambition-for-race-equality/ (accessed on 8 February 2024).
- You Need to Know. Available online: https://eveappeal.org.uk/news-awareness/you-need-to-know/ (accessed on 1 October 2023).
- Khadraoui, W.; Meade, C.E.; Backes, F.J.; Felix, A.S. Racial and Ethnic Disparities in Clinical Trial Enrollment Among Women with Gynecologic Cancer. JAMA Netw. Open 2023, 6, e2346494. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.N.; Staples, J.N.; Garcia, C.; Chatfield, L.; Ferriss, J.S.; Duska, L. Are Ethnic and Racial Minority Women Less Likely to Participate in Clinical Trials? Gynecol. Oncol. 2020, 157, 323–328. [Google Scholar] [CrossRef]
- Racial Disparities in Endometrial Cancer: Improving Diagnosis and Treatment. Available online: https://www.fredhutch.org/en/news/blog/2023/06/racial-disparities-endometrial-cancer-improving-diagnosis-treatment.html (accessed on 8 February 2024).



| Stage | Description | |
|---|---|---|
| Stage 1 | Confined to the uterine corpus and ovary | |
| 1A | Disease limited to the endometrium OR non-aggressive histological type, i.e., low-grade endometroid, with invasion of less than half of myometrium with no or focal LVSI OR good prognosis disease. 1A1: Non-aggressive histological type limited to an endometrial polyp OR confined to the endometrium. 1A2: Non-aggressive histological types involving less than half of the myometrium with no or focal LVSI. 1A3: Low-grade endometrioid carcinomas limited to the uterus and ovary. | |
| 1B | Non-aggressive histological types with invasion of half or more of the myometrium, and with no or focal LVSI | |
| 1C | Aggressive histological types limited to a polyp or confined to the endometrium | |
| Stage 2 | Invasion of cervical stroma without extrauterine extension OR with substantial LVSI OR aggressive histological types with myometrial invasion | |
| 2A | Invasion of the cervical stroma of non-aggressive histological types | |
| 2B | Substantial LVSI of non-aggressive histological types | |
| 2C | Aggressive histological types with any myometrial involvement | |
| Stage 3 | Local and/or regional spread of the tumour of any histological subtype | |
| 3A | Invasion of uterine serosa, adnexa, or both by direct extension or metastasis 3A1 Spread to ovary or fallopian tube (except when meeting stage 1A3 criteria) 3A2 Involvement of uterine subserosa or spread through the uterine serosa | |
| 3B | Metastasis or direct spread to the vagina and/or to the parametria or pelvic peritoneum 3B1 Metastasis or direct spread to the vagina and/or the parametria 3B2 Metastasis to the pelvic peritoneum | |
| 3C | Metastasis to the pelvic or para-aortic lymph nodes or both 3C1 Metastasis to the pelvic lymph nodes (3C1 i—micrometastasis; 3C1 ii—macrometastasis) 3C2 Metastasis to para-aortic lymph nodes up to the renal vessels, with or without metastasis to the pelvic lymph nodes (3C2 i—micrometastasis; 3C2 ii—macrometastasis) | |
| Stage 4 | Spread to the bladder mucosa and/or intestinal mucosa and/or distance metastasis | |
| 4A | Invasion of the bladder mucosa and/or the intestinal/bowel mucosa | |
| 4B | Abdominal peritoneal metastasis beyond the pelvis | |
| 4C | Distant metastasis, including metastasis to any extra-or intra-abdominal lymph nodes above the renal vessels, lungs, liver, brain, or bone | |
| Stage | Molecular findings in patients with early endometrial cancer (Stages I and II after surgical staging) | |
| Stage 1AmPOLEm | POLEmut endometrial carcinoma, confined to the uterine corpus or with cervical extension, regardless of the degree of LVSI or histological type | |
| Stage 2Cmp53abn | p53abn endometrial carcinoma, confined to the uterine corpus with any myometrial invasion, with or without cervical invasion, and regardless of the degree of LVSI or histological type | |
| Disparity | Summary | Proposed Solutions |
|---|---|---|
| Access to care | Socioeconomic differences between racioethnic groups are commonly attributed to disparities in outcomes. However, studies in equal-access healthcare systems demonstrate black women still have higher EC mortality. | Redistribution of resources to ensure equitable healthcare access. |
| Patient-related diagnostic delays | The following patient factors are more prevalent in black women and are associated with delays in clinical presentation:
| Increased population EC awareness through culturally sensitive campaigns. |
| Care-provider-related diagnostic delays | There are minimal data on EC-specific care-provider-related diagnostic delays; studies on other cancers show black patients require more consultations with primary care providers before specialist referral.
| Reinforcement of mandates that standardise clinical pathways to limit the impact of individual biases. |
| Disparities in EC diagnostic pathway | EC diagnosis is largely reliant on TVS detection of endometrial abnormalities. In black women, TVS is less reliable due to the following:
| Review of thresholds for endometrial sampling and the development of diagnostic tests with improved performance. |
| Histological and molecular differences | Black women are more likely to be diagnosed with more aggressive EC due to the following:
| Histological and molecular differences are biological and nonmodifiable. More scientific research into non-endometrioid ECs may generate more effective treatment options. |
| Disparities in EC treatment | Several disparities in EC treatment in black women have been identified:
| Reinforcement of mandates that standardise clinical pathways to limit the impact of individual biases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illah, O.; Adeeko, D.; Olaitan, A.; Gentry-Maharaj, A. Racioethnic Disparities in Endometrial Cancer Outcomes. Diagnostics 2024, 14, 417. https://doi.org/10.3390/diagnostics14040417
Illah O, Adeeko D, Olaitan A, Gentry-Maharaj A. Racioethnic Disparities in Endometrial Cancer Outcomes. Diagnostics. 2024; 14(4):417. https://doi.org/10.3390/diagnostics14040417
Chicago/Turabian StyleIllah, Ojone, Deborah Adeeko, Adeola Olaitan, and Aleksandra Gentry-Maharaj. 2024. "Racioethnic Disparities in Endometrial Cancer Outcomes" Diagnostics 14, no. 4: 417. https://doi.org/10.3390/diagnostics14040417
APA StyleIllah, O., Adeeko, D., Olaitan, A., & Gentry-Maharaj, A. (2024). Racioethnic Disparities in Endometrial Cancer Outcomes. Diagnostics, 14(4), 417. https://doi.org/10.3390/diagnostics14040417






