Abstract
Background: High cerebrospinal fluid (CSF) sampling frequency is considered a risk factor for external ventricular drain (EVD)-associated infections. To reduce manipulation at the proximal port and potentially minimize the risk of an infection, we aimed to analyze whether CSF parameters sampled from the far distal collection bag could provide reliable results compared to the proximal port. Methods: We included patients who were treated with an EVD at our neurosurgical intensive care unit (ICU) between June 2021 and September 2022. CSF sampling, including microbiological analysis, was performed simultaneously from the proximal port and the collection bag. Spearman’s correlation coefficients were calculated to assess the correlation of CSF cell count, protein, lactate and glucose between the two sample sites. Results: We analyzed 290 pairs of CSF samples in 77 patients. Ventriculitis was identified in 4/77 (5%) patients. In 3/4 patients, microbiological analysis showed the same bacterial species at both sample sites at the same time. Spearman’s correlation coefficient showed that CSF cell count (r = 0.762), lactate (r = 0.836) and protein (r = 0.724) had a high positive correlation between the two collection sites, while CSF glucose (r = 0.663) showed a moderate positive correlation. Conclusion: This study shows that biochemical CSF parameters can be reliably assessed from the EVD collection bag.
1. Introduction
External ventricular drain (EVD) placement is considered one of the most important life-saving treatment strategies in neurosurgical patients, particularly when dealing with patients facing acute hydrocephalus. The external ventricular drain provides continuous monitoring of intracranial pressure (ICP) and effective ICP reduction by a constant cerebrospinal fluid (CSF) release.
Despite its high diagnostic and treatment potential, EVDs are also associated with a high rate of infections [1,2,3,4,5]. Various risk factors have been extensively described in the literature [5,6,7]. A high CSF sampling frequency has been considered one of the most common risk factors in promoting EVD-associated infections [5,6,7]. To reduce infection rates, it has been proposed that CSF sampling should not be routinely performed and should be restricted to cases in which infections are suspected based on clinical parameters. However, predicting an EVD-associated infection in neurocritically ill patients is challenging since, in this patient cohort, laboratory and clinical parameters are often altered due to systemic infections or intraventricular hemorrhage [8]. Although microbiological analysis is considered to be the gold standard in identifying EVD-associated infections, the results are usually only available with considerable delay. Modern multiplex PCR systems have been developed to alleviate this drawback; however, they suffer from a limited spectrum of identifiable microorganisms [9]. Given the need for timely intervention and the limitations of current diagnostic approaches, sequential CSF parameter analysis remains of utmost importance. This approach can significantly enhance treatment strategies and consequently reduce infection-associated morbidity and duration of hospitalization.
Previous studies have explored CSF sampling from a more distal site, at the EVD drip chamber, aiming to reduce manipulation at the proximal port and potentially decrease the risk of EVD-associated infections while preserving data integrity [10,11]. These studies demonstrated that CSF samples from the EVD drip chamber yielded results comparable to those from the proximal port [10,11]. Nevertheless, to our knowledge, no previous study has analyzed the reliability of CSF parameters acquired from an even more distal site at the EVD collection bag. Hence, our objective was to evaluate the correlation between CSF parameters obtained from the far distal collection bag and those from the proximal port.
2. Materials and Methods
We retrospectively analyzed consecutive patients who were treated with an EVD at our neurosurgical intensive care unit (ICU) between June 2021 and September 2022. We included only patients who were 18 years or older with at least two concurrent CSF samples from the proximal port and the collection bag obtained on the same day. Patients who received an EVD due to meningitis or ventriculitis were excluded from our study.
We assessed the following variables: patient’s demographics, indication for EVD insertion (subarachnoid hemorrhage (SAH), tumor, trauma, intracerebral hemorrhage (ICH) or other), CSF cell count, protein, glucose, lactate and microbiological analysis of CSF from both collection sites and type of EVD-associated infection according to our previously published definition [7].
2.1. External Ventricular Drainage System
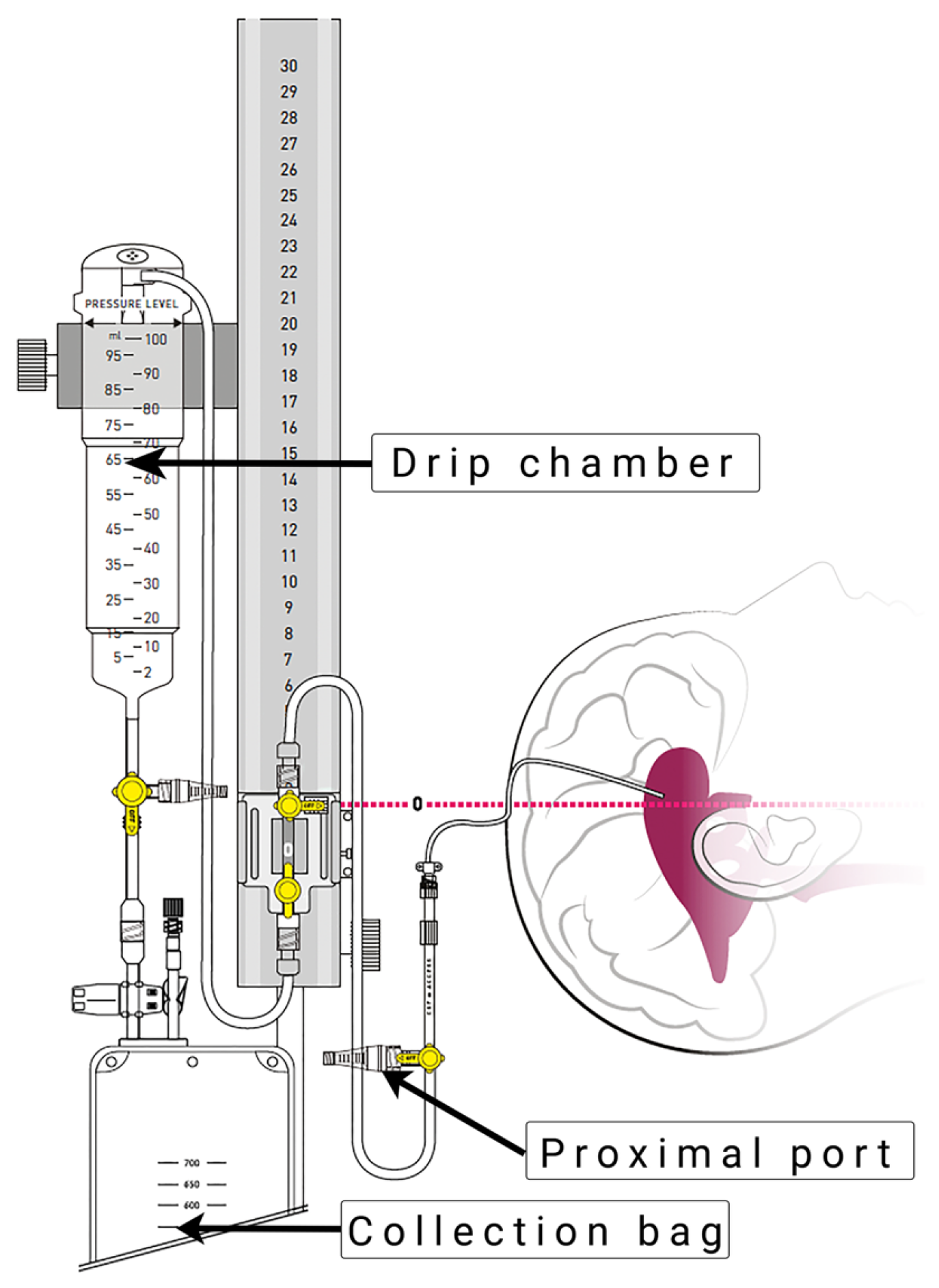
The EVD insertion technique and drainage system (Figure 1) were applied according to our routine hospital protocol, as previously described by Khalaveh et al. [7].
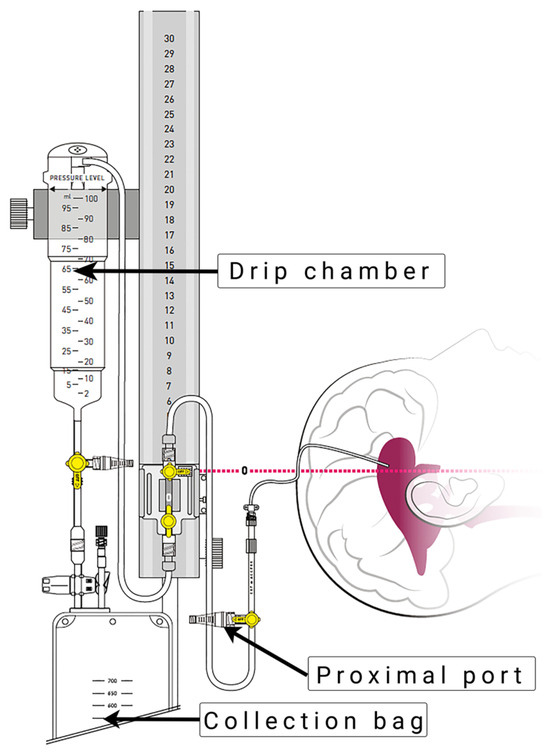
Figure 1.
External ventricular drain system (VentrEX® by Neuromedex®, Hamburg, Germany). Reprinted with permission from Neuromedex®. Copyright by Neuromedex®.
EVD insertion was performed by a neurosurgical consultant or resident in the operating room or in the ICU under sterile conditions. The procedures were performed under appropriate sedation and analgesia, with the patient in the supine position and the head of the bed elevated by 30–40 degrees. The area of insertions was shaved and prepared under sterile conditions. After identifying Kocher’s point, a 2 cm straight incision at the pupilar line was performed. A self-retaining retractor was placed, and a small burr hole was drilled. After coagulating and incising the dura, a regular non-antibiotic coated ventricular catheter (Straight Ventricular Catheter F8, Integra LifeSciences©, Princeton, NJ, USA) was passed toward the ipsilateral medial canthus and ipsilateral tragus into the frontal horn of the lateral ventricle. Subsequently, the catheter was tunneled under the galea and secured by multiple sutures.
2.2. CSF Sampling Protocol
According to our clinical protocol, CSF sampling was performed by the neurosurgeon assigned to the ICU from the 3-way stopcock at the proximal port twice a week. Additionally, since the EVD collection bag has to be changed every day, daily CSF samplings from the removed collection bag were performed as well. From each site, we withdrew 2 mL CSF to assess cell count, protein, glucose and lactate, and 4 mL for the microbiological analysis.
2.3. Definition of Infections
We used the definition of EVD-associated infections according to our previously published study (Table 1) [7]. According to our definition of EVD-associated infections, which is based on previously published studies and the guidelines of the Infectious Diseases Society of America (ISDA), patients were retrospectively classified into one of the five categories, depending on the microbiological results of testing CSF samples, which were drawn at the proximal port [4,8,9]. We further arranged our patients into the groups (1) “infection” or (2) “no-infection” (Table 1).

Table 1.
Classification of EVD-associated infection.
2.4. Statistical Analysis
Statistical analysis was performed using SPSS software version 28.0 (IBM, Armonk, NY, USA). Categorical data were presented as counts and percentages, and continuous parameters as median and range. To assess the correlation of CSF cell count, protein, lactate and glucose between two CSF samples, Spearman’s correlation coefficients were calculated. Interpretation of Spearman’s correlation coefficients was performed according to established ranges as previously described by Mukaka et al. [12]. A p-value < 0.05 was considered statistically significant for all performed tests.
3. Results
We analyzed 290 pairs of CSF samples in 77 patients treated with an EVD at a median age of 58 years (range: 18–80 years). There were 44 (57%) female patients. The reason for EVD placement was hydrocephalus due to SAH in 60 (78%) patients, intracerebral tumor in 8 (10%) patients and other diseases in 9 (12%) patients (including spontaneous intracerebral hemorrhage, ischemic stroke, trauma or autoimmune encephalitis).
3.1. CSF Sample Comparison between the Proximal Port and the Collection Bag
The median CSF cell count, glucose and lactate from the proximal port was 57 cells/µL (range: 0–17,720 cells/µL), 71 mg/dL (range: 11–113 mg/dL) and 2.8 mmol/L (range: 1.2–18.6 mmol/L), respectively. Quantitative protein values were available from 107 samples and showed a median value of 32 mg/dL (range: 0–1856 mg/dL).
The median CSF cell count, glucose, lactate and protein from the collection bag was 26 cells/µL (range: 0–2830 cells/µL), 69 mg/dL (range: 2–114 mg/dL), 2.9 mmol/L (range: 0.6–11.8 mmol/L) and 55.3 mg/dL (range: 4.3–797 mg/dL), respectively.
CSF cell count and glucose, acquired from the proximal site, showed median 1.84 (range: 0–910) and 1.01 (range: 0.39–6.2) times higher values compared to the distal site. In contrast, CSF lactate and protein showed median 0.93 (0.42–5.8) and 0.99 (range: 0–25.32) times lower values.
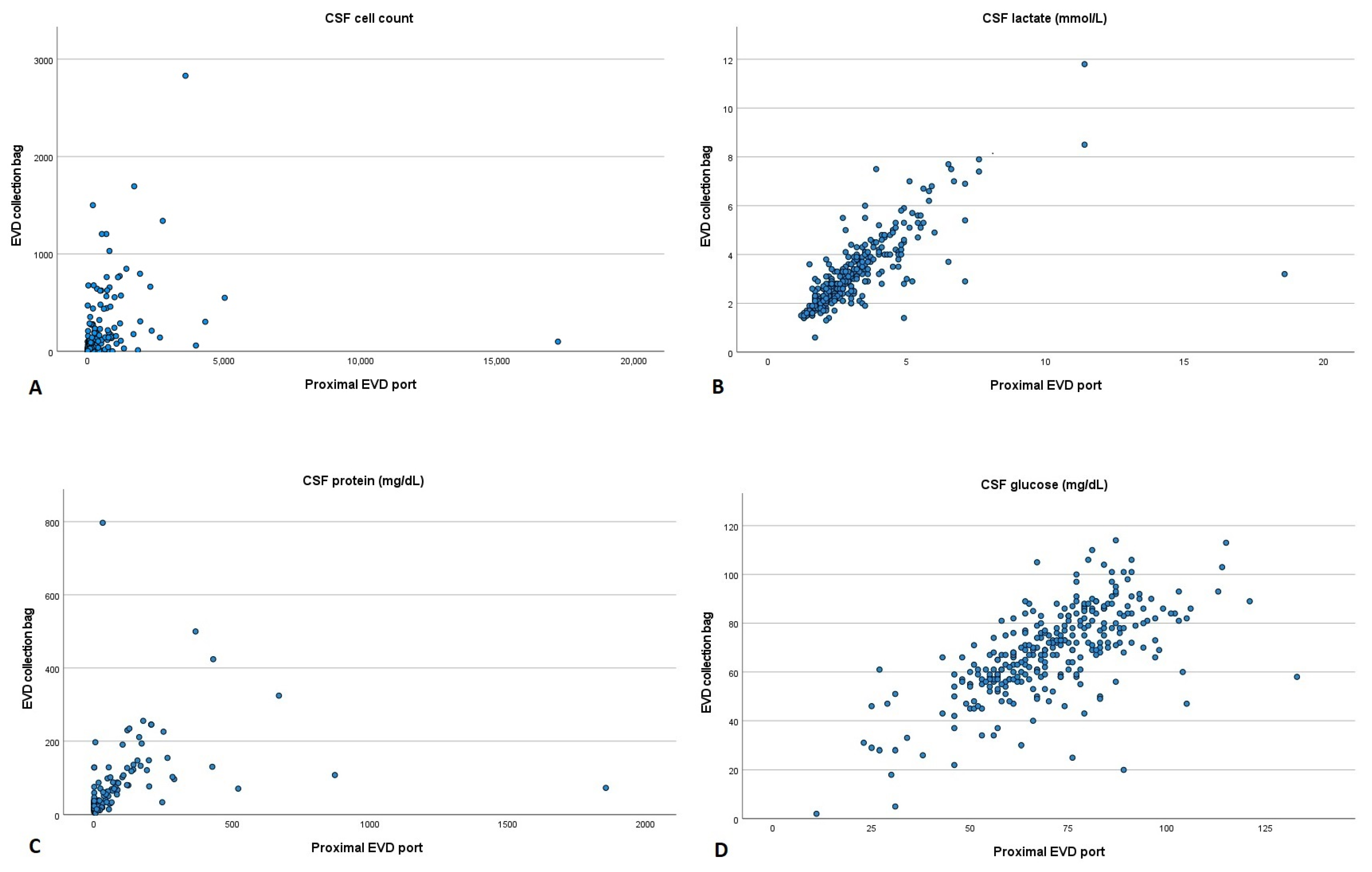
After calculating Spearman’s correlation coefficient, we could observe that CSF cell count (r = 0.762, p < 0.001), lactate (r = 0.836, p < 0.001) and protein (r = 0.724, p < 0.001) had a high positive correlation between the two collection sites. In contrast, CSF glucose (r = 0.663, p < 0.001) showed a moderate positive correlation (Figure 2).
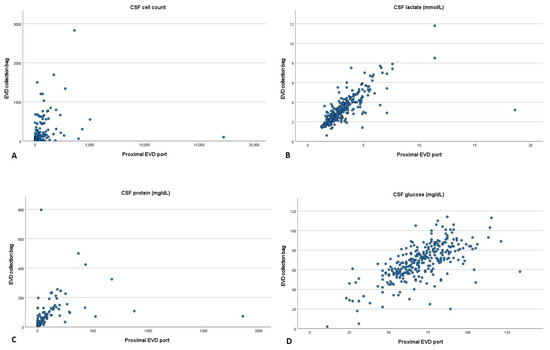
Figure 2.
Scatter plot comparing cerebrospinal fluid parameters acquired from the proximal port and collection bag of an external ventricular drain. (A) Cell count, (B) lactate, (C) protein and (D) glucose. CSF: cerebrospinal fluid; EVD: external ventricular drain.
3.2. Comparison of Microbiological Results of CSF between the Two Collection Sites
To analyze whether microbiological analysis could be reliably assessed from the collection bag as well, we further compared the microbiological results from the two collection sites. In regard to infection status, which was based on the proximal results of the microbiological analysis, 73/77 (95%) patients had no infections, with 9/77 (12%) classified as contamination and 4/77 (5%) patients as ventriculitis.
The results of the positive microbiological analysis from the proximal EVD port showed 19 bacterial species in 17 samples, with Staphylococcus epidermidis (7/19; 37%) being the most frequent species. Staphylococcus spp. was identified in all nine contamination cases. In the ventriculitis group, Candida albicans and Escherichia coli 4 MRGN were identified separately in three consecutive microbiological analyses in one patient, respectively. In the other two cases, the microbiological analysis revealed Serratia marcescens and Klebsiella pneumoniae.
In one patient with ventriculitis (Candida albicans and Staphylococcus epidermidis) and in one with contamination (Staphylococcus hominis and Staphylococcus epidermidis), two species were identified in the same microbiological analysis.
The microbiological analysis of the CSF from the collection bag showed Staphylococcus epidermidis (10/16; 63%) as the most frequent species. In three out of four cases of ventriculitis, microbiological analysis identified the same species as from the proximal EVD port on the same day. Moreover, in one case, Serratia marcescens was identified 2 days prior to the positive result of the proximal sample. We have summarized all microbiological results from the proximal EVD port and the collection bag and compared them to each other in Table 2.

Table 2.
Comparison of the microbiological results of CSF between the proximal EVD port and the collection bag.
4. Discussion
This study aimed to investigate whether CSF parameters acquired from the EVD collection bag would show comparable results to CSF parameters collected from the proximal port of the EVD system. We showed that CSF cell count, lactate and protein levels had a high positive correlation between the two sample sites. Therefore, these parameters could be reliably assessed from CSF drawn at the more distal EVD collection bag to potentially decrease EVD-associated infections by reducing manipulation at the proximal site of the EVD system [5,6,7].
There are various guidelines available on how to effectively utilize CSF parameters in the diagnosis of specific diseases affecting the central nervous system, including conditions like bacterial infections [13]. These recommendations may not be universally applicable, especially when dealing with a specific subset of patients—the neurocritically ill individuals who require treatment through an EVD. This becomes particularly pertinent in cases following intraventricular hemorrhage (IVH) or subarachnoid hemorrhage (SAH). In these patients, CSF parameters may be altered, which reduces their diagnostic reliability. Therefore, the Infectious Diseases Society of America (IDSA) has stressed that the primary basis for diagnosing EVD-associated infections should rest on the outcomes derived from CSF cultures [9]. This approach has been integrated into our department’s protocols. In addition to implementing these guidelines, we intended to analyze whether microbiological analyses from the CSF collection bag could yield results of comparable diagnostic value to those obtained from the proximal port of the EVD. Our data revealed that in three out of four (75%) ventriculitis cases, the microbiological findings from both collection sites (distal and proximal) concurred, and, moreover, in one case, the microbiological analysis from the distal site showed a positive result two days prior to the proximal sample. Furthermore, the rate of contamination species at the distal site was comparable to that at the proximal site, with 10 cases compared to 9, which minimizes the concerns about an elevated risk of contamination at the distal sample site. We believe that assessing CSF cultures from the collection bag could have the potential to be as reliable as the proximal assessment while concurrently mitigating the risk of inadvertently introducing bacterial species into the ventricles through the proximal EVD port. Thus, assessment from the collection bag is not limited by sample frequency as it would be from the proximal port. However, it is imperative to acknowledge that irrespective of the sampling site, the microbiological results may take several days to reach their conclusive stage, and these results may be influenced by any ongoing empirical antibiotic therapy. To improve the diagnosis of bacterial infections, contemporary techniques, such as polymerase chain reaction (PCR) or metagenomic next-generation sequencing, have been introduced into the clinical setting [14,15,16]. Yet, these techniques are either limited in their spectrum of detectable pathogens or are expensive and are, therefore, not routinely used in daily clinical practice.
Since a one-time CSF parameter analysis in neurosurgical patients is not specific enough to detect EVD-associated infections, clinicians at the neurosurgical ICU need to rely on the sequential analysis of CSF parameters to prevent a delay in antibiotic treatment and to guide treatment decisions [17,18]. Our study showed that CSF parameter analysis from the collection bag would generate comparable results to those from the proximal site with the potential to prevent a loss of information and guide treatment decisions. Similar results have been found in previous studies comparing CSF parameters from the EVD drip chamber to the proximal collection site [10,11]. While Wong et al. showed that CSF protein and glucose correlated between the two collection sites, Kinast et al. could demonstrate that CSF lactate, protein and glucose could be reliably determined from the distal site [10,11].
In contrast to these studies, our study showed only a moderate positive correlation of glucose values between the two collection sites. According to Deisenhammer et al., CSF glucose degrades during storage, which could explain our results [13]. Another explanation could be a dilution effect of constantly altered glucose values since sampling from the collection bag has been performed after CSF accumulation over 24 h. However, there are no data in regard to the appropriate method of CSF storage, with recommendations being inconsistent compared to published data [19]. While it has been emphasized by the Guidelines of the European Federation of Neurological Societies (EFNS) that glucose sample processing should be performed immediately after acquisition, others have shown that glucose concentrations remain stable over 2.5 h at room temperature or even over 24 h at 4 °C [13,20,21]. Nevertheless, studies that analyzed CSF glucose acquired from the EVD drip chamber have not specified the exact duration of CSF storage [10,11].
Compared to glucose, the stability of CSF lactate and protein concentrations seems to be less affected by storage time. While the data on the stability of these CSF parameters are scarce, it has been shown in previous studies that a time delay of 2.5 h in sample processing did not influence the analytical results [21]. Moreover, it has been shown that both remain stable for up to 3 days at room temperature [20,22,23]. Our results confirm previously published data and show that CSF lactate and protein samples can be reliably assessed from the EVD collection bag.
In regard to the CSF cell count, it appears that the in vitro biochemical process has been analyzed in more detail. Historical studies have shown that due to the hypotonic state of CSF, leukocytes lyse if they are exposed for an extended time period [24]. This cell degradation process has been confirmed by recent studies, in which it has been shown that even a short delay in CSF analysis leads to a statistically significant decrease in CSF cell count [21,25]. Since CSF collection at the drip chamber or collection bag occurs over many hours, one would assume that sampling CSF from these distal sites would result in lower cell count values compared to samples acquired from the proximal site. This has been observed by Kinast et al. and was confirmed by the results of the present study [10]. According to our results, the CSF cell count from the proximal site was median 1.84 times higher than the values from the distal site. This apparent cell count degradation process has not been previously analyzed in neurosurgical patients after SAH or IVH. Nevertheless, our study showed a high positive correlation of CSF cell count between the two sample sites, with similar results being described by Wong et al. [11]. Moreover, in studies with non-neurosurgical patients, it has been shown that a 5 h time delay of sample processing had no significant influence on the cell count analysis if a serum-containing medium was added to the CSF [25]. Thus, it could be hypothesized that in patients with SAH or IVH, the degradation process occurs only to a certain degree since, theoretically, these patients have a higher CSF osmolarity.
In summary, our data show that CSF parameters can be reliably assessed from the EVD collection bag. CSF sampling by this approach has the potential to reduce manipulation of the proximal site of the EVD system and, thus, theoretically reduce EVD-associated infections to a certain degree. However, since this is the first study analyzing the reliability of this CSF sampling approach, more studies are needed to validate our results.
5. Strength and Limitations
To our knowledge, this is the first study analyzing the analytic value of CSF parameters from the EVD collection bag. Moreover, the continuously applied standard CSF sampling protocol with a high amount of CSF sample pairs is the major strength of our study.
While we could show a moderate to high positive correlation of CSF values between the two collection sites, it must be pointed out that laboratory reference values are validated for CSF specimens acquired from the proximal site. Thus, future studies are needed to assess appropriate reference values for CSF parameters acquired from far distal collection sites.
Although we included neurosurgical patients only, our cohort still comprised a heterogeneous group of cases with a history of SAH in 78% of our patients. Moreover, due to our low infection rate (4/77 patients, 5%), we did not perform a comparison analysis of the microbiological results between the two collection sites. Thus, the comparison of the microbiological results should be interpreted with caution since this part of the study was purely descriptive.
6. Conclusions
Clinicians still depend on the sequential analysis of CSF parameters to detect EVD-associated infections due to (1) the lack of specific parameters and (2) the delayed availability of microbiological results. Moreover, microbiological analysis may be influenced by empiric antibiotic treatment. With the aim of reducing manipulation at the proximal port of the EVD system, we analyzed whether CSF parameters could be reliably detected from the far distal collection bag. Our results showed a high correlation of CSF cell count, lactate and protein between the two collection sites. Therefore, we are convinced that an increased CSF sampling frequency from the far distal collection bag is possible, with the potential to prevent a loss of information and minimize the risk of EVD-associated infections. This approach could guide the treating clinician to improve treatment strategies.
However, in cases of abnormal CSF values, CSF parameter analysis should always be performed from the proximal site to verify the analytical results. More importantly, future studies should focus on the development of newer techniques to expedite the determination of EVD-associated infections.
Author Contributions
Conceptualization, F.K. and J.H.; methodology, F.K. and J.H.; acquisition of data, F.K., M.M. and V.Z.; formal analysis, F.K., M.M. and J.H.; writing—original draft preparation, F.K. and J.H.; writing—review and editing, F.K., M.M., V.Z., M.G.V., A.R., C.D., K.R. and J.H.; analysis and interpretation of data, M.G.V., A.R., C.D. and K.R.; visualization, F.K.; supervision, J.H.; project administration, J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the local ethics committee of the Medical University of Vienna (EK 2236/2021, 11 May 2023).
Informed Consent Statement
As our study was retrospective and utilized anonymized data from our database, formal consent was not required.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Hagel, S.; Bruns, T.; Pletz, M.W.; Engel, C.; Kalff, R.; Ewald, C. External Ventricular Drain Infections: Risk Factors and Outcome. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 708531. [Google Scholar] [CrossRef] [PubMed]
- Lozier, A.P.; Sciacca, R.R.; Romagnoli, M.F.; Connolly, E.S., Jr. Ventriculostomy-related Infections: A Critical Review of the Literature. Neurosurgery 2002, 51, 170–182; discussion 181–182. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, M.; Lipman, J.; Shorr, A.; Shankar, A. A meta-analysis of ventriculostomy-associated cerebrospinal fluid infections. BMC Infect. Dis. 2015, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Hoefnagel, D.; Dammers, R.; Ter Laak-Poort, M.P.; Avezaat, C.J.J. Risk factors for infections related to external ventricular drainage. Acta Neurochir. 2008, 150, 209–214; discussion 214. [Google Scholar] [CrossRef] [PubMed]
- Camacho, E.F.; Boszczowski, I.; Basso, M.; Jeng, B.C.P.; Freire, M.P.; Guimarães, T.; Teixeira, M.J.; Costa, S.F. Infection rate and risk factors associated with infections related to external ventricular drain. Infection 2011, 39, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Park, P.; Garton, H.J.; Kocan, M.J.; Thompson, B.G. Risk of Infection with Prolonged Ventricular Catheterization. Neurosurgery 2004, 55, 594–601; discussion 599–601. [Google Scholar] [CrossRef] [PubMed]
- Khalaveh, F.; Fazel, N.; Mischkulnig, M.; Vossen, M.G.; Reinprecht, A.; Dorfer, C.; Roessler, K.; Herta, J. Risk Factors Promoting External Ventricular Drain Infections in Adult Neurosurgical Patients at the Intensive Care Unit—A Retrospective Study. Front. Neurol. 2021, 12, 734156. [Google Scholar] [CrossRef]
- Schade, R.P.; Schinkel, J.; Roelandse, F.W.C.; Geskus, R.B.; Visser, L.G.; van Dijk, M.C.; Voormolen, J.H.C.; van Pelt, H.; Kuijper, E.J. Lack of value of routine analysis of cerebrospinal fluid for prediction and diagnosis of external drainage–related bacterial meningitis. J. Neurosurg. 2006, 104, 101–108. [Google Scholar] [CrossRef]
- Tunkel, A.R.; Hasbun, R.; Bhimraj, A.; Byers, K.; Kaplan, S.L.; Scheld, W.M.; van de Beek, D.; Bleck, T.P.; Garton, H.J.; Zunt, J.R. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin. Infect. Dis. 2017, 64, e34–e65. [Google Scholar] [CrossRef]
- Kinast, C.B.; Paal, M.; Liebchen, U. Comparison of Cerebrospinal Fluid Collection Through the Proximal and Distal Port Below the Overflow System from an External Ventricular Drain. Neurocrit. Care 2022, 37, 775–778. [Google Scholar] [CrossRef]
- Wong, F.W.H. Cerebrospinal fluid collection: A comparison of different collection sites on the external ventricular drain. Dynamics 2011, 22, 19–24. [Google Scholar]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Deisenhammer, F.; Bartos, A.; Egg, R.; Gilhus, N.E.; Giovannoni, G.; Rauer, S.; Sellebjerg, F.; Force, E.T. Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force. Eur. J. Neurol. 2006, 13, 913–922. [Google Scholar] [CrossRef]
- Qian, L.; Shi, Y.; Li, F.; Wang, Y.; Ma, M.; Zhang, Y.; Shao, Y.W.; Zheng, G.; Zhang, G. Metagenomic Next-Generation Sequencing of Cerebrospinal Fluid for the Diagnosis of External Ventricular and Lumbar Drainage-Associated Ventriculitis and Meningitis. Front. Microbiol. 2020, 11, 596175. [Google Scholar] [CrossRef]
- Banks, J.T.; Bharara, S.; Tubbs, R.S.; Wolff, C.L.; Gillespie, G.Y.; Markert, J.M.; Blount, J.P. Polymerase Chain Reaction for the Rapid Detection of Cerebrospinal Fluid Shunt or Ventriculostomy Infections. Neurosurgery 2005, 57, 1237–1243; discussion 1237–1243. [Google Scholar] [CrossRef]
- Deutch, S.; Dahlberg, D.; Hedegaard, J.; Schmidt, M.B.; Møller, J.K.; Ostergaard, L. Diagnosis of ventricular drainage-related bacterial meningitis by broad-range real-time polymerase chain reaction. Neurosurgery 2007, 61, 306–312; discussion 311–312. [Google Scholar] [CrossRef]
- Hariri, O.; Farr, S.; Lawandy, S.; Zampella, B.; Miulli, D.; Siddiqi, J. Will clinical parameters reliably predict external ventricular drain-associated ventriculitis: Is frequent routine cerebrospinal fluid surveillance necessary? Surg. Neurol. Int. 2017, 8, 137. [Google Scholar] [CrossRef]
- Wiegand, J.; Hickson, L.; Merz, T.M. Indicators of external ventricular drainage-related infections—A retrospective observational study. Acta Neurochir. 2016, 158, 595–601; discussion 601. [Google Scholar] [CrossRef]
- Teunissen, C.E.; Petzold, A.; Bennett, J.L.; Berven, F.S.; Brundin, L.; Comabella, M.; Franciotta, D.; Frederiksen, J.L.; Fleming, J.O.; Furlan, R.; et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 2009, 73, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Dujmovic, I.; Deisenhammer, F. Stability of cerebrospinal fluid/serum glucose ratio and cerebrospinal fluid lactate concentrations over 24 h: Analysis of repeated measurements. Clin. Chem. Lab. Med. 2009, 48, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Mlinarić, A.; Vogrinc, Z.; Drenšek, Z. Effect of sample processing and time delay on cell count and chemistry tests in cerebrospinal fluid collected from drainage systems. Biochem. Medica 2018, 28, 030705. [Google Scholar] [CrossRef]
- Zhang, L.; Gabler, J.; Payto, D.; Wang, S. Stability of lactate and pyruvate in cerebrospinal fluid under typical clinical laboratory storage conditions. Clin. Biochem. 2015, 48, 631–632. [Google Scholar] [CrossRef]
- Janelidze, S.; Stomrud, E.; Brix, B.; Hansson, O. Towards a unified protocol for handling of CSF before β-amyloid measurements. Alzheimer’s Res. Ther. 2019, 11, 63. [Google Scholar] [CrossRef]
- Steele, R.W.; Marmer, D.J.; O’Brien, M.D.; Tyson, S.T.; Steele, C.R. Leukocyte survival in cerebrospinal fluid. J. Clin. Microbiol. 1986, 23, 965–966. [Google Scholar] [CrossRef]
- De Graaf, M.T.; van den Broek, P.D.; Kraan, J.; Luitwieler, R.L.; van den Bent, M.J.; Boonstra, J.G.; Schmitz, P.I.M.; Gratama, J.W.; Sillevis Smitt, P.A. Addition of serum-containing medium to cerebrospinal fluid prevents cellular loss over time. J. Neurol. 2011, 258, 1507–1512. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).