Landscape of Innovative Methods for Early Diagnosis of Gastric Cancer: A Systematic Review
Abstract
:1. Introduction
Classification
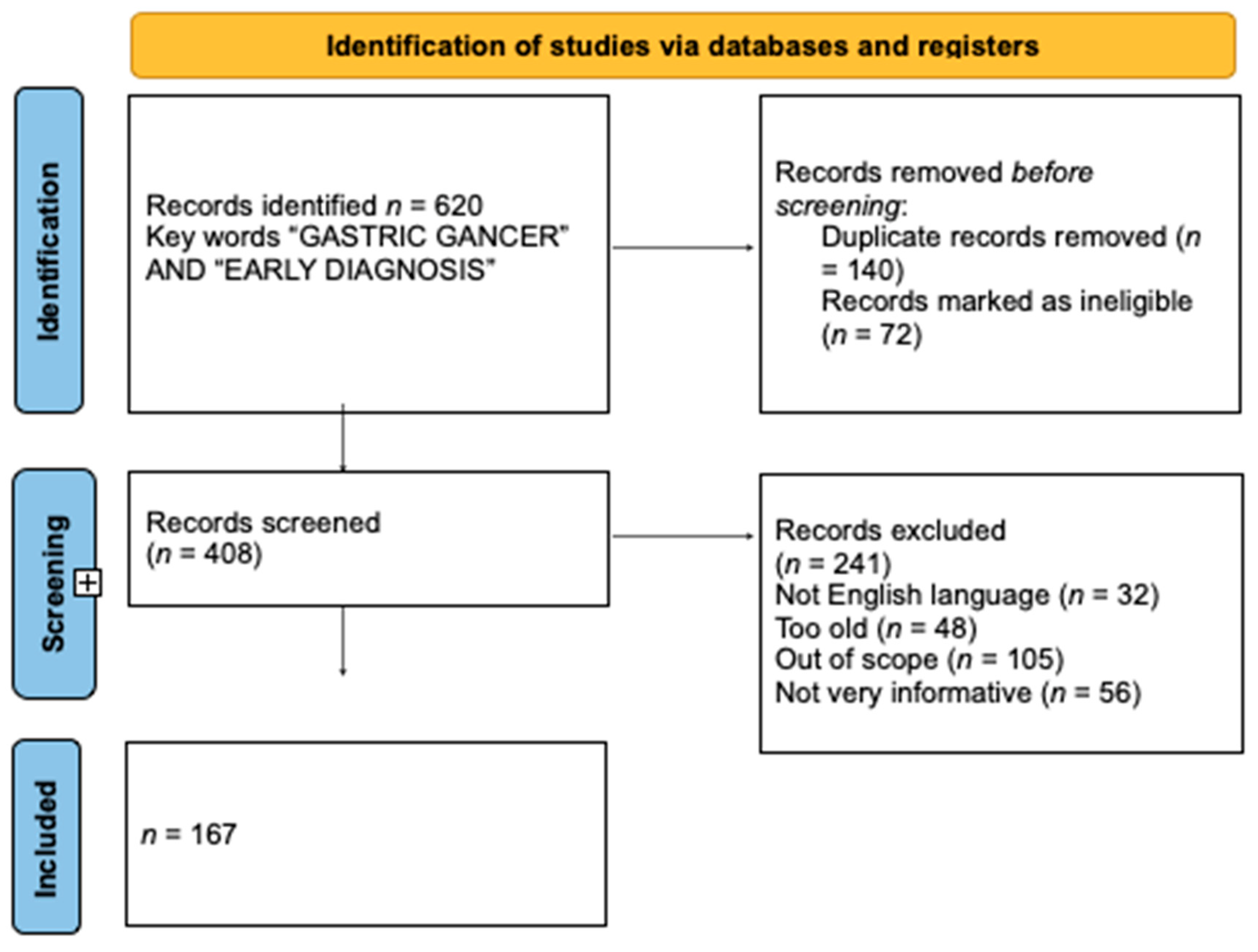
2. Material and Methods
2.1. Search Strategy
2.2. Eligibility Assessment and Data Extraction
2.3. Outcomes
2.4. Results
3. Classical Diagnosis Methods
3.1. Radiographic Diagnosis
3.2. Computed Tomography Imaging
3.3. Endoscopic Diagnosis
3.3.1. White Light Endoscopy
3.3.2. Chromoendoscopy
3.3.3. Narrowband Imaging
3.4. Classic Tumor Markers
4. Genetic Biomarkers
4.1. Circulating Tumor Cells
4.2. Circulating Tumor-DNA/Cell-Free DNA
Cell-Free DNA in Comparison to Traditional Biomarkers
4.3. DNA Methylation
4.4. MicroRNAs
4.5. Exosomes
4.6. Long Noncoding RNAs
4.7. CDH1 (E-Cadherin)
5. Metabolic Biomarkers of GC
5.1. Serum Biomarkers
Differential Diagnosis between CG vs. GC Using Metabolomics
5.2. Urinary Biomarkers
5.3. Fecal Biomarkers
6. Future Perspectives
6.1. Linked Color Imaging
6.1.1. LCI Used for Diagnosis of HP+ Gastritis and Premalignant Lesions
6.1.2. LCI Used for Detection of EGC Lesions
6.2. Machine Learning Models
7. Discussion
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 42–54. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Agency for Research on Cancer (IARC)—GLOBOCAN 2020: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2018. 2018. Available online: https://gco.iarc.fr/ (accessed on 23 January 2019).
- American Cancer Society. Cancer Facts and Figures 2019; American Cancer Society: Atlanta, GA, USA, 2019. [Google Scholar]
- SEER*Explorer: An Interactive Website for SEER Cancer Statistics. Surveillance Research Program, National Cancer Institute. Available online: https://seer.cancer.gov/explorer/ (accessed on 30 December 2019).
- Hamashima, C.; Shibuya, D.; Yamazaki, H.; Inoue, K.; Fukao, A.; Saito, H.; Sobue, T. The Japanese Guidelines for Gastric Cancer Screening. Jpn. J. Clin. Oncol. 2008, 38, 259–267. [Google Scholar] [CrossRef]
- Maruyama, K.; Kaminishi, M.; Hayashi, K.-I.; Isobe, Y.; Honda, I.; Katai, H.; Arai, K.; Kodera, Y.; Nashimoto, A. Gastric cancer treated in 1991 in Japan: Data analysis of nationwide registry. Gastric Cancer 2006, 9, 51–66. [Google Scholar] [CrossRef]
- Ono, H.; Kondo, H.; Gotoda, T.; Shirao, K.; Yamaguchi, H.; Saito, D.; Hosokawa, K.; Shimoda, T.; Yoshida, S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 2001, 48, 225–229. [Google Scholar] [CrossRef]
- Oliveira, F.J.; Ferrão, H.; Furtado, E.; Batista, H.; Conceição, L. Early gastric cancer: Report of 58 cases. Gastric Cancer 1998, 1, 51–56. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma—2nd English edition. Gastric Cancer 1998, 1, 10–24. [Google Scholar] [CrossRef]
- Katai, H.; Sano, T. Early gastric cancer: Concepts, diagnosis, and management. Int. J. Clin. Oncol. 2005, 10, 375–383. [Google Scholar] [CrossRef]
- The Paris endoscopic classification of superficial neoplastic lesions: Esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest. Endosc. 2003, 58 (Suppl. S6), S3–S43. [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Chan, A.W.; Mercier, P.; Schiller, D.; Bailey, R.; Robbins, S.; Eurich, D.T.; Sawyer, M.B.; Broadhurst, D. 1H-NMR urinary metabolomic profiling for diagnosis of gastric cancer. Br. J. Cancer 2016, 114, 59–62. [Google Scholar] [CrossRef]
- Kubota, K.; Kuroda, J.; Yoshida, M.; Ohta, K.; Kitajima, M. Medical image analysis: Computer-aided diagnosis of gastric cancer invasion on endoscopic images. Surg. Endosc. 2012, 26, 1485–1489. [Google Scholar] [CrossRef]
- Song, X.; Zhong, H.; Wu, Q.; Wang, M.; Zhou, J.; Zhou, Y.; Lu, X.; Ying, B. Association between SNPs in microRNA machinery genes and gastric cancer susceptibility, invasion, and metastasis in Chinese Han population. Oncotarget 2017, 8, 86435–86446. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Kim, G.H.; Jeon, H.K.; Kim, D.H.; Jeon, T.Y.; Park, D.Y.; Jeong, H.; Chun, W.J.; Kim, M.-H.; Park, J.; et al. Circulating tumor cells detected by lab-on-a-disc: Role in early diagnosis of gastric cancer. PLoS ONE 2017, 12, e0180251. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Q.-C.; Xu, M.-D.; Zhang, Z.; Cheng, J.; Zhong, Y.-S.; Zhang, Y.-Q.; Chen, W.-F.; Yao, L.-Q.; Zhou, P.-H.; et al. Application of convolutional neural network in the diagnosis of the invasion depth of gastric cancer based on conventional endoscopy. Gastrointest. Endosc. 2019, 89, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Ning, L.; Qiu, F.; Yu, Q.; Cao, B. Clinical significance of serum miR-25 as a diagnostic and prognostic biomarker in human gastric cancer. Cancer Biomark. 2019, 24, 477–483. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Q.; An, X.; Pan, J.; Zhao, L.; Shen, L.; Xu, Y.; Yuan, D. The Ratio of ssDNA to dsDNA in Circulating Cell-Free DNA Extract is a Stable Indicator for Diagnosis of Gastric Cancer. Pathol. Oncol. Res. 2020, 26, 2621–2632. [Google Scholar] [CrossRef]
- Qian, C.; Cai, R.; Zhang, W.; Wang, J.; Hu, X.; Zhang, Y.; Jiang, B.; Yuan, H.; Liu, F. Neutrophil-Lymphocyte Ratio and Circulating Tumor Cells Counts Predict Prognosis in Gastrointestinal Cancer Patients. Front. Oncol. 2021, 11, 710704. [Google Scholar] [CrossRef] [PubMed]
- Tuck, M.K.; Chan, D.W.; Chia, D.; Godwin, A.K.; Grizzle, W.E.; Krueger, K.E.; Rom, W.; Sanda, M.; Sorbara, L.; Stass, S.; et al. Standard Operating Procedures for Serum and Plasma Collection: Early Detection Research Network Consensus Statement Standard Operating Procedure Integration Working Group. J. Proteome Res. 2009, 8, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Dooley, C.P.; Larson, A.W.; Stace, N.H.; Renner, I.G.; Valenzuela, J.E.; Eliasoph, J.; Colletti, P.M.; Halls, J.M.; Weiner, J.M. Double-Contrast Barium Meal and Upper Gastrointestinal Endoscopy. A comparative study. Ann. Intern. Med. 1984, 101, 538–545. [Google Scholar] [CrossRef] [PubMed]
- E Longo, W.; A Zucker, K.; Zdon, M.J.; Modlin, I.M. Detection of early gastric cancer in an aggressive endoscopy unit. Am. Surg. 1989, 55, 100–104. [Google Scholar]
- Choi, K.S.; Jun, J.K.; Park, E.-C.; Park, S.; Jung, K.W.; Han, M.A.; Choi, I.J.; Lee, H.-Y. Performance of Different Gastric Cancer Screening Methods in Korea: A Population-Based Study. PLoS ONE 2012, 7, e50041. [Google Scholar] [CrossRef] [PubMed]
- Hamashima, C. Update version of the Japanese Guidelines for Gastric Cancer Screening. Jpn. J. Clin. Oncol. 2018, 48, 673–683. [Google Scholar] [CrossRef]
- Lee, K.; Inoue, M.; Otani, T.; Iwasaki, M.; Sasazuki, S.; Tsugane, S. Gastric cancer screening and subsequent risk of gastric cancer: A large-scale population-based cohort study, with a 13-year follow-up in Japan. Int. J. Cancer 2006, 118, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, A.; Kuriyama, S.; Nishino, Y.; Tsubono, Y.; Nakaya, N.; Ohmori, K.; Kurashima, K.; Shibuya, D.; Tsuji, I. Lower risk of death from gastric cancer among participants of gastric cancer screening in Japan: A population-based cohort study. Prev. Med. 2007, 44, 12–19. [Google Scholar] [CrossRef]
- Kwee, R.M.; Kwee, T.C. Imaging in Local Staging of Gastric Cancer: A Systematic Review. J. Clin. Oncol. 2007, 25, 2107–2116. [Google Scholar] [CrossRef]
- Graham, D.Y.; Schwartz, J.T.; Cain, G.; Gyorkey, F. Prospective evaluation of biopsy number in the diagnosis of esophageal and gastric carcinoma. Gastroenterology 1982, 82, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, K.; Sanft, C.; Zimmer, T.; Zeitz, M.; Felsenberg, D.; Stein, H.; Germer, C.; Deutschmann, C.; O’Riecken, E. Comparison of computed tomography, endosonography, and intraoperative assessment in TN staging of gastric carcinoma. Gut 1993, 34, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Redel, C.A.; Zweiner, R.J. Stomach. In Sleisenger & Fordtran’s Gastrointestinal and Liver Disease, 7th ed.; Feldman, M., Friedman, L.S., Sleisenger, M.H., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2002; pp. 557–560. [Google Scholar]
- Sano, T.; Okuyama, Y.; Kobori, O.; Shimizu, T.; Morioka, Y. Early gastric cancer: Endoscopic diagnosis of depth of invasion. Dig. Dis. Sci. 1990, 35, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, S.G.; Im, J.P.; Kim, J.S.; Jung, H.C.; Song, I.S. Endoscopic prediction of tumor invasion depth in early gastric cancer. Gastrointest. Endosc. 2011, 73, 917–927. [Google Scholar] [CrossRef]
- Tajiri, H.; Ohtsu, A.; Boku, N.; Muto, M.; Chin, K.; Matsumoto, S.; Yoshida, S. Routine endoscopy using electronic endoscopes for gastric cancer diagnosis: Retrospective study of inconsistencies between endoscopic and biopsy diagnoses. Cancer Detect. Prev. 2001, 25, 166–173. [Google Scholar]
- Kawahara, Y.; Takenaka, R.; Okada, H.; Kawano, S.; Inoue, M.; Tsuzuki, T.; Tanioka, D.; Hori, K.; Yamamoto, K. Novel chromoendoscopic method using an acetic acid–indigocarmine mixture for diagnostic accuracy in delineating the margin of early gastric cancers. Dig. Endosc. 2009, 21, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Ribeiro, M. Chromoendoscopy for early diagnosis of gastric cancer. Eur. J. Gastroenterol. Hepatol. 2006, 18, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Anagnostopoulos, G.; Ragunath, K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy 2009, 41, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Ezoe, Y.; Muto, M.; Uedo, N.; Doyama, H.; Yao, K.; Oda, I.; Kaneko, K.; Kawahara, Y.; Yokoi, C.; Sugiura, Y.; et al. Magnifying Narrowband Imaging Is More Accurate Than Conventional White-Light Imaging in Diagnosis of Gastric Mucosal Cancer. Gastroenterology 2011, 141, 2017–2025.e3. [Google Scholar] [CrossRef]
- Nagahama, T.; Yao, K.; Maki, S.; Yasaka, M.; Takaki, Y.; Matsui, T.; Tanabe, H.; Iwashita, A.; Ota, A. Usefulness of magnifying endoscopy with narrow-band imaging for determining the horizontal extent of early gastric cancer when there is an unclear margin by chromoendoscopy (with video). Gastrointest. Endosc. 2011, 74, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, S.; Gotoda, T.; Oda, I. Clinical imaging of early Gastric cancers—Conventional endoscopy: Including chromoendoscopy using indigo carmine. Stomach Intest. 2009, 44, 650–662. [Google Scholar] [CrossRef]
- Tsai, M.-M.; Wang, C.-S.; Tsai, C.-Y.; Huang, H.-W.; Chi, H.-C.; Lin, Y.-H.; Lu, P.-H.; Lin, K.-H. Potential Diagnostic, Prognostic and Therapeutic Targets of MicroRNAs in Human Gastric Cancer. Int. J. Mol. Sci. 2016, 17, 945. [Google Scholar] [CrossRef]
- Suo, J.; Zhang, D.Y.; Tong, W.; Ye, F.; He, L.; Cui, L.; Cui, M.; Hu, Y.; Li, W.; Jiang, J. Serum biomarker panels for diagnosis of gastric cancer. OncoTargets Ther. 2016, 9, 2455–2463. [Google Scholar] [CrossRef]
- Cisło, M.; Filip, A.A.; Offerhaus, G.J.A.; Ciseł, B.; Rawicz-Pruszyński, K.; Skierucha, M.; Polkowski, W.P. Distinct molecular subtypes of gastric cancer: From Laurén to molecular pathology. Oncotarget 2018, 9, 19427–19442. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.-M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef]
- Katona, B.W.; Rustgi, A.K. Gastric Cancer Genomics: Advances and Future Directions. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 211–217. [Google Scholar] [CrossRef]
- Chivu-Economescu, M.; Matei, L.; Necula, L.G.; Dragu, D.L.; Bleotu, C.; Diaconu, C.C. New therapeutic options opened by the molecular classification of gastric cancer. World J. Gastroenterol. 2018, 24, 1942–1961. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA A Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef]
- Tan, M.C.; Graham, D.Y. Screening for Gastric Cancer: Focus on the Ants Instead of the Ant Hill. Clin. Gastroenterol. Hepatol. 2021, 19, 1990–1991. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef] [PubMed]
- Salati, M.; Orsi, G.; Smyth, E.; Aprile, G.; Beretta, G.; De Vita, F.; Di Bartolomeo, M.; Fanotto, V.; Lonardi, S.; Morano, F.; et al. Gastric cancer: Translating novels concepts into clinical practice. Cancer Treat. Rev. 2019, 79, 101889. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Ling, S.; Zheng, S.; Xu, X. Liquid biopsy in hepatocellular carcinoma: Circulating tumor cells and circulating tumor DNA. Mol. Cancer 2019, 18, 114. [Google Scholar] [CrossRef]
- Hou, J.; Li, X.; Xie, K.-P. Coupled liquid biopsy and bioinformatics for pancreatic cancer early detection and precision prognostication. Mol. Cancer 2021, 20, 34. [Google Scholar] [CrossRef]
- Nagasaka, M.; Uddin, M.H.; Al-Hallak, M.N.; Rahman, S.; Balasubramanian, S.; Sukari, A.; Azmi, A.S. Liquid biopsy for therapy monitoring in early-stage non-small cell lung cancer. Mol. Cancer 2021, 20, 82. [Google Scholar] [CrossRef]
- Lim, S.Y.; Lee, J.H.; Diefenbach, R.J.; Kefford, R.F.; Rizos, H. Liquid biomarkers in melanoma: Detection and discovery. Mol. Cancer 2018, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef]
- Lengyel, C.G.; Hussain, S.; Trapani, D.; El Bairi, K.; Altuna, S.C.; Seeber, A.; Odhiambo, A.; Habeeb, B.S.; Seid, F. The Emerging Role of Liquid Biopsy in Gastric Cancer. J. Clin. Med. 2021, 10, 2018. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy in 2016: Circulating tumour cells and cell-free DNA in gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.N.; Roy, S.; Ravi, R. Gastric cancer and related epigenetic alterations. Ecancermedicalscience 2017, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Weiner, H.; Crabb, D.W. The mutation in the mitochondrial aldehyde dehydrogenase (ALDH2) gene responsible for alcohol-induced flushing increases turnover of the enzyme tetramers in a dominant fashion. J. Clin. Investig. 1996, 98, 2027–2032. [Google Scholar] [CrossRef]
- Kim, K.; Shin, D.G.; Park, M.K.; Baik, S.H.; Kim, T.H.; Kim, S.; Lee, S. Circulating cell-free DNA as a promising biomarker in patients with gastric cancer: Diagnostic validity and significant reduction of cfDNA after surgical resection. Ann. Surg. Treat. Res. 2014, 86, 136–142. [Google Scholar] [CrossRef]
- Kato, S.; Okamura, R.; Baumgartner, J.M.; Patel, H.; Leichman, L.; Kelly, K.; Sicklick, J.K.; Fanta, P.T.; Lippman, S.M.; Kurzrock, R. Analysis of Circulating Tumor DNA and Clinical Correlates in Patients with Esophageal, Gastroesophageal Junction, and Gastric Adenocarcinoma. Clin. Cancer Res. 2018, 24, 6248–6256. [Google Scholar] [CrossRef]
- Li, T.-T.; Liu, H.; Yu, J.; Shi, G.-Y.; Zhao, L.-Y.; Li, G.-X. Prognostic and predictive blood biomarkers in gastric cancer and the potential application of circulating tumor cells. World J. Gastroenterol. 2018, 24, 2236–2246. [Google Scholar] [CrossRef]
- Krebs, M.G.; Metcalf, R.L.; Carter, L.; Brady, G.; Blackhall, F.H.; Dive, C. Molecular analysis of circulating tumour cells—Biology and biomarkers. Nat. Rev. Clin. Oncol. 2014, 11, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Pantel, K. Tumor Cell Dissemination: Emerging Biological Insights from Animal Models and Cancer Patients. Cancer Cell 2013, 23, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Kim, G.H.; Jeon, H.K.; Park, S.J. Clinical Application of Circulating Tumor Cells in Gastric Cancer. Gut Liver 2019, 13, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Pierga, J.-Y.; Hajage, D.; Bachelot, T.; Delaloge, S.; Brain, E.; Campone, M.; Diéras, V.; Rolland, E.; Mignot, L.; Mathiot, C.; et al. High independent prognostic and predictive value of circulating tumor cells compared with serum tumor markers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patients. Ann. Oncol. 2012, 23, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zou, K.; Yuan, Z.; Guo, T.; Xiong, B. Prognostic value of circulating tumor cells detected with the CellSearch System in patients with gastric cancer: Evidence from a meta-analysis. OncoTargets Ther. 2018, 11, 1013–1023. [Google Scholar] [CrossRef]
- Gasch, C.; Bauernhofer, T.; Pichler, M.; Langer-Freitag, S.; Reeh, M.; Seifert, A.M.; Mauermann, O.; Izbicki, J.R.; Pantel, K.; Riethdorf, S. Heterogeneity of Epidermal Growth Factor Receptor Status and Mutations of KRAS/PIK3CA in Circulating Tumor Cells of Patients with Colorectal Cancer. Clin. Chem. 2013, 59, 252–260. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, J.; Kim, S.T.; Park, S.H.; Park, J.O.; Park, Y.S.; Lim, H.Y.; Kang, W.K. Circulating Tumor Cells are Predictive of Poor Response to Chemotherapy in Metastatic gastric cancer. Int. J. Biol. Markers 2015, 30, 382–386. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Mishima, Y.; Matsusaka, S.; Chin, K.; Mikuniya, M.; Minowa, S.; Takayama, T.; Shibata, H.; Kuniyoshi, R.; Ogura, M.; Terui, Y.; et al. Detection of HER2 Amplification in Circulating Tumor Cells of HER2-Negative Gastric Cancer Patients. Target. Oncol. 2017, 12, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, B.; Liu, Z.; Gong, J.; Shao, L.; Ren, J.; Niu, Y.; Bo, S.; Li, Z.; Lai, Y.; et al. HER2 copy number of circulating tumour DNA functions as a biomarker to predict and monitor trastuzumab efficacy in advanced gastric cancer. Eur. J. Cancer 2018, 88, 92–100. [Google Scholar] [CrossRef]
- Spellman, P.T.; Gray, J.W. Detecting cancer by monitoring circulating tumor DNA. Nat. Med. 2014, 20, 474–475. [Google Scholar] [CrossRef]
- Stroun, M.; Maurice, P.; Vasioukhin, V.; Lyautey, J.; Lederrey, C.; Lefort, F.; Rossier, A.; Chen, X.Q.; Anker, P. The Origin and Mechanism of Circulating DNA. Ann. N. Y. Acad. Sci. 2000, 906, 161–168. [Google Scholar] [CrossRef]
- Jogo, T.; Nakamura, Y.; Shitara, K.; Bando, H.; Yasui, H.; Esaki, T.; Terazawa, T.; Satoh, T.; Shinozaki, E.; Nishina, T.; et al. Circulating Tumor DNA Analysis Detects FGFR2 Amplification and Concurrent Genomic Alterations Associated with FGFR Inhibitor Efficacy in Advanced Gastric Cancer. Clin. Cancer Res. 2021, 27, 5619–5627. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wu, W.; Han, L.; Yu, W.; Du, Y. A quantitative analysis of the potential biomarkers of non-small cell lung cancer by circulating cell-free DNA. Oncol. Lett. 2018, 16, 4353–4360. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Ju, S.; Qi, J.; Zhao, J.; Shen, X.; Jing, R.; Yu, J.; Li, L.; Shi, Y.; Zhang, L.; et al. Alu-based cell-free DNA: A novel biomarker for screening of gastric cancer. Oncotarget 2017, 8, 54037–54045. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Chen, D.L.; Wang, F.; Yang, C.P.; Chen, X.X.; You, J.Q.; Huang, J.S.; Shao, Y.; Zhu, D.Q.; Ouyang, Y.M.; et al. The predicting role of circulating tumor DNA landscape in Gastric cancer patients treated with immune checkpoint inhibitors. Mol. Cancer. 2020, 19, 154. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.C.M.; Massie, C.; Garcia-Corbacho, J.; Mouliere, F.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, R.; Rosenfeld, N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer 2017, 17, 223–238. [Google Scholar] [CrossRef]
- Yang, J.; Gong, Y.; Lam, V.K.; Shi, Y.; Guan, Y.; Zhang, Y.; Ji, L.; Chen, Y.; Zhao, Y.; Qian, F.; et al. Deep sequencing of circulating tumor DNA detects molecular residual disease and predicts recurrence in gastric cancer. Cell Death Dis. 2020, 11, 346. [Google Scholar] [CrossRef]
- Ko, K.; Kananazawa, Y.; Yamada, T.; Kakinuma, D.; Matsuno, K.; Ando, F.; Kuriyama, S.; Matsuda, A.; Yoshida, H. Methylation status and long-fragment cell-free DNA are prognostic biomarkers for gastric cancer. Cancer Med. 2021, 10, 2003–2012. [Google Scholar] [CrossRef]
- Davies, R.J.; Miller, R.; Coleman, N. Colorectal cancer screening: Prospects for molecular stool analysis. Nat. Rev. Cancer 2005, 5, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Czeiger, D.; Shaked, G.; Eini, H.; Vered, I.; Belochitski, O.; Avriel, A.; Ariad, S.; Douvdevani, A. Measurement of Circulating Cell-Free DNA Levels by a New Simple Fluorescent Test in Patients with Primary Colorectal Cancer. Am. J. Clin. Pathol. 2011, 135, 264–270. [Google Scholar] [CrossRef]
- Skvortsova, K.; Stirzaker, C.; Taberlay, P. The DNA methylation landscape in cancer. Essays Biochem. 2019, 63, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, V.; Soleimanian, A.; Ebrahimi, T.; Azargun, R.; Yazdani, P.; Eyvazi, S.; Tarhriz, V. Epigenetic modifications in gastric cancer: Focus on DNA methylation. Gene 2020, 742, 144577. [Google Scholar] [CrossRef]
- Zeng, X.-Q.; Wang, J.; Chen, S.-Y. Methylation modification in gastric cancer and approaches to targeted epigenetic therapy (Review). Int. J. Oncol. 2017, 50, 1921–1933. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.-Q.; Lv, P.; Lu, X.-X.; Yu, J.-L.; Han, J.; Ying, L.-S.; Zhu, X.; Zhu, W.-Y.; Fang, X.-H.; Wang, S.; et al. Circulating Methylated XAF1 DNA Indicates Poor Prognosis for Gastric Cancer. PLoS ONE 2013, 8, e67195. [Google Scholar] [CrossRef]
- Pimson, C.; Ekalaksananan, T.; Pientong, C.; Promthet, S.; Putthanachote, N.; Suwanrungruang, K.; Wiangnon, S. Aberrant methylation of PCDH10 and RASSF1A genes in blood samples for non-invasive diagnosis and prognostic assessment of gastric cancer. PeerJ 2016, 4, e2112. [Google Scholar] [CrossRef] [PubMed]
- Karamitrousis, E.I.; Balgkouranidou, I.; Xenidis, N.; Amarantidis, K.; Biziota, E.; Koukaki, T.; Trypsianis, G.; Karayiannakis, A.; Bolanaki, H.; Kolios, G.; et al. Prognostic Role of RASSF1A, SOX17 and Wif-1 Promoter Methylation Status in Cell-Free DNA of Advanced Gastric Cancer Patients. Technol. Cancer Res. Treat. 2021, 20, 1533033820973279. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-L.; Lv, P.; Han, J.; Zhu, X.; Hong, L.-L.; Zhu, W.-Y.; Wang, X.-B.; Wu, Y.-C.; Li, P.; Ling, Z.-Q. Methylated TIMP-3 DNA in Body Fluids Is an Independent Prognostic Factor for Gastric Cancer. Arch. Pathol. Lab. Med. 2014, 138, 1466–1473. [Google Scholar] [CrossRef]
- Quirico, L.; Orso, F. The power of microRNAs as diagnostic and prognostic biomarkers in liquid biopsies. Cancer Drug Resist. 2020, 3, 117–139. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, L.-A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561. [Google Scholar] [CrossRef]
- Shin, V.Y. MiRNA as potential biomarkers and therapeutic targets for gastric cancer. World J. Gastroenterol. 2014, 20, 10432–10439. [Google Scholar] [CrossRef]
- Yu, L.; Wu, D.; Gao, H.; Balic, J.J.; Tsykin, A.; Han, T.-S.; Liu, Y.D.; Kennedy, C.L.; Li, J.K.; Mao, J.Q.; et al. Clinical Utility of a STAT3-Regulated miRNA-200 Family Signature with Prognostic Potential in Early Gastric Cancer. Clin. Cancer Res. 2018, 24, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Lujambio, A.; Lowe, S.W. The microcosmos of cancer. Nature 2012, 482, 347–355. [Google Scholar] [CrossRef]
- McLean, M.H.; El-Omar, E.M. Genetics of gastric cancer. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Schirrmeister, W.; Langner, C.; Varbanova, M.; Bornschein, J.; Wex, T.; Malfertheiner, P. Differential expression of microRNAs in preneoplastic gastric mucosa. Sci. Rep. 2015, 5, 8270. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The MicroRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Necula, L.; Matei, L.; Dragu, D.; I Neagu, A.; Mambet, C.; Nedeianu, S.; Bleotu, C.; Diaconu, C.C.; Chivu-Economescu, M. Recent advances in gastric cancer early diagnosis. World J. Gastroenterol. 2019, 25, 2029–2044. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Goel, A. MicroRNA in gastrointestinal cancer: A step closer to reality. Adv Clin Chem. 2013, 62, 221–268. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.M.; Xie, J.M.; Liu, Y.M.; Tang, F.M.; Long, Z.M.; Wang, Y.M.; Luo, J.M.; Li, J.M.; Li, G. MicroRNA expression profiling and target gene analysis in gastric cancer. Medicine 2020, 99, e21963. [Google Scholar] [CrossRef]
- Raad, M.; Salehi, Z.; Baalsini, M.H.; Mashayekhi, F.; Saedi, H.S. Association of rs2620381 polymorphism in miR-627 and gastric cancer. Br. J. Biomed. Sci. 2020, 77, 76–80. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Exosomes as a new frontier of cancer liquid biopsy. Mol. Cancer 2022, 21, 56. [Google Scholar] [CrossRef]
- Fu, M.; Gu, J.; Jiang, P.; Qian, H.; Xu, W.; Zhang, X. Exosomes in gastric cancer: Roles, mechanisms, and applications. Mol. Cancer 2019, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Yu, T.; Jing, X.; Ma, L.; Fan, Y.; Yang, F.; Ma, P.; Jiang, H.; Wu, X.; Shu, Y.; et al. Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol. Cancer 2020, 19, 112. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qiu, R.; Yu, S.; Xu, X.; Gu, R.; Tan, C.; Zhu, W.; Shen, B.; Li, G. Paclitaxelresistant gastric cancer MGC803 cells promote epithelialtomesenchymal transition and chemoresistance in paclitaxelsensitive cells via exosomal delivery of miR1555p. Int. J. Oncol. 2019, 54, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and Functions of Long Noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Song, J.H.; Cheng, Y.; Abraham, J.M.; Ibrahim, S.; Sun, Z.; Ke, X.; Meltzer, S.J. Long non-coding antisense RNA KRT7-AS is activated in gastric cancers and supports cancer cell progression by increasing KRT7 expression. Oncogene 2016, 35, 4927–4936. [Google Scholar] [CrossRef]
- Matouk, I.J.; Abbasi, I.; Hochberg, A.; Galun, E.; Dweik, H.; Akkawi, M. Highly upregulated in liver cancer noncoding RNA is overexpressed in hepatic colorectal metastasis. Eur. J. Gastroenterol. Hepatol. 2009, 21, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Schalken, J.; Dijkstra, S.; Baskin-Bey, E.; van Oort, I. Potential utility of cancer-specific biomarkers for assessing response to hormonal treatments in metastatic prostate cancer. Ther. Adv. Urol. 2014, 6, 245–252. [Google Scholar] [CrossRef]
- Wu, M.-S.; Wang, H.-P.; Lin, C.-C.; Sheu, J.-C.; Shun, C.-T.; Lee, W.-J.; Lin, J.-T. Loss of imprinting and overexpression of IGF2 gene in gastric adenocarcinoma. Cancer Lett. 1997, 120, 9–14. [Google Scholar] [CrossRef]
- Fei, Z.-H.; Yu, X.-J.; Zhou, M.; Su, H.-F.; Zheng, Z.; Xie, C.-Y. Upregulated expression of long non-coding RNA LINC00982 regulates cell proliferation and its clinical relevance in patients with gastric cancer. Tumor Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 1983–1993. [Google Scholar] [CrossRef]
- Zheng, L.; Cao, J.; Liu, L.; Xu, H.; Chen, L.; Kang, L.; Gao, L. Long noncoding RNA LINC00982 upregulates CTSF expression to inhibit gastric cancer progression via the transcription factor HEY1. Am. J. Physiol. Liver Physiol. 2021, 320, G816–G828. [Google Scholar] [CrossRef] [PubMed]
- Arita, T.; Ichikawa, D.; Konishi, H.; Komatsu, S.; Shiozaki, A.; Shoda, K.; Kawaguchi, T.; Hirajima, S.; Nagata, H.; Kubota, T.; et al. Circulating long non-coding RNAs in plasma of patients with Gastric cancer. Anticancer Res. 2013, 33, 3185–3193. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yin, C.; Dang, Y.; Ye, F.; Zhang, G. Identification of the long non-coding RNA H19 in plasma as a novel biomarker for diagnosis of gastric cancer. Sci. Rep. 2015, 5, 11516. [Google Scholar] [CrossRef] [PubMed]
- Hashad, D.; Elbanna, A.; Ibrahim, A.; Khedr, G. Evaluation of the Role of Circulating Long Non-Coding RNA H19 as a Promising Novel Biomarker in Plasma of Patients with Gastric Cancer. J. Clin. Lab. Anal. 2016, 30, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Yörüker, E.E.; Keskin, M.; Kulle, C.B.; Holdenrieder, S.; Gezer, U. Diagnostic and prognostic value of circulating lncRNA H19 in gastric cancer. Biomed. Rep. 2018, 9, 181–186. [Google Scholar] [CrossRef]
- Lecuit, T.; Yap, A.S. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nature 2015, 17, 533–539. [Google Scholar] [CrossRef]
- Xicola, R.M.; Li, S.; Rodriguez, N.; Reinecke, P.; Karam, R.; Speare, V.; Black, M.H.; LaDuca, H.; Llor, X. Clinical features and cancer risk in families with pathogenic CDH1 variants irrespective of clinical criteria. J. Med. Genet. 2019, 56, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, V.; Pensotti, V.; Marabelli, M.; Feroce, I.; Barile, M.; Pozzi, S.; Laghi, L.; Serrano, D.; Bernard, L.; Bonanni, B.; et al. Complementary molecular approaches reveal heterogeneous CDH1 germline defects in Italian patients with hereditary diffuse gastric cancer (HDGC) syndrome. Genes Chromosom. Cancer 2014, 53, 432–445. [Google Scholar] [CrossRef]
- Lynch, H.T.; Nustas, R.; Kassim, T.; Snyder, C.; Shaw, T.; Diab, O. The benefits of a model of interval comprehensive assessments (MICA) in hereditary cancer Syndromes: Hereditary diffuse gastric cancer (HDGC) as an example. Cancer Genet. 2019, 233–234, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, P.; Fenton, H.; Morris-Stiff, G.; Ahmad, N.; Fisher, J.; Prasad, K.R. Metabonomics: A Useful Tool for the Future Surgeon. J. Surg. Res. 2010, 160, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Burton, C.; Ma, Y. Current Trends in Cancer Biomarker Discovery Using Urinary Metabolomics: Achievements and New Challenges. Curr. Med. Chem. 2019, 26, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Wijeyesekera, A.; Selman, C.; Barton, R.H.; Holmes, E.; Nicholson, J.K.; Withers, D.J. Metabotyping of Long-Lived Mice using 1H NMR Spectroscopy. J. Proteome Res. 2012, 11, 2224–2235. [Google Scholar] [CrossRef]
- Emwas, A.H. The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Methods Mol. Biol. 2015, 1277, 161–193. [Google Scholar]
- Yu, L.; Lai, Q.; Feng, Q.; Li, Y.; Feng, J.; Xu, B. Serum Metabolic Profiling Analysis of Chronic Gastritis and Gastric Cancer by Untargeted Metabolomics. Front. Oncol. 2021, 11, 636917. [Google Scholar] [CrossRef] [PubMed]
- Killampalli, L.K.; Reddy, A.V.; Prakash, A.R.; Naag, S.; Sreenath, G.; Biraggari, S.K. Analysis of lipid profile in cancer patients, smokers, and nonsmokers. Dent. Res. J. 2016, 13, 494–499. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, E.Y.; Yoo, H.M.; Park, C.H.; Song, K.Y. Changes of lipid profiles after radical gastrectomy in patients with gastric cancer. Lipids Health Dis. 2015, 14, 21. [Google Scholar] [CrossRef]
- Graham, D.Y.; Shiotani, A. The time to eradicate gastric cancer is now. Gut 2005, 54, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Correa, P. Human gastric carcinogenesis: A multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992, 52, 6735–6740. [Google Scholar]
- Hanh, T.T.H.; Hang, D.T.T.; Van Minh, C.; Dat, N.T. Anti-inflammatory effects of fatty acids isolated from Chromolaena odorata. Asian Pac. J. Trop. Med. 2011, 4, 760–763. [Google Scholar] [CrossRef]
- Zhang, T.; Duran, V.; Vanarsa, K.; Mohan, C. Targeted urine proteomics in lupus nephritis—A meta-analysis. Expert Rev. Proteom. 2020, 17, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.L.; Knowles, M.A.; Thompson, D.; Selby, P.J.; Banks, R.E. Proteomic studies of urinary biomarkers for prostate, bladder and kidney cancers. Nat. Rev. Urol. 2013, 10, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Bax, C.; Lotesoriere, B.J.; Sironi, S.; Capelli, L. Review and Comparison of Cancer Biomarker Trends in Urine as a Basis for New Diagnostic Pathways. Cancers 2019, 11, 1244. [Google Scholar] [CrossRef]
- Pejcic, M.; Stojnev, S.; Stefanovic, V. Urinary Proteomics—A Tool for Biomarker Discovery. Ren. Fail. 2010, 32, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Guo, L.; Liu, L.; Wen, J.; Xu, L.; Yan, M.; Li, Z.; Zhang, X.; Nan, P.; et al. A characteristic biosignature for discrimination of gastric cancer from healthy population by high throughput GC-MS analysis. Oncotarget 2016, 7, 87496–87510. [Google Scholar] [CrossRef]
- Monleón, D.; Morales, J.M.; Barrasa, A.; López, J.A.; Vázquez, C.; Celda, B. Metabolite profiling of fecal water extracts from human colorectal cancer. NMR Biomed. 2009, 22, 342–348. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, C.; Liu, C.; Wang, Z.; Yang, J.; Liu, X.; Shen, Z.; Wu, R. NMR-based fecal metabolomics fingerprinting as predictors of earlier diagnosis in patients with colorectal cancer. Oncotarget 2016, 7, 29454–29464. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, C.; Bezabeh, T.; Wang, Z.; Liang, J.; Huang, Y.; Zhao, J.; Liu, X.; Ye, W.; Tang, W.; et al. 1H NMR-based metabolomics reveal overlapping discriminatory metabolites and metabolic pathway disturbances between colorectal tumor tissues and fecal samples. Int. J. Cancer 2019, 145, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. ‘Why do tumour cells glycolyse?’: From glycolysis through citrate to lipogenesis. Mol. Cell. Biochem. 2005, 280, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kaltenbach, T.; Sano, Y.; Friedland, S.; Soetikno, R. American Gastroenterological Association (AGA) Institute Technology Assessment on Image-Enhanced Endoscopy. Gastroenterology 2008, 134, 327–340. [Google Scholar] [CrossRef]
- Dohi, O.; Yagi, N.; Naito, Y.; Fukui, A.; Gen, Y.; Iwai, N.; Ueda, T.; Yoshida, N.; Kamada, K.; Uchiyama, K.; et al. Blue laser imaging-bright improves the real-time detection rate of early gastric cancer: A randomized controlled study. Gastrointest. Endosc. 2019, 89, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Nagahama, T.; Matsui, T.; Iwashita, A. Detection and characterization of early gastric cancer for curative endoscopic submucosal dissection. Dig. Endosc. 2013, 25 (Suppl. S1), 44–54. [Google Scholar] [CrossRef] [PubMed]
- Yao, K. The endoscopic diagnosis of early Gastric cancer. Ann. Gastroenterol. 2013, 26, 12–23. [Google Scholar] [CrossRef]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the Development of Gastric Cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef]
- Osawa, H.; Yamamoto, H. Present and future status of flexible spectral imaging color enhancement and blue laser imaging technology. Dig. Endosc. 2014, 26 (Suppl. S1), 105–115. [Google Scholar] [CrossRef]
- Osawa, H.; Yamamoto, H.; Miura, Y.; Sasao, W.; Ino, Y.; Satoh, H.; Satoh, K.; Sugano, K. Blue Laser Imaging Provides Excellent Endoscopic Images of Upper Gastrointestinal Lesions. Video J. Encycl. GI Endosc. 2014, 1, 607–610. [Google Scholar] [CrossRef]
- Dohi, O.; Yagi, N.; Onozawa, Y.; Kimura-Tsuchiya, R.; Majima, A.; Kitaichi, T.; Horii, Y.; Suzuki, K.; Tomie, A.; Okayama, T.; et al. Linked color imaging improves endoscopic diagnosis of active Helicobacter pylori infection. Endosc. Int. Open 2016, 4, E800–E805. [Google Scholar] [CrossRef]
- Mizukami, K.; Ogawa, R.; Okamoto, K.; Shuto, M.; Fukuda, K.; Sonoda, A.; Matsunari, O.; Hirashita, Y.; Okimoto, T.; Kodama, M.; et al. Objective Endoscopic Analysis with Linked Color Imaging regarding Gastric Mucosal Atrophy: A Pilot Study. Gastroenterol. Res. Pract. 2017, 2017, 5054237. [Google Scholar] [CrossRef]
- Ono, S.; Kato, M.; Tsuda, M.; Miyamoto, S.; Abiko, S.; Shimizu, Y.; Sakamoto, N. Lavender Color in Linked Color Imaging Enables Noninvasive Detection of Gastric Intestinal Metaplasia. Digestion 2018, 98, 222–230. [Google Scholar] [CrossRef]
- Osawa, H.; Miura, Y.; Takezawa, T.; Ino, Y.; Khurelbaatar, T.; Sagara, Y.; Lefor, A.K.; Yamamoto, H. Linked color imaging and blue laser imaging for upper gastrointestinal screening. Clin. Endosc. 2018, 51, 513–526. [Google Scholar] [CrossRef]
- Fukuda, H.; Miura, Y.; Hayashi, Y.; Takezawa, T.; Ino, Y.; Okada, M.; Osawa, H.; Lefor, A.K.; Yamamoto, H. Linked color imaging technology facilitates early detection of flat gastric cancers. Clin. J. Gastroenterol. 2015, 8, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Miura, Y.; Osawa, H.; Takezawa, T.; Ino, Y.; Okada, M.; Khurelbaatar, T.; Lefor, A.K.; Yamamoto, H. Linked color imaging can enhance recognition of early gastric cancer by high color contrast to surrounding gastric intestinal metaplasia. J. Gastroenterol. 2019, 54, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Maeta, K.; Nishiyama, Y.; Fujibayashi, K.; Gunji, T.; Sasabe, N.; Iijima, K.; Naito, T. Prediction of Glucose Metabolism Disorder Risk Using a Machine Learning Algorithm: Pilot Study. JMIR Diabetes 2018, 3, e10212. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Lee, H.-C.; Diao, K.-Y.; Huang, M.; Lin, B.; Liu, C.; Xie, Z.; Ma, Y.; Robson, P.M.; Chung, M.; et al. Artificial intelligence–enabled rapid diagnosis of patients with COVID-19. Nat. Med. 2020, 26, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.-H.; Zhao, L.-L.; Wu, H.-L.; Zhao, D.-B.; Chen, Y.-T. Artificial intelligence in gastric cancer: Application and future perspectives. World J. Gastroenterol. 2020, 26, 5408–5419. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, J.; Wang, Y.; Ji, M.-H.; Tong, J.; Yang, J.-J.; Xia, H. A machine learning-based predictor for the identification of the recurrence of patients with gastric cancer after operation. Sci. Rep. 2021, 11, 1571. [Google Scholar] [CrossRef] [PubMed]
- Afrash, M.R.; Khalili, M.; Salekde, M.S. A comparison of data mining methods for diagnosis and prognosis of heart disease. Int. J. Adv. Intell. Paradig. 2020, 16, 88–97. [Google Scholar] [CrossRef]
- Jordan, M.I.; Mitchell, T.M. Machine learning: Trends, perspectives, and prospects. Science 2015, 349, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Shichijo, S.; Nomura, S.; Aoyama, K.; Nishikawa, Y.; Miura, M.; Shinagawa, T.; Takiyama, H.; Tanimoto, T.; Ishihara, S.; Matsuo, K.; et al. Application of Convolutional Neural Networks in the Diagnosis of Helicobacter pylori Infection Based on Endoscopic Images. EBioMedicine 2017, 25, 106–111. [Google Scholar] [CrossRef]
- Itoh, T.; Kawahira, H.; Nakashima, H.; Yata, N. Deep learning analyzes Helicobacter pylori infection by upper gastrointestinal endoscopy images. Endosc. Int. Open 2018, 6, E139–E144. [Google Scholar] [CrossRef]
- Ba, W.; Wang, S.; Shang, M.; Zhang, Z.; Wu, H.; Yu, C.; Xing, R.; Wang, W.; Wang, L.; Liu, C.; et al. Assessment of deep learning assistance for the pathological diagnosis of gastric cancer. Mod. Pathol. 2022, 35, 1262–1268. [Google Scholar] [CrossRef] [PubMed]

| Population | Patients | Sensitivity % | Specificity % | Diagnosis Method | Sample Type | Biomarker | |
|---|---|---|---|---|---|---|---|
| Chan et al. (2016) [13] | Mixed (GC + CG + healthy controls) | 43 + 40 + 40 | 95% | 80% | Metabolic | Urine | Multiple |
| Shichijo et al. (2017) [14] | Mixed (GC + healthy controls) | 753 + 1015 | 81.9% | 83.4% | Endoscopic | - | Machine learning models |
| Song et al. (2017) [15] | Mixed (GC + healthy controls) | 628 + 502 | Missing data | Missing data | Genetic | Serum | MicroRNA |
| Hwa Mi Kang et al. (2017) [16] | Mixed (GC + healthy controls) | 116 + 31 | 85.3% | 90.3% | Genetic | Serum | Circulating tumor cells |
| Itoh et al. (2018) [17] | Mixed (HP+ and HP-) | 65 + 74 | 86.7% | 86.7% | Endoscopic | - | Machine learning models |
| Kong et al. (2019) [18] | Mixed (GC + CG + healthy controls) | 184 + 56 + 78 | 67.3–69.4% | 80.4–81.0% | Genetic | Serum | MicroRNA |
| Huang et al. (2020) [19] | Mixed (GC + healthy controls) | 106 + 118 | 83.96% | 94.07% | Genetic | Serum | Circulating cell-free DNA |
| Qian et al. (2021) [20] | GI cancer patients | 72 | Missing data | Missing data | Genetic | Serum | Circulating tumor cells |
| Yu et al. (2021) [21] | Mixed (GC + healthy controls) | 72 + 51 | 75.73% | 70.36% | Metabolic | Serum | Multiple |
| Advantages | Disadvantages | |
|---|---|---|
| Blood |
|
|
| Urine |
|
|
| Fecal water |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orășeanu, A.; Brisc, M.C.; Maghiar, O.A.; Popa, H.; Brisc, C.M.; Șolea, S.F.; Maghiar, T.A.; Brisc, C. Landscape of Innovative Methods for Early Diagnosis of Gastric Cancer: A Systematic Review. Diagnostics 2023, 13, 3608. https://doi.org/10.3390/diagnostics13243608
Orășeanu A, Brisc MC, Maghiar OA, Popa H, Brisc CM, Șolea SF, Maghiar TA, Brisc C. Landscape of Innovative Methods for Early Diagnosis of Gastric Cancer: A Systematic Review. Diagnostics. 2023; 13(24):3608. https://doi.org/10.3390/diagnostics13243608
Chicago/Turabian StyleOrășeanu, Alexandra, Mihaela Cristina Brisc, Octavian Adrian Maghiar, Horia Popa, Ciprian Mihai Brisc, Sabina Florina Șolea, Teodor Andrei Maghiar, and Ciprian Brisc. 2023. "Landscape of Innovative Methods for Early Diagnosis of Gastric Cancer: A Systematic Review" Diagnostics 13, no. 24: 3608. https://doi.org/10.3390/diagnostics13243608
APA StyleOrășeanu, A., Brisc, M. C., Maghiar, O. A., Popa, H., Brisc, C. M., Șolea, S. F., Maghiar, T. A., & Brisc, C. (2023). Landscape of Innovative Methods for Early Diagnosis of Gastric Cancer: A Systematic Review. Diagnostics, 13(24), 3608. https://doi.org/10.3390/diagnostics13243608






