Low Vitamin D Levels Are Associated with Increased Cardiac Iron Uptake in Beta-Thalassemia Major
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Biochemical Analysis
2.3. Magnetic Resonance Imaging
2.4. Diagnostic Criteria
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Correlates of Vitamin D Levels
3.3. Correlates of PTH Levels
3.4. Determinants of Global Heart T2* Values
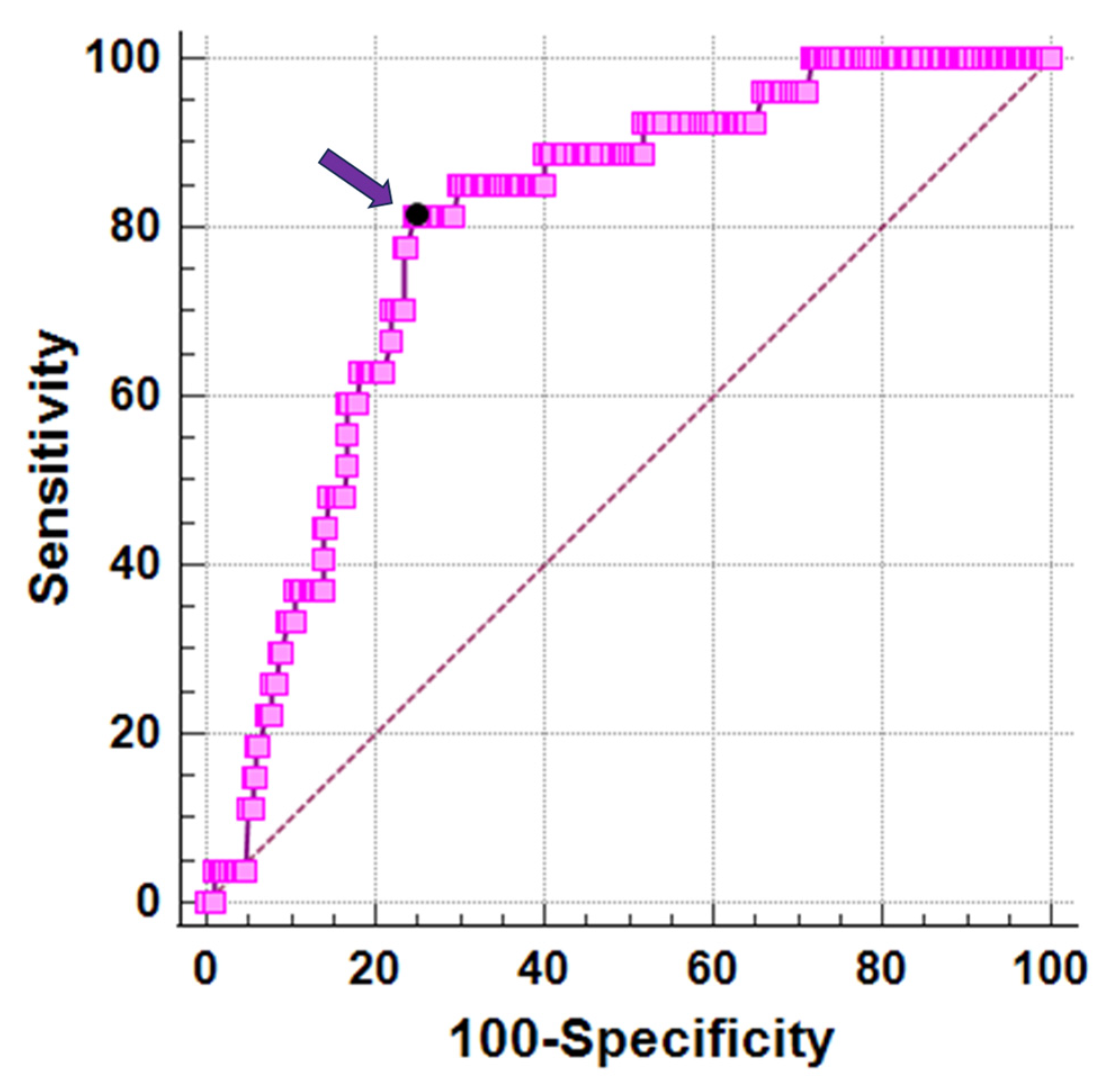
3.5. Best Cut-Off of Vitamin D for Cardiac Iron
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weatherall, D.J.; Clegg, J.B. The Thalassemia Syndromes; Blackwell Science: Oxford, UK, 2001. [Google Scholar] [CrossRef]
- Cao, A.; Galanello, R. Beta-thalassemia. Genet. Med. 2010, 12, 61–76. [Google Scholar] [CrossRef]
- Origa, R. Beta-Thalassemia. Genet. Med. 2017, 19, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Nienhuis, A.W.; Nathan, D.G. Pathophysiology and Clinical Manifestations of the β-Thalassemias. Cold Spring Harb. Perspect. Med. 2012, 2, a011726. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.; Piolatto, A.; Ferrero, G.B.; Piga, A. Ineffective Erythropoiesis in β-Thalassaemia: Key Steps and Therapeutic Options by Drugs. Int. J. Mol. Sci. 2021, 22, 7229. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Cohen, A.; Porter, J.; Taher, A.; Viprakasit, V. Guidelines for the Management of Transfusion Dependent Thalassaemia (TDT) [Internet], 3rd ed.; Thalassaemia International Federation: Nicosia, Cyprus, 2014. [Google Scholar]
- Alli, N.A.; Patel, M.; Poole, J.; Goga, Y.; Fazel, F.; Novitzky, N.; Parasnath, S.; Bassa, F. Thalassaemia (part 2): Management. S. Afr. Med. J. 2021, 111, 825–833. [Google Scholar] [CrossRef]
- Andrews, P.A. Disorders of iron metabolism. N. Engl. J. Med. 2000, 342, 1293. [Google Scholar] [PubMed]
- Ozment, C.P.; Turi, J.L. Iron overload following red blood cell transfusion and its impact on disease severity. Biochim. Biophys. Acta 2009, 1790, 694–701. [Google Scholar] [CrossRef]
- Rund, D.; Rachmilewitz, E. Beta-thalassemia. N. Engl. J. Med. 2005, 353, 1135–1146. [Google Scholar] [CrossRef]
- Saliba, A.; Taher, A. Iron overload in transfusion-dependent thalassemia. Hematology 2015, 20, 311–312. [Google Scholar] [CrossRef]
- Coates, T.D. Iron overload in transfusion-dependent patients. Hematol. Am. Soc. Hematol. Educ. Program. 2019, 2019, 337–344. [Google Scholar] [CrossRef]
- Neufeld, E.J. Update on Iron Chelators in Thalassemia. Hematology 2010, 2010, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Di Maggio, R.; Maggio, A. The new era of chelation treatments: Effectiveness and safety of 10 different regimens for controlling iron overloading in thalassaemia major. Br. J. Haematol. 2017, 178, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.B. Concepts and goals in the management of transfusional iron overload. Am. J. Hematol. 2007, 82, 1136–1139. [Google Scholar] [CrossRef] [PubMed]
- Entezari, S.; Haghi, S.M.; Norouzkhani, N.; Sahebnazar, B.; Vosoughian, F.; Akbarzadeh, D.; Islampanah, M.; Naghsh, N.; Abbasalizadeh, M.; Deravi, N. Iron Chelators in Treatment of Iron Overload. J. Toxicol. 2022, 2022, 4911205. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.-P.; Pennell, D.J. Role of T2* Magnetic Resonance in Monitoring Iron Chelation Therapy. Acta Haematol. 2009, 122, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.A.; Galanello, R.; Dessi, C.; Smith, G.C.; Westwood, M.A.; Agus, A.; Roughton, M.; Assomull, R.; Nair, S.V.; Walker, J.M.; et al. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation 2007, 115, 1876–1884. [Google Scholar] [CrossRef] [PubMed]
- Pennell, D.J.; Berdoukas, V.; Karagiorga, M.; Ladis, V.; Piga, A.; Aessopos, A.; Gotsis, E.D.; Tanner, M.A.; Smith, G.C.; Westwood, M.A.; et al. Randomized controlled trial of deferiprone or deferoxamine in beta-thalassemia major patients with asymptomatic myocardial siderosis. Blood 2006, 107, 3738–3744. [Google Scholar] [CrossRef]
- Pennell, D.J.; Porter, J.B.; Piga, A.; Lai, Y.; El-Beshlawy, A.; Belhoul, K.M.; Elalfy, M.; Yesilipek, A.; Kilinc, Y.; Lawniczek, T.; et al. A 1-year randomized controlled trial of deferasirox vs deferoxamine for myocardial iron removal in beta-thalassemia major (CORDELIA). Blood 2014, 123, 1447–1454. [Google Scholar] [CrossRef]
- Pepe, A.; Meloni, A.; Pistoia, L.; Cuccia, L.; Gamberini, M.R.; Lisi, R.; D’Ascola, D.G.; Rosso, R.; Allo, M.; Spasiano, A.; et al. MRI multicentre prospective survey in thalassaemia major patients treated with deferasirox versus deferiprone and desferrioxamine. Br. J. Haematol. 2018, 183, 783–795. [Google Scholar] [CrossRef]
- Berdoukas, V.; Chouliaras, G.; Moraitis, P.; Zannikos, K.; Berdoussi, E.; Ladis, V. The efficacy of iron chelator regimes in reducing cardiac and hepatic iron in patients with thalassaemia major: A clinical observational study. J. Cardiovasc. Magn. Reson. 2009, 11, 20. [Google Scholar] [CrossRef]
- Origa, R.; Cinus, M.; Pilia, M.P.; Gianesin, B.; Zappu, A.; Orecchia, V.; Clemente, M.G.; Pitturru, C.; Denotti, A.R.; Corongiu, F.; et al. Safety and Efficacy of the New Combination Iron Chelation Regimens in Patients with Transfusion-Dependent Thalassemia and Severe Iron Overload. J. Clin. Med. 2022, 11, 2010. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.C. Magnetic resonance imaging measurement of iron overload. Curr. Opin. Hematol. 2007, 14, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Pennell, D.J.; Udelson, J.E.; Arai, A.E.; Bozkurt, B.; Cohen, A.R.; Galanello, R.; Hoffman, T.M.; Kiernan, M.S.; Lerakis, S.; Piga, A.; et al. Cardiovascular function and treatment in beta-thalassemia major: A consensus statement from the American Heart Association. Circulation 2013, 128, 281–308. [Google Scholar] [CrossRef]
- Fernandes, J.L. MRI for Iron Overload in Thalassemia. Hematol./Oncol. Clin. 2018, 32, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Bayraktaroglu, S.; Karadas, N.; Onen, S.; Karapinar, D.Y.; Aydinok, Y. Modern management of iron overload in thalassemia major patients guided by MRI techniques: Real-world data from a long-term cohort study. Ann. Hematol. 2022, 101, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Modell, B.; Khan, M.; Darlison, M.; Westwood, M.A.; Ingram, D.; Pennell, D.J. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2008, 10, 42. [Google Scholar] [CrossRef]
- Pepe, A.; Pistoia, L.; Gamberini, M.R.; Cuccia, L.; Lisi, R.; Cecinati, V.; Maggio, A.; Sorrentino, F.; Filosa, A.; Rosso, R.; et al. National networking in rare diseases and reduction of cardiac burden in thalassemia major. Eur. Heart J. 2022, 43, 2482–2492. [Google Scholar] [CrossRef]
- Borgna-Pignatti, C.; Cappellini, M.D.; De Stefano, P.; Del Vecchio, G.C.; Forni, G.L.; Gamberini, M.R.; Ghilardi, R.; Origa, R.; Piga, A.; Romeo, M.A.; et al. Survival and complications in thalassemia. Ann. N. Y. Acad. Sci. 2005, 1054, 40–47. [Google Scholar] [CrossRef]
- Wood, J.C. Cardiac iron across different transfusion-dependent diseases. Blood Rev. 2008, 22 (Suppl. S2), S14–S21. [Google Scholar] [CrossRef]
- Kremastinos, D.T.; Farmakis, D.; Aessopos, A.; Hahalis, G.; Hamodraka, E.; Tsiapras, D.; Keren, A. Beta-thalassemia cardiomyopathy: History, present considerations, and future perspectives. Circ. Heart Fail. 2010, 3, 451–458. [Google Scholar] [CrossRef]
- Wood, J.C.; Claster, S.; Carson, S.; Menteer, J.D.; Hofstra, T.; Khanna, R.; Coates, T. Vitamin D deficiency, cardiac iron and cardiac function in thalassaemia major. Br. J. Haematol. 2008, 141, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, M.; Begerow, B.; Minne, H.W. Vitamin D and muscle function. Osteoporos. Int. 2002, 13, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, R.G.; Wickenden, A.D.; Bouchard, R.A.; Oudit, G.Y.; Liu, P.P.; Backx, P.H. Modulation of iron uptake in heart by L-type Ca2+ channel modifiers: Possible implications in iron overload. Circ. Res. 1999, 84, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Oudit, G.Y.; Trivieri, M.G.; Khaper, N.; Liu, P.P.; Backx, P.H. Role of L-type Ca2+ channels in iron transport and iron-overload cardiomyopathy. J. Mol. Med. 2006, 84, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Bresgen, N.; Eckl, P.M. Oxidative stress and the homeodynamics of iron metabolism. Biomolecules 2015, 5, 808–847. [Google Scholar] [CrossRef]
- Mancardi, D.; Mezzanotte, M.; Arrigo, E.; Barinotti, A.; Roetto, A. Iron Overload, Oxidative Stress, and Ferroptosis in the Failing Heart and Liver. Antioxidants 2021, 10, 1864. [Google Scholar] [CrossRef] [PubMed]
- Oudit, G.Y.; Sun, H.; Trivieri, M.G.; Koch, S.E.; Dawood, F.; Ackerley, C.; Yazdanpanah, M.; Wilson, G.J.; Schwartz, A.; Liu, P.P.; et al. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat. Med. 2003, 9, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadou, M.; Christoforidis, A.; Economou, M.; Tsatra, I.; Vlachaki, E.; Fidani, L.; Katzos, G.; Athanassiou-Metaxa, M. Elevated serum parathormone levels are associated with myocardial iron overload in patients with beta-thalassaemia major. Eur. J. Haematol. 2010, 84, 64–71. [Google Scholar] [CrossRef]
- Shaykhbaygloo, R.; Moradabadi, A.; Taherahmadi, H.; Rafiei, M.; Lotfi, F.; Eghbali, A. Correlation of Cardiac and Liver Iron Level with T2*MRI and Vitamin D3 Serum Level in Patients with Thalassemia Major. J. Blood Med. 2020, 11, 83–87. [Google Scholar] [CrossRef]
- Noetzli, L.J.; Papudesi, J.; Coates, T.D.; Wood, J.C. Pancreatic iron loading predicts cardiac iron loading in thalassemia major. Blood 2009, 114, 4021–4026. [Google Scholar] [CrossRef]
- Meloni, A.; Restaino, G.; Missere, M.; De Marchi, D.; Positano, V.; Valeri, G.; Giuseppe D’Ascola, D.; Peluso, A.; Caterina Putti, M.C.; Lendini, M.; et al. Pancreatic iron overload by T2* MRI in a large cohort of well treated thalassemia major patients: Can it tell us heart iron distribution and function? Am. J. Hematol. 2015, 90, E189–E190. [Google Scholar] [CrossRef] [PubMed]
- Pepe, A.; Pistoia, L.; Gamberini, M.R.; Cuccia, L.; Peluso, A.; Messina, G.; Spasiano, A.; Allo, M.; Bisconte, M.G.; Putti, M.C.; et al. The Close Link of Pancreatic Iron with Glucose Metabolism and With Cardiac Complications in Thalassemia Major: A Large, Multicenter Observational Study. Diabetes Care 2020, 43, 2830–2839. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; De Marchi, D.; Pistoia, L.; Grassedonio, E.; Peritore, G.; Preziosi, P.; Restaino, G.; Righi, R.; Riva, A.; Renne, S.; et al. Multicenter validation of the magnetic resonance T2* technique for quantification of pancreatic iron. Eur. Radiol. 2019, 29, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Luciani, A.; Positano, V.; De Marchi, D.; Valeri, G.; Restaino, G.; Cracolici, E.; Caruso, V.; Dell’amico, M.C.; Favilli, B.; et al. Single region of interest versus multislice T2* MRI approach for the quantification of hepatic iron overload. J. Magn. Reson. Imaging 2011, 33, 348–355. [Google Scholar] [CrossRef]
- Restaino, G.; Meloni, A.; Positano, V.; Missere, M.; Rossi, G.; Calandriello, L.; Keilberg, P.; Mattioni, O.; Maggio, A.; Lombardi, M.; et al. Regional and global pancreatic T*(2) MRI for iron overload assessment in a large cohort of healthy subjects: Normal values and correlation with age and gender. Magn. Reson. Med. 2011, 65, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Positano, V.; Pepe, A.; Rossi, G.; Dell’Amico, M.; Salvatori, C.; Keilberg, P.; Filosa, A.; Sallustio, G.; Midiri, M.; et al. Preferential patterns of myocardial iron overload by multislice multiecho T*2 CMR in thalassemia major patients. Magn. Reson. Med. 2010, 64, 211–219. [Google Scholar] [CrossRef]
- Positano, V.; Pepe, A.; Santarelli, M.F.; Scattini, B.; De Marchi, D.; Ramazzotti, A.; Forni, G.; Borgna-Pignatti, C.; Lai, M.E.; Midiri, M.; et al. Standardized T2* map of normal human heart in vivo to correct T2* segmental artefacts. NMR Biomed. 2007, 20, 578–590. [Google Scholar] [CrossRef]
- Meloni, A.; De Marchi, D.; Positano, V.; Neri, M.G.; Mangione, M.; Keilberg, P.; Lendini, M.; Cirotto, C.; Pepe, A. Accurate estimate of pancreatic T2* values: How to deal with fat infiltration. Abdom. Imaging 2015, 40, 3129–3136. [Google Scholar] [CrossRef]
- Wood, J.C.; Enriquez, C.; Ghugre, N.; Tyzka, J.M.; Carson, S.; Nelson, M.D.; Coates, T.D. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 2005, 106, 1460–1465. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002, 105, 539–542. [Google Scholar]
- He, T.; Gatehouse, P.D.; Smith, G.C.; Mohiaddin, R.H.; Pennell, D.J.; Firmin, D.N. Myocardial T2* measurements in iron-overloaded thalassemia: An in vivo study to investigate optimal methods of quantification. Magn. Reson. Med. 2008, 60, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Righi, R.; Missere, M.; Renne, S.; Schicchi, N.; Gamberini, M.R.; Cuccia, L.; Lisi, R.; Spasiano, A.; Roberti, M.G.; et al. Biventricular Reference Values by Body Surface Area, Age, and Gender in a Large Cohort of Well-Treated Thalassemia Major Patients Without Heart Damage Using a Multiparametric CMR Approach. J. Magn. Reson. Imaging 2021, 53, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Pepe, A.; Positano, V.; Capra, M.; Maggio, A.; Lo Pinto, C.; Spasiano, A.; Forni, G.; Derchi, G.; Favilli, B.; Rossi, G.; et al. Myocardial scarring by delayed enhancement cardiovascular magnetic resonance in thalassaemia major. Heart 2009, 95, 1688–1693. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, E.; Brittenham, G.M.; McLaren, C.E.; Ripalti, M.; Baronciani, D.; Giardini, C.; Galimberti, M.; Polchi, P.; Lucarelli, G. Hepatic iron concentration and total body iron stores in thalassemia major. N. Engl. J. Med. 2000, 343, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.J.; Holden, S.; Davis, B.; Prescott, E.; Charrier, C.C.; Bunce, N.H.; Firmin, D.N.; Wonke, B.; Porter, J.; Walker, J.M.; et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur. Heart J. 2001, 22, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.M.; Papadea, N.; Wentz, B.; Hollis, B.; Willi, S.; Bell, N.H. Increased serum 1,25-dihydroxyvitamin D after growth hormone administration is not parathyroid hormone-mediated. Calcif. Tissue Int. 1997, 61, 101–103. [Google Scholar] [CrossRef]
- Manolopoulos, P.P.; Lavranos, G.; Mamais, I.; Angouridis, A.; Giannakou, K.; Johnson, E.O. Vitamin D and bone health status in beta thalassemia patients—Systematic review. Osteoporos. Int. 2021, 32, 1031–1040. [Google Scholar] [CrossRef]
- Bernicke, B.; Engelbogen, N.; Klein, K.; Franzenburg, J.; Borzikowsky, C.; Peters, C.; Janssen, O.; Junker, R.; Serrano, R.; Kabelitz, D. Analysis of the Seasonal Fluctuation of γδ T Cells and Its Potential Relation with Vitamin D(3). Cells 2022, 11, 1460. [Google Scholar] [CrossRef]
- Anika, A.; Manisha, G.; Jagdish, S.; Priyanshu, M.; Khurshida, K. A comparative study of 25 hydroxy vitamin D levels in patients of thalassemia and healthy children. Pediatr. Rev. Int. J. Pediatr. Res. 2016, 3, 9. [Google Scholar] [CrossRef]
- Fahim, F.M.; Saad, K.; Askar, E.A.; Eldin, E.N.; Thabet, A.F. Growth Parameters and Vitamin D status in Children with Thalassemia Major in Upper Egypt. Int. J. Hematol. Oncol. Stem Cell Res. 2013, 7, 10–14. [Google Scholar] [PubMed]
- Santra, S.; Sharma, K.; Dash, I.; Mondal, S.; Mondal, H. Bone Mineral Density, Serum Calcium, and Vitamin D Levels in Adult Thalassemia Major Patients: Experience from a Single Center in Eastern India. Cureus 2022, 14, e26688. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, B.E.; Thomas, C.M.; Schultz, C.G. Liver density measured by DEXA correlates with serum ferritin in patients with beta-Thalassemia Major. J. Clin. Densitom. 2003, 6, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.H.; Frei, J.V.; Hodsman, A.B.; Valberg, L.S. Low serum 25-hydroxyvitamin D in hereditary hemochromatosis: Relation to iron status. Gastroenterology 1985, 88, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Dresner Pollack, R.; Rachmilewitz, E.; Blumenfeld, A.; Idelson, M.; Goldfarb, A.W. Bone mineral metabolism in adults with beta-thalassaemia major and intermedia. Br. J. Haematol. 2000, 111, 902–907. [Google Scholar] [PubMed]
- Bajoria, R.; Rekhi, E.; Almusawy, M.; Chatterjee, R. Hepatic Hemosiderosis Contributes to Abnormal Vitamin D-PTH Axis in Thalassemia Major. J. Pediatr. Hematol. Oncol. 2019, 41, e83–e89. [Google Scholar] [CrossRef]
- Darvishi-Khezri, H.; Karami, H.; Naderisorki, M.; Zahedi, M.; Razavi, A.; Kosaryan, M.; Aliasgharian, A. Moderate to severe liver siderosis and raised AST are independent risk factors for vitamin D insufficiency in β-thalassemia patients. Sci. Rep. 2020, 10, 21164. [Google Scholar] [CrossRef]
- Saadatifar, H.; Mohaghegh, H.; Ahmadi, M.; Moshkani Farahani, M. Relationship Between the Plasma Levels of Vitamin D and Magnesium and Cardiac Involvement and Iron Overload in Thalassemia Major Patients Referred to Ganjavian Hospital of Dezful in 2018. Iran. Heart J. 2021, 22, 15–24. [Google Scholar]
- Chen, S.; Glenn, D.J.; Ni, W.; Grigsby, C.L.; Olsen, K.; Nishimoto, M.; Law, C.S.; Gardner, D.G. Expression of the vitamin d receptor is increased in the hypertrophic heart. Hypertension 2008, 52, 1106–1112. [Google Scholar] [CrossRef]
- Pörsti, I.H. Expanding targets of vitamin D receptor activation: Downregulation of several RAS components in the kidney. Kidney Int. 2008, 74, 1371–1373. [Google Scholar] [CrossRef]
- Schroten, N.F.; Ruifrok, W.P.; Kleijn, L.; Dokter, M.M.; Silljé, H.H.; Lambers Heerspink, H.J.; Bakker, S.J.; Kema, I.P.; van Gilst, W.H.; van Veldhuisen, D.J.; et al. Short-term vitamin D3 supplementation lowers plasma renin activity in patients with stable chronic heart failure: An open-label, blinded end point, randomized prospective trial (VitD-CHF trial). Am. Heart J. 2013, 166, 357–364.e352. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T.; Weglicki, W.B.; Simpson, R.U. Macro- and micronutrient dyshomeostasis in the adverse structural remodelling of myocardium. Cardiovasc. Res. 2009, 81, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Tomaschitz, A.; Drechsler, C.; Dekker, J.M.; März, W. Vitamin D deficiency and myocardial diseases. Mol. Nutr. Food Res. 2010, 54, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Fanari, Z.; Hammami, S.; Hammami, M.B.; Hammami, S.; Abdellatif, A. Vitamin D deficiency plays an important role in cardiac disease and affects patient outcome: Still a myth or a fact that needs exploration? J. Saudi Heart Assoc. 2015, 27, 264–271. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.T.; Canatan, D.; Elsedfy, H.; Karimi, M.; Daar, S.; Rimawi, H.; Christou, S.; Skordis, N.; Tzoulis, P.; et al. An ICET- A survey on Hypoparathyroidism in Patients with Thalassaemia Major and Intermedia: A preliminary report. Acta Biomed. 2018, 88, 435–444. [Google Scholar] [CrossRef]
| β-TM Patients (n = 278) | |
|---|---|
| Age (years) | 39.04 ± 8.58 |
| Females, n (%) | 158 (56.8) |
| Age at start of regular transfusions (months) | 17.87 ± 16.97 |
| Chelation starting age (years) | 4.41 ± 4.69 |
| BMI (kg/m2) | 22.81 ± 3.37 |
| Mean pre-transfusion hemoglobin (g/dL) | 9.69 ± 0.50 |
| Mean ferritin (ng/L) | 1086.75 ± 1252.93 |
| Vitamin D levels (ng/dL) | 23.73 ± 10.90 |
| Parathyroid hormone (pg/mL) | 17.13 ± 7.66 |
| Alanine aminotransferase (U/L) | 30.34 ± 24.41 |
| Aspartate aminotransferase (U/L) | 28.33 ± 19.15 |
| Gamma-glutamyl transferase (U/L) | 25.81 ± 23.31 |
| MRI LIC (mg/g dw) | 7.38 ± 11.06 |
| Hepatic iron overload, n (%) | 145 (52.2) |
| Global pancreas T2* values (ms) | 10.68 ± 8.39 |
| Pancreatic iron overload, n (%) | 259 (93.2) |
| Global heart T2* values (ms) | 36.96 ± 10.11 |
| Myocardial iron overload, n (%) | 27 (9.7) |
| Number of segments with T2* < 20 ms | 1.78 ± 4.46 |
| LV EDVI (mL/m2) | 84.70 ± 17.94 |
| LV mass index (g/m2) | 60.54 ± 13.18 |
| LV EF (%) | 63.46 ± 6.90 |
| RV EDVI (mL/m2) | 84.92 ± 20.74 |
| RV EF (%) | 61.54 ± 6.96 |
| Replacement myocardial fibrosis, n (%) | 77/191 (40.3) |
| Normal Vitamin D (n = 75) | Insufficient Vitamin D (n = 96) | Deficient Vitamin D (n = 107) | p-Value | |
|---|---|---|---|---|
| Age (years) | 41.26 ± 8.19 | 39.63 ± 9.39 | 36.96 ± 7.64 | 0.001 |
| Females, n (%) | 47 (62.7) | 59 (61.5) | 52 (48.6) | 0.089 |
| BMI (kg/m2) | 22.89 ± 3.41 | 23.05 ± 3.47 | 22.54 ± 3.25 | 0.538 |
| Mean pre-transfusion hemoglobin (g/dL) | 9.71 ± 0.40 | 9.74 ± 0.43 | 9.62 ± 0.64 | 0.411 |
| Mean ferritin (ng/L) | 797.08 ± 765.10 | 1014.99 ± 788.01 | 1425.75 ± 1822.79 | 0.049 |
| Alanine aminotransferase (U/L) | 23.49 ± 13.26 | 28.17 ± 20.80 | 36.59 ± 30.64 | 0.018 |
| Aspartate aminotransferase (U/L) | 24.12 ± 12.67 | 25.31 ± 15.35 | 33.45 ± 23.79 | 0.016 |
| Gamma-glutamyl transferase (U/L) | 22.50 ± 19.46 | 20.38 ± 14.94 | 31.06 ± 28.16 | 0.055 |
| MRI LIC (mg/g dw) | 3.68 ± 4.58 | 6.10 ± 6.62 | 11.11 ± 15.51 | <0.0001 |
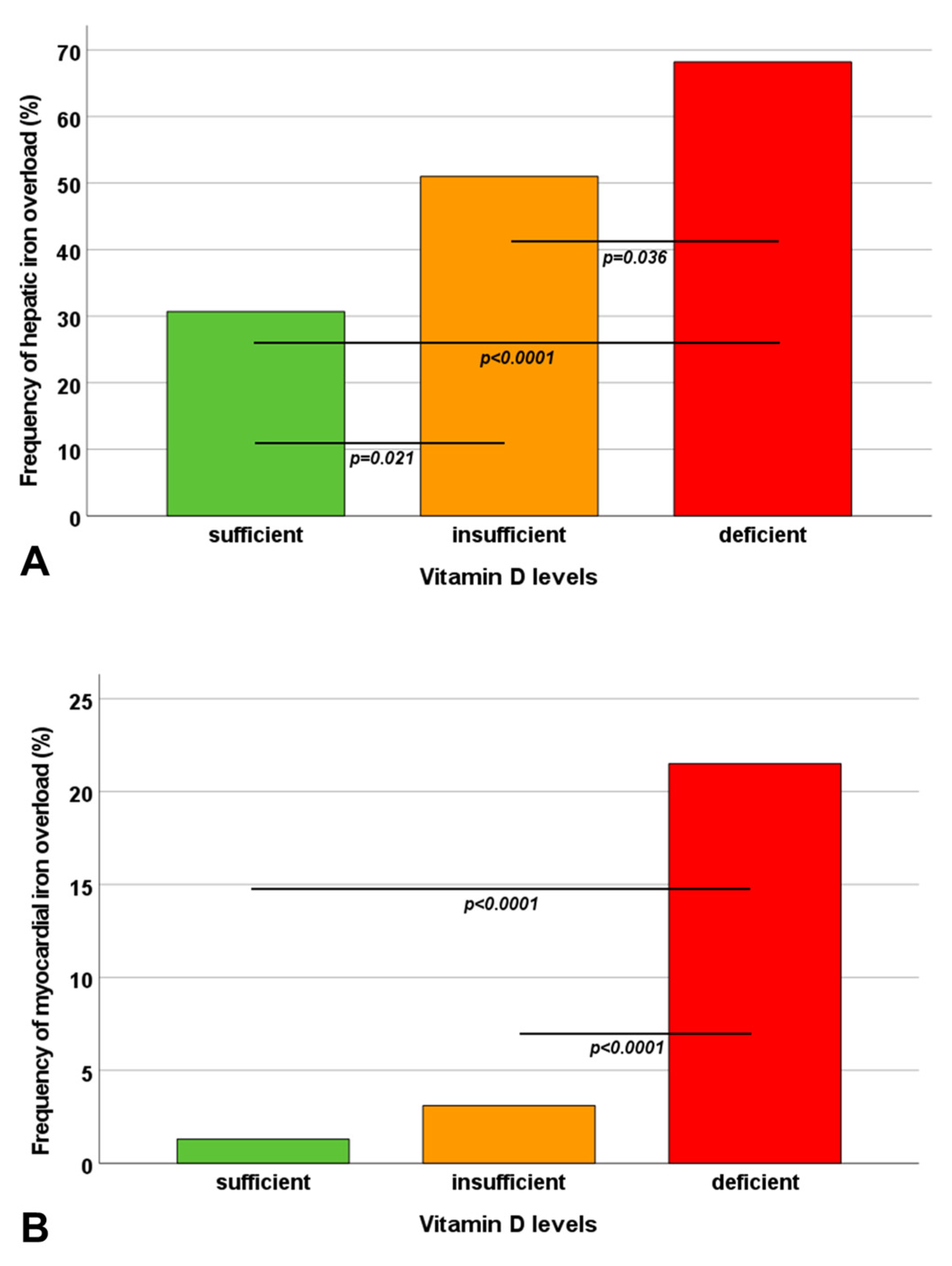
| Hepatic iron overload, n (%) | 23 (30.7) | 49 (51.0) | 73 (68.2) | <0.0001 |
| Global pancreas T2* values (ms) | 12.69 ± 7.65 | 11.03 ± 9.03 | 8.97 ± 8.01 | <0.0001 |
| Pancreatic iron overload, n (%) | 71 (94.7) | 88 (91.7) | 100 (93.5) | 0.734 |
| Global heart T2* values (ms) | 40.92 ± 5.96 | 38.25 ± 7.76 | 33.03 ± 12.66 | <0.0001 |
| Myocardial iron overload, n (%) | 1 (1.3) | 3 (3.1) | 23 (21.5) | <0.0001 |
| Number of segments with T2* < 20 ms | 0.32 ± 1.56 | 0.89 ± 2.82 | 3.59 ± 6.14 | <0.0001 |
| LV EDVI (mL/m2) | 79.17 ± 14.27 | 85.54 ± 18.32 | 87.61 ± 19.19 | 0.003 |
| LV mass index (g/m2) | 57.45 ± 12.90 | 59.84 ± 12.47 | 63.39 ± 13.54 | 0.005 |
| LV EF (%) | 64.41 ± 6.74 | 63.45 ± 6.79 | 62.77 ± 7.11 | 0.360 |
| RV EDVI (mL/m2) | 81.99 ± 21.97 | 84.18 ± 18.55 | 87.67 ± 21.60 | 0.056 |
| RV EF (%) | 61.38 ± 7.68 | 62.49 ± 6.77 | 60.79 ± 6.55 | 0.275 |
| Replacement myocardial fibrosis, n (%) | 24/49 (49.0) | 25/64 (39.1) | 28/78 (35.9) | 0.332 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| β | p-Value | β | p-Value | |
| Female sex | 0.025 | 0.679 | ||
| Age | 0.189 | 0.002 | ||
| Pre-transfusion hemoglobin | −0.141 | 0.072 | ||
| Serum ferritin | −0.431 | <0.0001 | −0.344 | <0.0001 |
| Vitamin D levels | 0.315 | <0.0001 | 0.168 | 0.018 |
| Parathyroid hormone | −0.053 | 0.387 | ||
| MRI LIC | −0.428 | <0.0001 | ||
| Global pancreas T2* | 0.316 | <0.0001 | 0.241 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meloni, A.; Pistoia, L.; Vassalle, C.; Spasiano, A.; Fotzi, I.; Bagnato, S.; Putti, M.C.; Cossu, A.; Massei, F.; Giovangrossi, P.; et al. Low Vitamin D Levels Are Associated with Increased Cardiac Iron Uptake in Beta-Thalassemia Major. Diagnostics 2023, 13, 3656. https://doi.org/10.3390/diagnostics13243656
Meloni A, Pistoia L, Vassalle C, Spasiano A, Fotzi I, Bagnato S, Putti MC, Cossu A, Massei F, Giovangrossi P, et al. Low Vitamin D Levels Are Associated with Increased Cardiac Iron Uptake in Beta-Thalassemia Major. Diagnostics. 2023; 13(24):3656. https://doi.org/10.3390/diagnostics13243656
Chicago/Turabian StyleMeloni, Antonella, Laura Pistoia, Cristina Vassalle, Anna Spasiano, Ilaria Fotzi, Sergio Bagnato, Maria Caterina Putti, Antonella Cossu, Francesco Massei, Piera Giovangrossi, and et al. 2023. "Low Vitamin D Levels Are Associated with Increased Cardiac Iron Uptake in Beta-Thalassemia Major" Diagnostics 13, no. 24: 3656. https://doi.org/10.3390/diagnostics13243656
APA StyleMeloni, A., Pistoia, L., Vassalle, C., Spasiano, A., Fotzi, I., Bagnato, S., Putti, M. C., Cossu, A., Massei, F., Giovangrossi, P., Maffei, S., Positano, V., & Cademartiri, F. (2023). Low Vitamin D Levels Are Associated with Increased Cardiac Iron Uptake in Beta-Thalassemia Major. Diagnostics, 13(24), 3656. https://doi.org/10.3390/diagnostics13243656











