R4Alz-Revised: A Tool Able to Strongly Discriminate ‘Subjective Cognitive Decline’ from Healthy Cognition and ‘Minor Neurocognitive Disorder’
Abstract
1. Introduction
2. The Purpose and the Hypotheses of the Study
- Hypothesis 1: The battery’s subtasks would not be affected by demographic variables such as age or education.
- Hypothesis 2: The extended R4Alz battery, namely, the R4Alz-Revised (R4Alz-R), would adequately differentiate adults of advanced age with SCD from healthy controls and people with MCI, as well as healthy controls from people with MCI.
3. Method
3.1. Design
3.2. Ethics
3.3. Participants
3.4. Exclusion Criteria
3.5. Inclusion Criteria
3.6. Tools
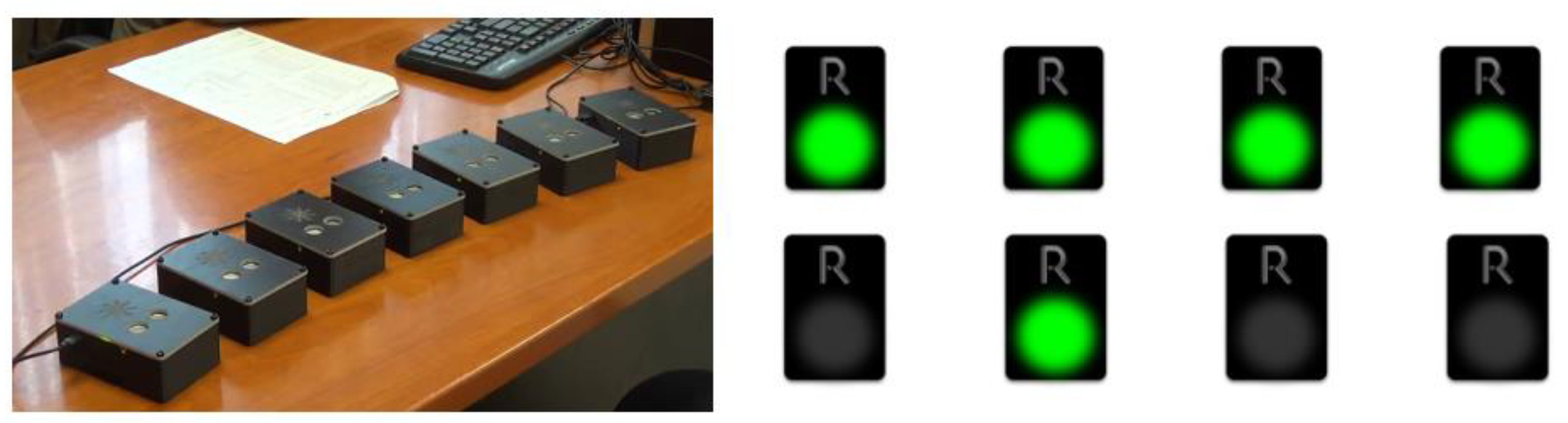
3.6.1. The R4Alz Battery via the Physical, Three-Dimensional Devices (REMEDES Pads)
3.6.2. The Procedure of Developing the New Digital-Designed and -Performed R4Alz’s-R Tasks
3.7. Description of the New Tasks
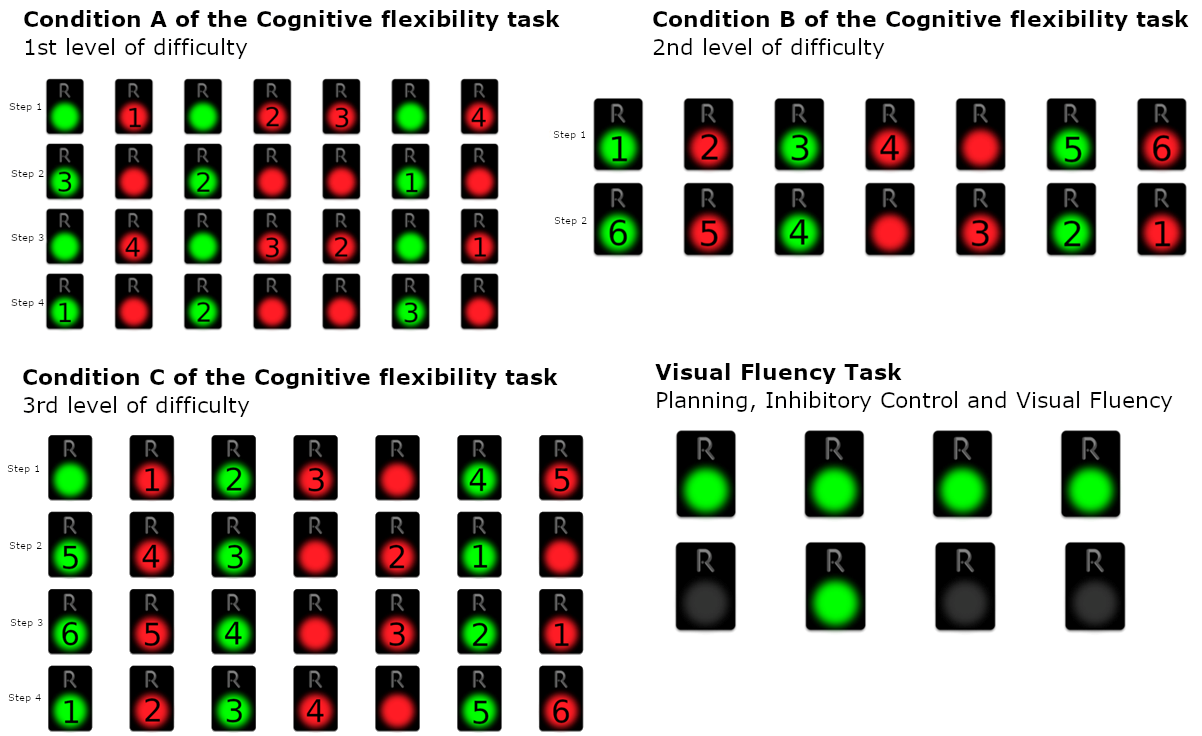
3.7.1. Cognitive Flexibility Task Part 2 (CFT2): Inhibitory Control plus Task/Rule Switching
- Step 1: All the red pads starting from the left and moving to the right
- Step 2: All the green starting from the right and moving to the left
- Step 3: All the red pads starting from the right and moving to the left
- Step 4: All the green pads starting from the left and moving to the right
- Step 1: Deactivate green and red pads from left to right, with alternating colors, skipping continuous occurrences of the same color, starting from green (green, the next red, the next green, etc.)
- Step 2: Deactivate green and red pads from right to left, with alternating colors, skipping continuous occurrences of the same color, starting from red (red, the next green, the next red, etc.)
- Step 1: Deactivate green and red pads from left to right, with alternating colors, skipping continuous occurrences of the same color, starting from red (red, the next green, the next red, etc.)
- Step 2: Deactivate green and red pads from right to left, with alternating colors, skipping continuous occurrences of the same color, starting from green (green, the next red, the next green, etc.)
- Step 3: Deactivate green and red pads from right to left, with alternating colors, skipping continuous occurrences of the same color, starting from red (red, the next green, the next red, etc.)
- Step 4: Deactivate green and red pads from left to right, with alternating colors, skipping continuous occurrences of the same color, starting from green (green, the next red, the next green, etc.)
3.7.2. Episodic Memory Task—Windows (EMT-W)
3.8. Statistical Analysis
4. Results
4.1. Mediation Analyses
- (a)
- Mediation analysis in SCD and HC groups
- (b)
- Mediation analysis in SCD and MCI groups
- (c)
- Mediation analysis in HC and MCI groups
4.2. Discriminant Validity
4.2.1. R4Alz-R Scoring
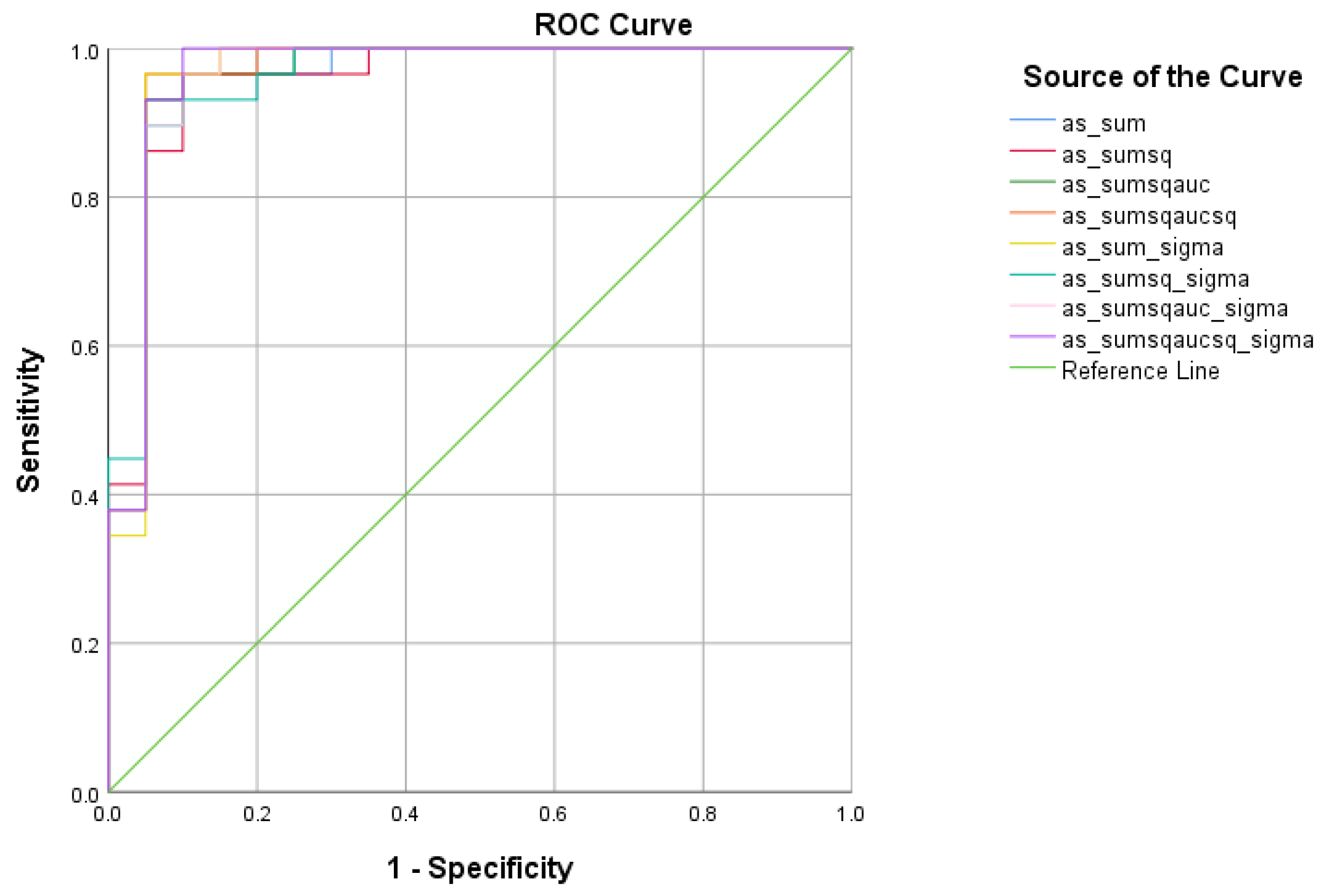
4.2.2. SCD vs. HC Analysis
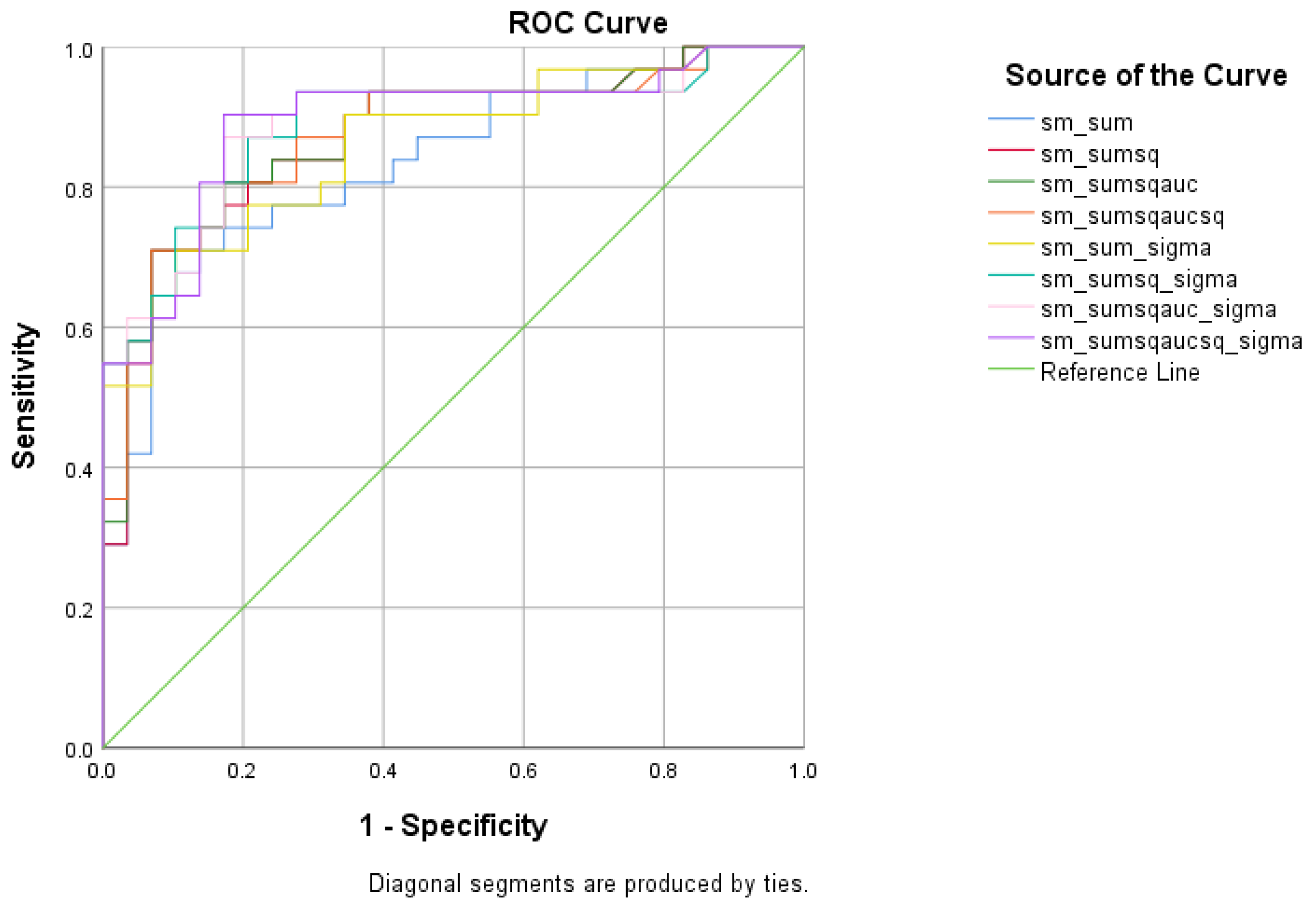
4.2.3. SCD vs. MCI Analysis
4.2.4. HC vs. MCI Analysis
5. Discussion
5.1. Age and Educational Level Effects
5.2. R4Alz-R’s Differential Capacity
5.3. Differential Capacity between HC and SCD
5.4. Differential Capacity between SCD and MCI
5.5. Differential Capacity between HC and MCI
5.6. Comments on the Total Score Creation Process
5.7. Limitations and Future Work
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reisberg, B. Dementia: A systematic approach to identifying reversible causes. Geriatrics 1986, 41, 30–46. [Google Scholar]
- Rostamzadeh, A.; Bohr, L.; Wagner, M.; Baethge, C.; Jessen, F. Progression of Subjective Cognitive Decline to MCI or Dementia in Relation to Biomarkers for Alzheimer Disease: A Meta-Analysis. Neurology 2022, 99, e1866–e1874. [Google Scholar] [CrossRef]
- Jessen, F.; Amariglio, R.E.; van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G. Subjective Cognitive Decline Initiative (SCD-I) Working Group. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sheng, X.; Luo, C.; Qin, R.; Ye, Q.; Zhao, H.; Xu, Y.; Bai, F. The compensatory phenomenon of the functional connectome related to pathological biomarkers in individuals with subjective cognitive decline. Transl. Neurodegener. 2020, 9, 21. [Google Scholar] [CrossRef]
- Petrazzuoli, F.; Vestberg, S.; Midlöv, P.; Thulesius, H.; Stomrud, E.; Palmqvist, S. Brief Cognitive Tests Used in Primary Care Cannot Accurately Differentiate Mild Cognitive Impairment from Subjective Cognitive Decline. J. Alzheimer’s Dis. 2020, 75, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Tsentidou, G.; Moraitou, D.; Tsolaki, M. Cognition in Vascular Aging and Mild Cognitive Impairment. J. Alzheimer’s Dis. 2019, 72, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Lazarou, I.; Moraitou, D.; Papatheodorou, M.; Vavouras, I.; Lokantidou, C.; Agogiatou, C.; Gialaoutzis, M.; Nikolopoulos, S.; Stavropoulos, T.G.; Kompatsiaris, I.; et al. Adaptation and Validation of the Memory Alteration Test (M@T) in Greek Middle-Aged, Older, and Older-Old Population with Subjective Cognitive Decline and Mild Cognitive Impairment. J. Alzheimer’s Dis. 2021, 84, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Jessen, F.; Amariglio, R.E.; Buckley, R.F.; van der Flier, W.M.; Han, Y.; Molinuevo, J.L.; Wagner, M. The char-acterisation of subjective cognitive decline. Lancet Neurol. 2020, 19, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Poptsi, E.; Moraitou, D.; Tsardoulias, E.; Symeonidisd, A.L.; Tsolaki, M. Is the Discrimination of Subjective Cognitive Decline from Cognitively Healthy Adulthood and Mild Cognitive Impairment Possible? A Pilot Study Utilizing the R4Alz Battery. J. Alzheimer’s Dis. 2020, 77, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Poptsi, E.; Tsardoulias, E.; Moraitou, D.; Symeonidis, A.L.; Tsolaki, M. REMEDES for Alzheimer-R4Alz Battery: Design and Development of a New Tool of Cognitive Control Assessment for the Diagnosis of Minor and Major Neurocognitive Disorders. J. Alzheimer’s Dis. 2019, 72, 783–801. [Google Scholar] [CrossRef]
- Wolfsgruber, S.; Kleineidam, L.; Guski, J.; Polcher, A.; Frommann, I.; Roeske, S.; Spruth, E.J.; Franke, C.; Priller, J.; Kilimann, I.; et al. Minor neuropsychological deficits in patients with subjective cognitive decline. Neurology 2020, 95, e1134–e1143. [Google Scholar] [CrossRef] [PubMed]
- Huff, M.J.; Balota, D.A.; Aschenbrenner, A.J.; Duchek, J.M.; Fagan, A.M.; Holtzman, D.M.; Morris, J.C. P2-085: Task-switching errors show sensitivity to preclinical Alzheimer’s disease biomarkers. Alzheimer’s Dement 2015, 11, P516. [Google Scholar] [CrossRef]
- López-Higes, R.; Prados, J.M.; Rubio, S.; Montejo, P.; Del Río, D. Executive functions and linguistic performance in SCD older adults and healthy controls. Aging Neuropsychol. Cogn. 2017, 24, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Irish, M.; Hornberger, M. Episodic memory in neurodegenerative disorders: Past, present, and future. In Wiley Handbook on the Cognitive Neuroscience of Memory; Addis, D.R., Barense, M., Duarte, A., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2015; pp. 415–433. [Google Scholar]
- Raz, N. Ageing of the brain and its impact on cognitive performance: Integration of structural and functional findings. In Handbook of Ageing and Cognition, 2nd ed.; Craik, F.I.M., Salthouse, T.A., Eds.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 2000. [Google Scholar]
- Clarys, D.; Bugaiska, A.; Tapia, G.; Alexia Baudouin, A. Ageing, remembering, and executive func-tion. Memory 2009, 17, 158–168. [Google Scholar] [CrossRef]
- Yuan, B.; Chen, J.; Gong, L.; Shu, H.; Liao, W.; Wang, Z.; Liu, D.; Xie, C.; Zhang, Z. Mediation of episodic memory performance by the executive function network in patients with amnestic mild cognitive impairment: A resting-state functional MRI study. Oncotarget 2016, 7, 64711–64725. [Google Scholar] [CrossRef]
- Schacter, D.L.; Tulving, E. (Eds.) What are the memory systems of 1994? In Memory Systems; MIT Press: Cambridge, MA, USA, 1994; pp. 1–38. [Google Scholar]
- Tulving, E. What Is Epi Miyake sodic Memory? Curr. Dir. Psychol. Sci. 1993, 2, 67–70. [Google Scholar] [CrossRef]
- Tromp, D.; Dufour, A.; Lithfous, S.; Pebayle, T.; Després, O. Episodic memory in normal aging and Alzheimer disease: Insights from imaging and behavioral studies. Ageing Res. Rev. 2015, 24, 232–262. [Google Scholar] [CrossRef]
- West, R.L. An application of prefrontal cortex function theory to cognitive aging. Psychol. Bull. 1996, 120, 272–292. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef]
- Irish, M.; Lawlor, B.A.; Coen, R.F.; O’Mara, S.M. Everyday episodic memory in amnestic mild cognitive impairment: A preliminary investigation. BMC Neurosci. 2011, 12, 80. [Google Scholar] [CrossRef]
- Quaranta, D.; Gainotti, G.; Di Giuda, D.; Vita, M.G.; Cocciolillo, F.; Lacidogna, G.; Guglielmi, V.; Masullo, C.; Giordano, A.; Marra, C. Predicting progression of amnesic MCI: The integration of episodic memory impairment with perfusion SPECT. Psychiatry Res. Neuroimaging 2018, 271, 43–49. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1983, 17, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Fountoulakis, K.N.; Tsolaki, M.; Iacovides, A.; Yesavage, J.; O’Hara, R.; Kazis, A.; Ierodiakonou, C. The validation of the short form of the Geriatric Depression Scale (GDS) in Greece. Aging Clin. Exp. Res. 1999, 11, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Ward, C.; Mendelson, M.; Mock, J.; Erbaugh, J. Beck depression inventory (BDI). Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Sinoff, G.; Ore, L.; Zlotogorsky, D.; Tamir, A. Short anxiety screening test—A brief instrument for detecting anxiety in the elderly. Int. J. Geriatr. Psychiatry 1999, 14, 1062–1071. [Google Scholar] [CrossRef]
- Grammatikopoulos, I.A.; Sinoff, G.; Alegakis, A.; Kounalakis, D.; Antonopoulou, M.; Lionis, C. The Short Anxiety Screening Test in Greek: Translation and validation. Ann. Gen. Psychiatry 2010, 9, 1–8. [Google Scholar] [CrossRef]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893. [Google Scholar] [CrossRef]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.A.; Gornbein, J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994, 44, 2308. [Google Scholar] [CrossRef]
- Politis, A.M.; Mayer, L.S.; Passa, M.; Maillis, A.; Lyketsos, C.G. Validity and reliability of the newly translated Hellenic Neuropsychiatric Inventory (H-NPI) applied to Greek outpatients with Alzheimer’s disease: A study of disturbing behaviors among referrals to a memory clinic. Int. J. Geriatr. Psychiatry 2004, 19, 203–208. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Fountoulakis, K.N.; Tsolaki, M.; Chantzi, H.; Kazis, A. Mini Mental State Examination (MMSE): A validation study in Greece. Am. J. Alzheimer’s Dis. Other Dementias 2000, 15, 342–345. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Poptsi, E.; Moraitou, D.; Eleftheriou, M.; Kounti-Zafeiropoulou, F.; Papasozomenou, C.; Agogiatou, C.; Bakoglidou, E.; Batsila, G.; Liapi, D.; Markou, N.; et al. Normative Data for the Montreal Cognitive Assessment in Greek Older Adults with Subjective Cognitive Decline, Mild Cognitive Impairment and Dementia. J. Geriatr. Psychiatry Neurol. 2019, 32, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Kounti, F.; Tsolaki, M.; Kiosseoglou, G. Functional cognitive assessment scale (FUCAS): A new scale to assess executive cognitive function in daily life activities in patients with dementia and mild cognitive impairment. Hum. Psychopharmacol. Clin. Exp. 2006, 21, 305–311. [Google Scholar] [CrossRef]
- Reisberg, B.; Ferris, S.H.; de Leon, M.J.; Crook, T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar]
- Rami, L.; Mollica, M.A.; García-Sanchez, C.; Saldaña, J.; Sanchez, B.; Sala, I.; Valls-Pedret, C.; Castellví, M.; Olives, J.; Molinuevo, J.L. The Subjective Cognitive Decline Questionnaire (SCD-Q): A Validation Study. J. Alzheimer’s Dis. 2014, 41, 453–466. [Google Scholar] [CrossRef]
- Tsolaki, M.; Poptsi, E.; Aggogiatou, C.; Markou, N.; Zafeiropoulos, S.; Kounti, F. Computer-Based Cognitive Training Versus Paper and Pencil Training: Which is more effective? A Randomized Controlled Trial in People with Mild Cognitive Impairment. JSM Alzheimers Disord. Relat. Dement. 2017, 4, 1032. [Google Scholar]
- Baddeley, A.D.; Emslie, H.; Nimmo-Smith, I. Doors and People: A Test of Visual and Verbal Recall and Recognition; Harcourt Assessment: San Antonio, TX, USA, 2006. [Google Scholar]
- Delis, D.C.; Kaplan, E.; Kramer, J.H. Delis-Kaplan Executive Function System®(D-KEFS®): Examiner’s Manual: Flexibility of Thinking, Concept Formation, Problem Solving, Planning, Creativity, Impluse Control, Inhibition; Pearson: London, UK, 2001. [Google Scholar]
- Armstrong, R.A.; Hilton, A. Post hoc ANOVA tests. Microbiologist 2006, 6, 34–36. [Google Scholar]
- Jeffreys, H. Theory of Probability, 3rd ed.; MR0187257; Oxford University Press: Oxford, UK, 1961; p. 432. [Google Scholar]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; Sage: Thousand Oaks, CA, USA, 2013. [Google Scholar]
- Preacher, K.J.; Hayes, A.F. Asymptotic and Resampling Strategies for Assessing and Comparing Indirect Effects in Multiple Mediator Models. Behav. Res. Methods 2008, 40, 879–891. [Google Scholar] [CrossRef]
- Biesanz, J.C.; Falk, C.F.; Savalei, V. Assessing mediational models: Testing and interval estimation for indirect effects. Multivar. Behav. Res. 2010, 45, 661–701. [Google Scholar] [CrossRef]
- Spiro, A.; Brady, C. Integrating Health into Cognitive Aging: Toward a Preventive Cognitive Neuroscience of Aging. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2011, 66B, i17–i25. [Google Scholar] [CrossRef] [PubMed]
- Youden, W. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zalonis, I.; Kararizou, E.; Triantafyllou, N.; Kapaki, E.; Papageorgiou, S.; Sgouropoulos, P.; Vassilopoulos, D. A Normative Study of the Trail Making Test A and B in Greek Adults. Clin. Neuropsychol. 2008, 22, 842–850. [Google Scholar] [CrossRef]
- Tombaugh, T.N. Trail Making Test A and B: Normative data stratified by age and education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef]
- Zalonis, I.; Christidi, F.; Bonakis, A.; Kararizou, E.; Triantafyllou, N.I.; Paraskevas, G.; Kapaki, E.; Vasilopoulos, D. The Stroop Effect in Greek Healthy Population: Normative Data for the Stroop Neuropsychological Screening Test. Arch. Clin. Neuropsychol. 2009, 24, 81–88. [Google Scholar] [CrossRef]
- Van der Elst, W.; Van Boxtel, M.P.; Van Breukelen, G.J.; Jolles, J. The Stroop color-word test: Influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment 2006, 13, 62–79. [Google Scholar] [CrossRef]
- Molinuevo, J.L.; Rabin, L.A.; Amariglio, R.; Buckley, R.; Dubois, B.; Ellis, K.A.; Ewers, M.; Hampel, H.; Klöppel, S.; Rami, L.; et al. Implementation of subjective cognitive decline criteria in research studies. Alzheimer’s Dement. 2016, 13, 296–311. [Google Scholar] [CrossRef]
- Abeare, C.; Sabelli, A.; Taylor, B.; Holcomb, M.; Dumitrescu, C.; Kirsch, N.; Erdodi, L. The importance of demo-graphically adjusted cutoffs: Age and education bias in raw score cutoffs within the Trail Making Test. Psychol. Inj. Law 2019, 12, 170–182. [Google Scholar] [CrossRef]
- Salthouse, T.A. Aging and measures of processing speed. Biol. Psychol. 2000, 54, 35–54. [Google Scholar] [CrossRef]
- Wecker, N.S.; Kramer, J.H.; Hallam, B.J.; Delis, D.C. Mental Flexibility: Age Effects on Switching. Neuropsychology 2005, 19, 345–352. [Google Scholar] [CrossRef]
- Albinet, C.; Boucard, G.; Bouquet, C.; Audiffren, M. Processing speed and executive functions in cognitive aging: How to disentangle their mutual relationship? Brain Cogn. 2012, 79, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Manard, M.; Carabin, D.; Jaspar, M.; Collette, F. Age-related decline in cognitive control: The role of fluid intelligence and processing speed. BMC Neurosci. 2014, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Joanette, Y.; Monchi, O. The implications of age-related neurofunctional compensatory mechanisms in executive function and language processing including the new Temporal Hypothesis for Compensation. Front. Hum. Neurosci. 2015, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Fristoe, N.M.; Salthouse, T.A.; Woodard, J.L. Examination of age-related deficits on the Wisconsin Card Sorting Test. Neuropsychology 1997, 11, 428. [Google Scholar] [CrossRef]
- Parkin, A.J.; Java, R.I. Deterioration of frontal lobe function in normal aging: Influences of fluid intelligence versus perceptual speed. Neuropsychology 1999, 13, 539. [Google Scholar] [CrossRef]
- Kuhn, E.; Moulinet, I.; Perrotin, A.; La Joie, R.; Landeau, B.; Tomadesso, C.; Chételat, G. Cross-sectional and longitudinal characterization of SCD patients recruited from the community versus from a memory clinic: Subjective cognitive decline, psychoaffective factors, cognitive performances, and atrophy progression over time. Alzheimer’s Res. Ther. 2019, 11, 1–16. [Google Scholar] [CrossRef]
- Libon, D.J.; Xie, S.X.; Eppig, J.; Wicas, G.; Lamar, M.; Lippa, C.; Bettcher, B.M.; Price, C.C.; Giovannetti, T.; Swenson, R.; et al. The heterogeneity of mild cognitive impairment: A neuropsychological analysis. J. Int. Neuropsychol. Soc. 2009, 16, 84–93. [Google Scholar] [CrossRef]
- Pantsiou, K.; Sfakianaki, O.; Papaliagkas, V.; Savvoulidou, D.; Costa, V.; Papantoniou, G.; Moraitou, D. Inhibitory Control, Task/Rule Switching, and Cognitive Planning in Vascular Dementia: Are There Any Differences from Vascular Aging? Front. Aging Neurosci. 2018, 10, 330. [Google Scholar] [CrossRef]
- Mulligan, B.P.; Smart, C.M.; Ali, J.I. Relationship of subjective and objective performance indicators in subjective cognitive decline. Psychol. Neurosci. 2016, 9, 362–378. [Google Scholar] [CrossRef]
- Mattsson, N.; Insel, P.S.; Nosheny, R.; Tosun, D.; Trojanowski, J.Q.; Shaw, L.M.; Jack, C.R.; Donohue, M.C.; Weiner, M.W.; Alzheimer’s Disease Neuroimaging Initiative. Emerging β-amyloid pathology and accelerated cortical atrophy. JAMA Neurol. 2014, 71, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Mormino, E.C.; Madison, C.; Hayenga, A.; Smiljic, A.; Jagust, W.J. Amyloid affects frontal and posterior brain networks in normal aging. Neuroimage 2011, 54, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Vlassenko, A.G.; Mintun, M.A.; Xiong, C.; Sheline, Y.I.; Goate, A.M.; Benzinger, T.L.S.; Morris, J.C. Amyloid-beta plaque growth in cognitively normal adults: Longitudinal [11C] Pittsburgh compound B data. Ann. Neurol. 2011, 70, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M.G.; Chiang, J.; Pogoda, J.M.; Gomez, M.; Thomas, K.; Marion, S.D.; Miller, K.J.; Siddarth, P.; Yi, X.; Zhou, F.; et al. Executive function changes before memory in preclinical Alzheimer’s pathology: A prospective, cross-sectional, case control study. PLoS ONE 2013, 8, e79378. [Google Scholar] [CrossRef]
- Ohlhauser, L.; Parker, A.F.; Smart, C.M.; Gawryluk, J.R.; Alzheimer’s Disease Neuroimaging Initiative. White matter and its relationship with cognition in subjective cognitive decline. Alzheimer’s Dementia Diagn. Assess. Dis. Monit. 2018, 11, 28–35. [Google Scholar] [CrossRef]
- Valech, N.; Tort-Merino, A.; Coll-Padrós, N.; Olives, J.; León, M.; Rami, L.; Molinuevo, J.L. Executive and Language Subjective Cognitive Decline Complaints Discriminate Preclinical Alzheimer’s Disease from Normal Aging. J. Alzheimer’s Dis. 2017, 61, 689–703. [Google Scholar] [CrossRef]
- Ibnidris, A.; Robinson, J.N.; Stubbs, M.; Piumatti, G.; Govia, I.; Albanese, E. Evaluating measurement properties of subjective cognitive decline self-reported outcome measures: A systematic review. Syst. Rev. 2022, 11, 1–17. [Google Scholar] [CrossRef]
- Smart, C.M.; Krawitz, A. The impact of subjective cognitive decline on Iowa Gambling Task perfor-mance. Neuropsychology 2015, 29, 971–987. [Google Scholar] [CrossRef]
- Campos-Magdaleno, M.; Leiva, D.; Pereiro, A.X.; Lojo-Seoane, C.; Mallo, S.C.; Facal, D.; Juncos-Rabadán, O. Changes in visual memory in mild cognitive impairment: A longitudinal study with CANTAB. Psychol. Med. 2020, 51, 2465–2475. [Google Scholar] [CrossRef]
- Viviano, R.P.; Damoiseaux, J.S. Longitudinal change in hippocampal and dorsal anterior insulae functional con-nectivity in subjective cognitive decline. Alzheimer’s Res. Ther. 2021, 13, 1–15. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, K.; Zhao, Q.; Li, F.; Guo, Q. Short-term delayed recall of auditory verbal learning test provides equiv-alent value to long-term delayed recall in predicting MCI clinical outcomes: A longitudinal follow-up study. Appl. Neuropsychol. Adult 2018, 27, 73–81. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The Diagnosis of Mild Cognitive Impairment due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Focus 2013, 11, 96–106. [Google Scholar] [CrossRef]
- Gonçalves, A.P.B.; Tarrasconi, M.A.; Holz, M.R.; Kochhann, R.; Fonseca, R.P. Cognitive flexibility and inhibition in single-versus multiple-domain mild cognitive impairment: A comparative and discriminative analysis. Psychol. Neurosci. 2019, 12, 209–223. [Google Scholar] [CrossRef]
- Guarino, A.; Forte, G.; Giovannoli, J.; Casagrande, M. Executive functions in the elderly with mild cognitive im-pairment: A systematic review on motor and cognitive inhibition, conflict control and cognitive flexibility. Aging Ment. Health 2020, 24, 1028–1045. [Google Scholar] [CrossRef] [PubMed]
- Klekociuk, S.Z.; Summers, M.J. Exploring the validity of mild cognitive impairment (MCI) subtypes: Multiple-domain amnestic MCI is the only identifiable subtype at longitudinal follow-up. J. Clin. Exp. Neuropsychol. 2014, 36, 290–301. [Google Scholar] [CrossRef]
- Junquera, A.; García-Zamora, E.; Olazarán, J.; Parra, M.A.; Fernández-Guinea, S. Role of Executive Functions in the Conversion from Mild Cognitive Impairment to Dementia. J. Alzheimer’s Dis. 2020, 77, 641–653. [Google Scholar] [CrossRef]
- Tsatali, M.; Fotiadou, F.; Giaglis, G.; Tsolaki, M. The repeatable battery for the assessment of the neuropsychological status (RBANS): A diagnostic validity study in Greek elderly. Aging Clin. Exp. Res. 2018, 31, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Sun, K.; Phi, H.Q.; Ko, B.; Parsey, C.M.; Huang, B.; Ghomi, R.H. Validation of a Computerized Cognitive Test Battery for Detection of Dementia and Mild Cognitive Impairment. medRxiv 2021. [Google Scholar] [CrossRef]

| Diagnostic Groups | ||||
|---|---|---|---|---|
| Characteristics | HC (n =20) | SCD (n = 29) | MCI (n = 31) | p |
| Age M (SD) | 61.60 (6.58) | 61.17 (6.58) | 68.67 (8.43) | <0.05 |
| Gender (Male/Female) | 6 M/14 F | 9 M/20 F | 7 M/24 F | >0.05 |
| Education M (SD) | 16.30 (3.04) | 13.31 (4.20) | 13.45 (4.31) | <0.05 |
| MoCA M (SD) | 28.29 (1.35) | 27.17 (3.60) | 25.14 (1.88) | <0.05 |
| (a) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Direct Effects | 95% Confidence Interval | |||||||||
| b | SE | z-Value | p | Lower | Upper | |||||
| diagnosis | → | WMCUT S 1 | 0.039 | 0.299 | 0.131 | 0.896 | −0.545 | 0.771 | ||
| diagnosis | → | WMCUT S 2 | −0.682 | 0.286 | −2.383 | 0.017 | −1.310 | −0.028 | ||
| diagnosis | → | WMCUT S 3 | −1.999 | 0.665 | −3.005 | 0.003 * | −3.025 | −1.123 | ||
| diagnosis | → | ACT | 4.242 | 1.726 | 2.459 | 0.014 | 1.333 | 7.329 | ||
| diagnosis | → | ICT−RST 1 & 2 | 7.270 | 2.197 | 3.309 | <0.001 * | 3.626 | 10.540 | ||
| diagnosis | → | ICT−RST 1 & 2 SE | 0.437 | 0.200 | 2.178 | 0.029 | 0.057 | 0.772 | ||
| diagnosis | → | ICT−RST 1 & 2 FS | 1.188 | 0.398 | 2.983 | 0.003 * | 0.390 | 1.851 | ||
| diagnosis | → | CFT | 3.735 | 0.849 | 4.398 | <0.001 * | 1.761 | 5.522 | ||
| diagnosis | → | CFT Con. a | 0.371 | 0.516 | 0.718 | 0.473 | −0.591 | 1.094 | ||
| diagnosis | → | CFT Con. b | 1.758 | 0.559 | 3.145 | 0.002 * | 0.610 | 2.876 | ||
| diagnosis | → | CFT Con. c | 1.262 | 0.644 | 1.961 | 0.050 | −0.176 | 2.681 | ||
| diagnosis | → | VFT | −1.653 | 0.624 | −2.649 | 0.008 | −3.052 | −0.551 | ||
| diagnosis | → | EMT−W Con. a | 0.838 | 0.800 | 1.048 | 0.294 | −0.868 | 2.509 | ||
| diagnosis | → | EMT−W Con. b | 1.203 | 0.598 | 2.014 | 0.044 | −0.114 | 2.557 | ||
| (b) | ||||||||||
| Indirect Effects | 95% Confidence Interval | |||||||||
| b | SE | z-Value | p | Lower | Upper | |||||
| diagnosis | → | Age | → | WMCUT S 1 | −0.402 | 0.177 | −2.269 | 0.023 | −0.869 | −0.177 |
| diagnosis | → | Education | → | WMCUT S 1 | −0.053 | 0.107 | −0.498 | 0.619 | −0.369 | 0.083 |
| diagnosis | → | Age | → | WMCUT S 2 | −0.297 | 0.147 | −2.028 | 0.043 | −0.641 | −0.095 |
| diagnosis | → | Education | → | WMCUT S 2 | −0.172 | 0.118 | −1.459 | 0.144 | −0.588 | −0.007 |
| diagnosis | → | Age | → | WMCUT S 3 | −0.268 | 0.258 | −1.037 | 0.300 | −0.880 | 0.034 |
| diagnosis | → | Education | → | WMCUT S 3 | −0.028 | 0.234 | −0.121 | 0.904 | −0.650 | 0.439 |
| diagnosis | → | Age | → | ACT | 1.240 | 0.759 | 1.633 | 0.102 | −0.035 | 3.383 |
| diagnosis | → | Education | → | ACT | 0.274 | 0.614 | 0.447 | 0.655 | −1.108 | 1.627 |
| diagnosis | → | Age | → | ICT−RST 1 & 2 | 0.906 | 0.855 | 1.060 | 0.289 | −0.427 | 4.029 |
| diagnosis | → | Education | → | ICT−RST 1 & 2 | 1.816 | 1.011 | 1.797 | 0.072 | 0.330 | 4.619 |
| diagnosis | → | Age | → | ICT−RST 1 & 2 SE | 0.022 | 0.073 | 0.298 | 0.766 | −0.139 | 0.248 |
| diagnosis | → | Education | → | ICT−RST 1 & 2 SE | 0.200 | 0.101 | 1.990 | 0.047 | 0.047 | 0.489 |
| diagnosis | → | Age | → | ICT−RST 1 & 2 FS | 0.125 | 0.150 | 0.828 | 0.408 | −0.137 | 0.585 |
| diagnosis | → | Education | → | ICT−RST 1 & 2 FS | 0.275 | 0.171 | 1.607 | 0.108 | 0.066 | 0.705 |
| diagnosis | → | Age | → | CFT | 0.583 | 0.368 | 1.582 | 0.114 | −0.067 | 1.718 |
| diagnosis | → | Education | → | CFT | 0.268 | 0.313 | 0.855 | 0.393 | −0.129 | 1.244 |
| diagnosis | → | Age | → | CFT2 Con. a | 0.106 | 0.190 | 0.555 | 0.579 | −0.227 | 0.537 |
| diagnosis | → | Education | → | CFT2 Con. a | 0.341 | 0.219 | 1.558 | 0.119 | 0.008 | 1.069 |
| diagnosis | → | Age | → | CFT2 Con. b | 0.128 | 0.207 | 0.621 | 0.535 | −0.226 | 0.530 |
| diagnosis | → | Education | → | CFT2 Con. b | 0.364 | 0.236 | 1.543 | 0.123 | 0.021 | 1.044 |
| diagnosis | → | Age | → | CFT2 Con. c | 0.418 | 0.275 | 1.523 | 0.128 | −0.031 | 1.228 |
| diagnosis | → | Education | → | CFT2 Con. c | 0.418 | 0.271 | 1.539 | 0.124 | 0.035 | 1.115 |
| diagnosis | → | Age | → | VFT | −0.573 | 0.302 | −1.901 | 0.057 | −1.376 | −0.051 |
| diagnosis | → | Education | → | VFT | −0.240 | 0.235 | −1.018 | 0.309 | −1.088 | 0.126 |
| diagnosis | → | Age | → | EMT−W Con. a | 0.228 | 0.300 | 0.760 | 0.447 | −0.203 | 0.931 |
| diagnosis | → | Education | → | EMT−W Con. a | 0.186 | 0.289 | 0.643 | 0.520 | −0.189 | 1.115 |
| diagnosis | → | Age | → | EMT−W Con. b | 0.350 | 0.248 | 1.409 | 0.159 | −0.098 | 1.012 |
| diagnosis | → | Education | → | EMT−W Con. b | 0.188 | 0.220 | 0.855 | 0.393 | −0.245 | 0.622 |
| (a) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Direct Effects | 95% Confidence Interval | |||||||||
| b | SE | z-Value | p | Lower | Upper | |||||
| diagnosis | → | WMCUT S 1 | −0.253 | 0.204 | −1.236 | 0.217 | −0.698 | 0.164 | ||
| diagnosis | → | WMCUT S 2 | −0.483 | 0.211 | −2.292 | 0.022 | −0.932 | −0.061 | ||
| diagnosis | → | WMCUT S 3 | −1.040 | 0.655 | −1.587 | 0.112 | −2.288 | 0.415 | ||
| diagnosis | → | ACT | 5.439 | 1.950 | 2.789 | 0.005 | 1.813 | 9.321 | ||
| diagnosis | → | ICT-RST 1 & 2 | 9.710 | 2.690 | 3.610 | <0.001 * | 4.057 | 14.934 | ||
| diagnosis | → | ICT-RST 1 & 2 SE | 1.153 | 0.257 | 4.485 | <0.001 * | 0.678 | 1.671 | ||
| diagnosis | → | ICT-RST 1 & 2 FS | 1.078 | 0.406 | 2.655 | 0.008 | 0.219 | 1.848 | ||
| diagnosis | → | CFT | 2.099 | 0.794 | 2.643 | 0.008 | 0.588 | 3.757 | ||
| diagnosis | → | CFT2 Con. a | 1.597 | 0.498 | 3.204 | 0.001 * | 0.551 | 2.546 | ||
| diagnosis | → | CFT2 Con. b | 1.066 | 0.467 | 2.284 | 0.022 | −0.041 | 2.007 | ||
| diagnosis | → | CFT2 Con. c | 0.561 | 0.446 | 1.257 | 0.209 | −0.374 | 1.471 | ||
| diagnosis | → | VFT | −0.945 | 0.458 | −2.064 | 0.039 | −1.883 | −0.017 | ||
| diagnosis | → | EMT-W Con. a | 2.374 | 0.558 | 4.257 | <0.001 * | 1.222 | 3.419 | ||
| diagnosis | → | EMT-W Con. b | 0.564 | 0.519 | 1.087 | 0.277 | −0.507 | 1.496 | ||
| (b) | ||||||||||
| 95% Confidence Interval | ||||||||||
| Indirect Effects | b | SE | z-Value | P | Lower | Upper | ||||
| diagnosis | → | Age | → | WMCUT S 1 | −0.072 | 0.097 | −0.745 | 0.456 | −0.301 | 0.124 |
| diagnosis | → | Education | → | WMCUT S 1 | 2.377 | 0.004 | 0.061 | 0.952 | −0.044 | 0.072 |
| diagnosis | → | Age | → | WMCUT S 2 | −0.063 | 0.085 | −0.739 | 0.460 | −0.259 | 0.103 |
| diagnosis | → | Education | → | WMCUT S 2 | 0.001 | 0.009 | 0.120 | 0.905 | −0.051 | 0.081 |
| diagnosis | → | Age | → | WMCUT S 3 | −0.174 | 0.238 | −0.733 | 0.463 | −0.816 | 0.245 |
| diagnosis | → | Education | → | WMCUT S 3 | 0.011 | 0.085 | 0.129 | 0.897 | −0.193 | 0.337 |
| diagnosis | → | Age | → | ACT | 0.645 | 0.868 | 0.743 | 0.457 | −1.066 | 2.767 |
| diagnosis | → | Education | → | ACT | −0.033 | 0.257 | −0.130 | 0.897 | −1.153 | 0.517 |
| diagnosis | → | Age | → | ICT−RST 1 & 2 | 0.664 | 0.912 | 0.729 | 0.466 | −1.081 | 3.191 |
| diagnosis | → | Education | → | ICT−RST 1 & 2 | −0.067 | 0.515 | −0.130 | 0.896 | −1.757 | 1.132 |
| diagnosis | → | Age | → | ICT−RST 1 & 2 SE | 0.041 | 0.059 | 0.689 | 0.491 | −0.061 | 0.324 |
| diagnosis | → | Education | → | ICT−RST 1 & 2 SE | −0.017 | 0.133 | −0.131 | 0.896 | −0.304 | 0.274 |
| diagnosis | → | Age | → | ICT−RST 1 & 2 FS | 0.090 | 0.124 | 0.721 | 0.471 | −0.128 | 0.511 |
| diagnosis | → | Education | → | ICT−RST 1 & 2 FS | −0.016 | 0.121 | −0.130 | 0.896 | −0.273 | 0.271 |
| diagnosis | → | Age | → | CFT | 0.269 | 0.362 | 0.744 | 0.457 | −0.490 | 1.101 |
| diagnosis | → | Education | → | CFT | −0.019 | 0.145 | −0.130 | 0.897 | −0.546 | 0.280 |
| diagnosis | → | Age | → | CFT2 Con. a | 0.035 | 0.067 | 0.520 | 0.603 | −0.083 | 0.435 |
| diagnosis | → | Education | → | CFT2 Con. a | −0.020 | 0.157 | −0.130 | 0.896 | −0.428 | 0.314 |
| diagnosis | → | Age | → | CFT2 Con. b | 0.020 | 0.053 | 0.376 | 0.707 | −0.074 | 0.394 |
| diagnosis | → | Education | → | CFT2 Con. b | −0.022 | 0.165 | −0.130 | 0.896 | −0.365 | 0.356 |
| diagnosis | → | Age | → | CFT2 Con. c | 0.064 | 0.095 | 0.675 | 0.500 | −0.068 | 0.472 |
| diagnosis | → | Education | → | CFT2 Con. c | −0.009 | 0.068 | −0.130 | 0.897 | −0.233 | 0.148 |
| diagnosis | → | Age | → | VFT | −0.068 | 0.100 | −0.680 | 0.497 | −0.476 | 0.095 |
| diagnosis | → | Education | → | VFT | 0.011 | 0.085 | 0.130 | 0.897 | −0.155 | 0.301 |
| diagnosis | → | Age | → | EMT−W Con. a | 0.007 | 0.056 | 0.131 | 0.896 | −0.112 | 0.275 |
| diagnosis | → | Education | → | EMT−W Con. a | 0.002 | 0.020 | 0.115 | 0.908 | −0.102 | 0.215 |
| diagnosis | → | Age | → | EMT−W Con. b | 0.004 | 0.052 | 0.069 | 0.945 | −0.195 | 0.259 |
| diagnosis | → | Education | → | EMT−W Con. b | −0.003 | 0.024 | −0.122 | 0.903 | −0.215 | 0.153 |
| (a) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 95% Confidence Interval | ||||||||||
| Direct Effects | b | SE | z-Value | p | Lower | Upper | ||||
| diagnosis | → | WMCUT S 1 | −0.191 | 0.122 | −1.564 | 0.118 | −0.472 | 0.205 | ||
| diagnosis | → | WMCUT S 2 | −0.604 | 0.130 | −4.646 | <0.001 * | −0.893 | −0.298 | ||
| diagnosis | → | WMCUT S 3 | −1.212 | 0.337 | −3.593 | <0.001 * | −1.821 | −0.699 | ||
| diagnosis | → | ACT | 3.668 | 1.086 | 3.377 | <0.001 * | 1.968 | 5.581 | ||
| diagnosis | → | ICT-RST 1 & 2 | 8.102 | 1.495 | 5.421 | <0.001 * | 5.175 | 10.491 | ||
| diagnosis | → | ICT-RST 1 & 2 SE | 0.656 | 0.154 | 4.261 | <0.001 * | 0.324 | 0.937 | ||
| diagnosis | → | ICT-RST 1 & 2 FS | 1.016 | 0.217 | 4.689 | <0.001 * | 0.468 | 1.407 | ||
| diagnosis | → | CFT | 2.562 | 0.468 | 5.476 | <0.001 * | 1.703 | 3.362 | ||
| diagnosis | → | CFT2 Con. a | 0.994 | 0.280 | 3.549 | <0.001 * | 0.455 | 1.503 | ||
| diagnosis | → | CFT2 Con. b | 1.387 | 0.237 | 5.853 | <0.001 * | 0.731 | 1.877 | ||
| diagnosis | → | CFT2 Con. c | 0.988 | 0.289 | 3.417 | <0.001 * | 0.160 | 1.639 | ||
| diagnosis | → | VFT | −1.302 | 0.276 | −4.723 | <0.001 * | −1.835 | −0.749 | ||
| diagnosis | → | EMT-W Con. a | 1.732 | 0.374 | 4.638 | <0.001 * | 1.063 | 2.488 | ||
| diagnosis | → | EMT-W Con. b | 1.186 | 0.339 | 3.499 | <0.001 * | 0.430 | 1.788 | ||
| (b) | ||||||||||
| 95% Confidence Interval | ||||||||||
| Indirect Effects | b | SE | z-Value | P | Lower | Upper | ||||
| diagnosis | → | Age | → | WMCUT S 1 | −0.140 | 0.067 | −2.075 | 0.038 | −0.350 | −0.043 |
| diagnosis | → | Education | → | WMCUT S 1 | −0.040 | 0.044 | −0.898 | 0.369 | −0.203 | 0.038 |
| diagnosis | → | Age | → | WMCUT S 2 | −0.214 | 0.086 | −2.486 | 0.013 | −0.396 | −0.076 |
| diagnosis | → | Education | → | WMCUT S 2 | −0.030 | 0.046 | −0.653 | 0.514 | −0.200 | 0.048 |
| diagnosis | → | Age | → | WMCUT S 3 | −0.418 | 0.193 | −2.170 | 0.030 | −0.893 | −0.092 |
| diagnosis | → | Education | → | WMCUT S 3 | −0.120 | 0.124 | −0.964 | 0.335 | −0.507 | 0.082 |
| diagnosis | → | Age | → | ACT | 1.896 | 0.744 | 2.546 | 0.011 | 0.536 | 3.870 |
| diagnosis | → | Education | → | ACT | 0.341 | 0.394 | 0.865 | 0.387 | −0.280 | 1.628 |
| diagnosis | → | Age | → | ICT−RST 1 & 2 | 1.917 | 0.867 | 2.211 | 0.027 | 0.484 | 4.212 |
| diagnosis | → | Education | → | ICT−RST 1 & 2 | 0.130 | 0.514 | 0.252 | 0.801 | −1.042 | 1.744 |
| diagnosis | → | Age | → | ICT−RST 1 & 2 SE | 0.093 | 0.071 | 1.305 | 0.192 | −0.083 | 0.281 |
| diagnosis | → | Education | → | ICT−RST 1 & 2 SE | 0.168 | 0.083 | 2.025 | 0.043 | 0.017 | 0.447 |
| diagnosis | → | Age | → | ICT−RST 1 & 2 FS | 0.230 | 0.116 | 1.980 | 0.048 | 0.010 | 0.560 |
| diagnosis | → | Education | → | ICT−RST 1 & 2 FS | 0.124 | 0.088 | 1.411 | 0.158 | −0.009 | 0.415 |
| diagnosis | → | Age | → | CFT | 0.624 | 0.276 | 2.256 | 0.024 | 0.172 | 1.328 |
| diagnosis | → | Education | → | CFT | 0.283 | 0.193 | 1.462 | 0.144 | −0.064 | 0.880 |
| diagnosis | → | Age | → | CFT2 Con. a | 0.062 | 0.120 | 0.512 | 0.609 | −0.231 | 0.381 |
| diagnosis | → | Education | → | CFT2 Con. a | 0.159 | 0.114 | 1.403 | 0.161 | −0.029 | 0.467 |
| diagnosis | → | Age | → | CFT2 Con. b | 0.039 | 0.101 | 0.381 | 0.703 | −0.229 | 0.301 |
| diagnosis | → | Education | → | CFT2 Con. b | 0.232 | 0.120 | 1.930 | 0.054 | 0.025 | 0.510 |
| diagnosis | → | Age | → | CFT2 Con. c | 0.325 | 0.158 | 2.052 | 0.040 | 0.071 | 0.800 |
| diagnosis | → | Education | → | CFT2 Con. c | 0.044 | 0.101 | 0.436 | 0.663 | −0.138 | 0.334 |
| diagnosis | → | Age | → | VFT | −0.307 | 0.150 | −2.038 | 0.042 | −0.711 | −0.056 |
| diagnosis | → | Education | → | VFT | −0.125 | 0.106 | −1.182 | 0.237 | −0.432 | 0.035 |
| diagnosis | → | Age | → | EMT−W Con. a | 0.013 | 0.159 | 0.082 | 0.935 | −0.361 | 0.336 |
| diagnosis | → | Education | → | EMT−W Con. a | 0.072 | 0.131 | 0.552 | 0.581 | −0.152 | 0.427 |
| diagnosis | → | Age | → | EMT−W Con. b | −0.034 | 0.144 | −0.235 | 0.814 | −0.427 | 0.291 |
| diagnosis | → | Education | → | EMT−W Con. b | 0.001 | 0.116 | 0.010 | 0.992 | −0.337 | 0.260 |
| R4Alz-R Tasks | Std. Deviation after Min–Max Normalization | AUC |
|---|---|---|
| WMCUT S3 | 0.23166 | 0.839 |
| ICT-RST 1 & 2 | 0.26460 | 0.832 |
| ICT-RST FS | 0.31163 | 0.791 |
| CFT | 0.23456 | 0.876 |
| CFT Con. B | 0.31586 | 0.832 |
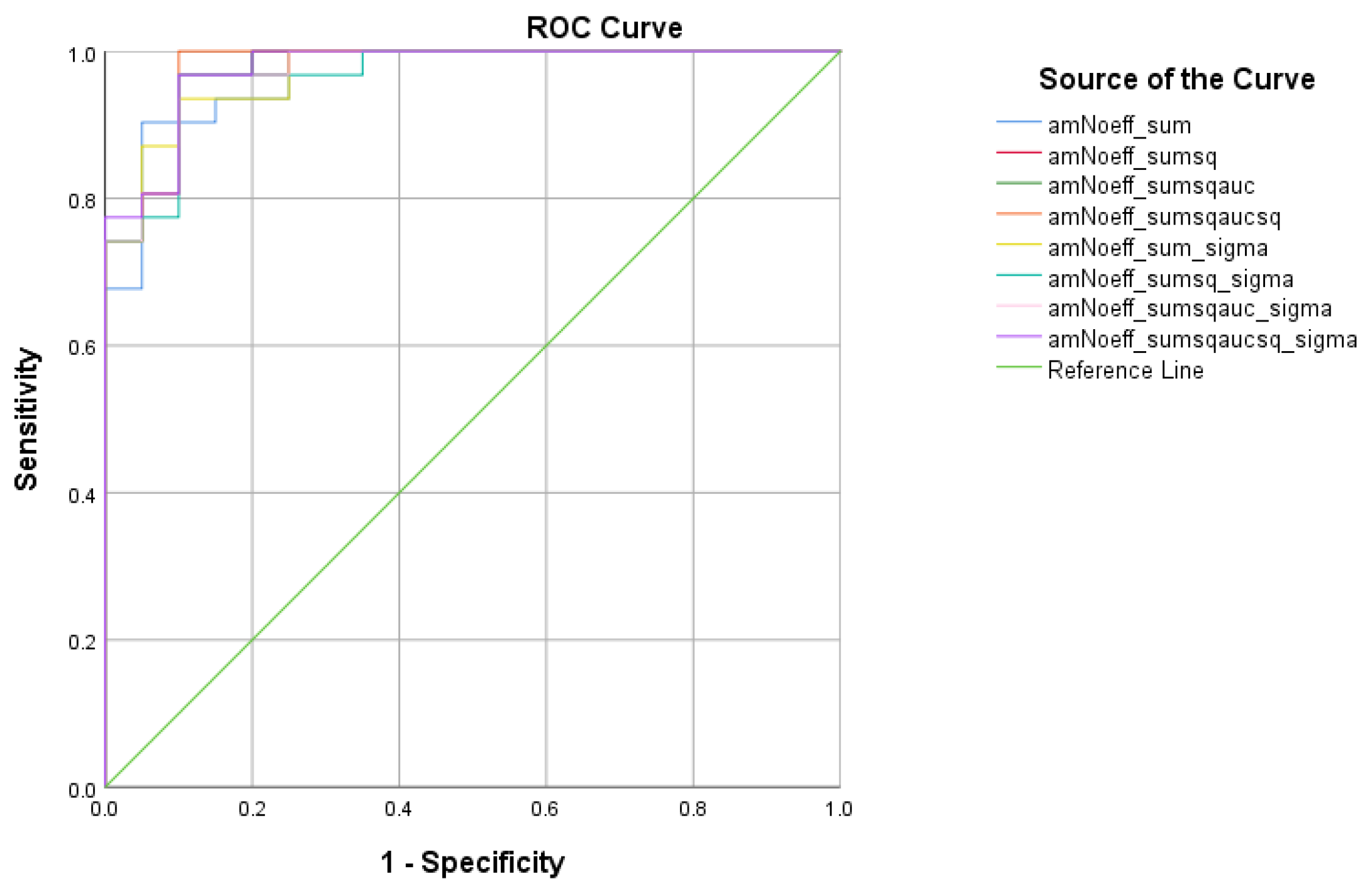
| Total Scores between SCD and HC | Cutoff | AUC | Sensitivity | Specificity | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| 1.0263 | 0.964 | 96.6% | 95% | 0.902–1.000 | <0.001 | |
| 0.3149 | 0.972 | 100% | 95% | 0.918–1.000 | <0.001 | |
| 0.2689 | 0.974 | 100% | 95% | 0.923–1.000 | <0.001 | |
| 0.2297 | 0.974 | 100% | 95% | 0.923–1.000 | <0.001 | |
| 3.7292 | 0.971 | 100% | 95% | 0.913–1.000 | <0.001 | |
| 1.2236 | 0.976 | 100% | 95% | 0.928–1.000 | <0.001 | |
| 1.0479 | 0.974 | 100% | 95% | 0.923–1.000 | <0.001 | |
| 0.8982 | 0.972 | 100% | 95% | 0.918–1.000 | <0.001 |
| R4Alz-R Tasks | Std. Deviation after Min–Max Normalization | AUC |
|---|---|---|
| ICT-RST 1 & 2 | 0.26460 | 0.734 |
| ICT-RST SE | 0.25238 | 0.744 |
| CFT Con. A | 0.30099 | 0.692 |
| EMT-W Con. A | 0.21215 | 0.800 |
| Total Scores between SCD and MCI | Cutoff | AUC | Sensitivity | Specificity | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| 1.5171 | 0.835 | 74.2% | 82.8% | 0.733–0.937 | <0.001 | |
| 0.8293 | 0.872 | 74.2% | 86.2% | 0.780–0.963 | <0.001 | |
| 0.5336 | 0.874 | 80.6% | 82.8% | 0.783–0.964 | <0.001 | |
| 0.4075 | 0.873 | 80.6% | 82.8% | 0.781–0.964 | <0.001 | |
| 6.8129 | 0.860 | 71% | 89.7% | 0.768–0.953 | <0.001 | |
| 13.9660 | 0.887 | 87.1% | 79.3% | 0.800–0.975 | <0.001 | |
| 11.0347 | 0.889 | 87.1% | 82.8% | 0.803–0.976 | <0.001 | |
| 8.4165 | 0.892 | 90.3% | 82.8% | 0.806–0.977 | <0.001 |
| R4Alz-R Tasks | Std. Deviation after Min–Max Normalization | AUC |
|---|---|---|
| ICT-RST 1 & 2 | 0.26460 | 0.931 |
| CFT Con. A | 0.30099 | 0.798 |
| CFT Con. B | 0.31586 | 0.923 |
| EMT-W Con. A | 0.21215 | 0.857 |
| EMT-W Con. B | 0.24559 | 0.773 |
| Total Scores between HC and MCI | Cutoff | AUC | Sensitivity | Specificity | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| 1.7635 | 0.968 | 90.3% | 95% | 0.926–1.000 | <0.001 | |
| 0.7512 | 0.973 | 96.8% | 90% | 0.935–1.000 | <0.001 | |
| 0.6167 | 0.974 | 96.8% | 90% | 0.938–1.000 | <0.001 | |
| 0.4804 | 0.977 | 100% | 90% | 0.943–1.000 | <0.001 | |
| 6.5582 | 0.971 | 93.5% | 90% | 0.934–1.000 | <0.001 | |
| 3.0873 | 0.968 | 96.8% | 90% | 0.926–1.000 | <0.001 | |
| 2.5263 | 0.973 | 96.8% | 90% | 0.935–1.000 | <0.001 | |
| 2.0760 | 0.976 | 96.8% | 90% | 0.942–1.000 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poptsi, E.; Moraitou, D.; Tsardoulias, E.; Symeonidis, A.L.; Papaliagkas, V.; Tsolaki, M. R4Alz-Revised: A Tool Able to Strongly Discriminate ‘Subjective Cognitive Decline’ from Healthy Cognition and ‘Minor Neurocognitive Disorder’. Diagnostics 2023, 13, 338. https://doi.org/10.3390/diagnostics13030338
Poptsi E, Moraitou D, Tsardoulias E, Symeonidis AL, Papaliagkas V, Tsolaki M. R4Alz-Revised: A Tool Able to Strongly Discriminate ‘Subjective Cognitive Decline’ from Healthy Cognition and ‘Minor Neurocognitive Disorder’. Diagnostics. 2023; 13(3):338. https://doi.org/10.3390/diagnostics13030338
Chicago/Turabian StylePoptsi, Eleni, Despina Moraitou, Emmanouil Tsardoulias, Andreas L. Symeonidis, Vasileios Papaliagkas, and Magdalini Tsolaki. 2023. "R4Alz-Revised: A Tool Able to Strongly Discriminate ‘Subjective Cognitive Decline’ from Healthy Cognition and ‘Minor Neurocognitive Disorder’" Diagnostics 13, no. 3: 338. https://doi.org/10.3390/diagnostics13030338
APA StylePoptsi, E., Moraitou, D., Tsardoulias, E., Symeonidis, A. L., Papaliagkas, V., & Tsolaki, M. (2023). R4Alz-Revised: A Tool Able to Strongly Discriminate ‘Subjective Cognitive Decline’ from Healthy Cognition and ‘Minor Neurocognitive Disorder’. Diagnostics, 13(3), 338. https://doi.org/10.3390/diagnostics13030338











