Prenatal Diagnosis and Fetopsy Validation of Complete Atrioventricular Septal Defects Using the Fetal Intelligent Navigation Echocardiography Method
Abstract
:1. Introduction
2. Materials and Methods
3. Results
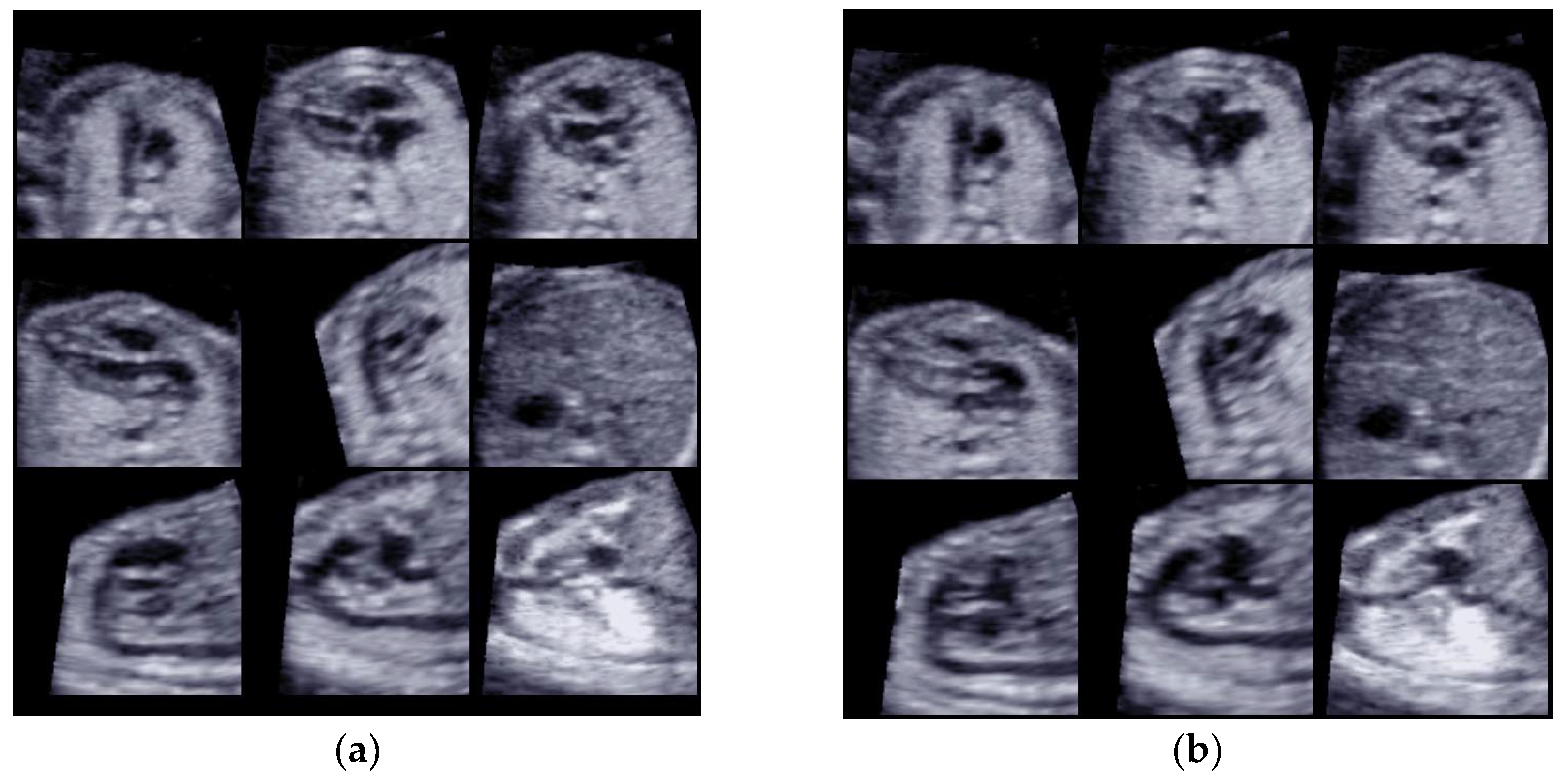
- Case 1: A 30-year-old woman (G2, P1001) referred to our maternal fetal unit at 20 weeks of gestation. She had received amniocentesis at 16 weeks of gestation after a combined test at high risk for Down’s syndrome. The fetal karyotype result was 47 XY + 21. The pregnancy had arisen spontaneously.
- Case 2: A 35-year-old woman (G2, P1001) referred to our maternal fetal unit at 20 weeks of gestation. She had received amniocentesis at 16 weeks of gestation due to maternal choice. The fetal karyotype result was 47 XX + 21. The pregnancy had arisen spontaneously.
- Case 3: A 28-year-old woman (G1, P 0000) referred to our unit at 18 weeks of gestation. She had received chorionic villus sampling at 13 weeks of gestation after a first-trimester scan showed a fetal cystic hygroma. The fetal karyotype result was 47 XX + 21. The pregnancy had arisen spontaneously.
- Case 4: A 38-year-old woman (G3, P 0020) referred to our unit at 17 weeks of gestation. She had received chorionic villus sampling at 12 weeks of gestation due to her maternal age. The fetal karyotype result was 47 XX + 21. The pregnancy was obtained with the FIVET procedure with a homologous biological material.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huggon, I.C.; Cook, A.C.; Smeeton, N.C.; Magee, A.G.; Sharland, G.K. Atrioventricular septal defects diagnosed in fetal life: Associated cardiac and extra-cardiac abnormalities and outcome. J. Am. Coll. Cardiol. 2000, 36, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Macris, M.P.; Ott, D.A.; Cooley, D.A. Complete atrioventricular canal defect: Surgical considerations. Tex. Heart Inst. J. 1992, 19, 239–243. [Google Scholar] [PubMed]
- Samaánek, M. Prevalence at birth, “natural” risk and survival with atrioventricular septal defect. Cardiol Young 1991, 1, 285–289. [Google Scholar] [CrossRef]
- Fyler, D.C.; Buckley, L.P.; Hellenbrand, W.E. Endocardial cushion defect: Report of the New England Regional Infant Cardiac Program. J. Pediatr. 1980, 65, 441–444. [Google Scholar]
- Araujo Júnior, E.; Rolo, L.C.; Rocha, L.A.; Nardozza, L.M.M.; Moron, A.F. The value of 3D and 4D assessments of the fetal heart. Int. J. Women's Health 2014, 6, 501–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yagel, S.; Cohen, S.M.; Shapiro, I.; Valsky, D.V. 3D and 4D ultrasound in fetal cardiac scanning: A new look at the fetal heart. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2007, 29, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Chaoui, R.; Hoffmann, J.; Heling, K.S. Three-dimensional (3D) and 4D color Doppler fetal echocardiography using spatio-temporal image correlation (STIC). Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2004, 23, 535–545. [Google Scholar] [CrossRef]
- Gonçalves, L.F.; Espinoza, J.; Lee, W.; Mazor, M.; Romero, R. Three- and four-dimensional reconstruction of the aortic and ductal arches using inversion mode: A new rendering algorithm for visualization of fluid-filled anatomical structures. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2004, 24, 696–698. [Google Scholar] [CrossRef]
- Devore, G.R.; Polanko, B. Tomographic ultrasound imaging of the fetal heart: A new technique for identifying normal and abnormal cardiac anatomy. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2005, 24, 1685–1696. [Google Scholar] [CrossRef]
- Espinoza, J.; Gonçalves, L.F.; Lee, W.; Mazor, M.; Romero, R. A novel method to improve prenatal diagnosis of abnormal systemic venous connections using three- and four-dimensional ultrasonography and ‘inversion mode’. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2005, 25, 428–434. [Google Scholar] [CrossRef]
- Paladini, D.; Volpe, P.; Sglavo, G.; Vassallo, M.; De Robertis, V.; Marasini, M.; Russo, M.G. Transposition of the great arteries in the fetus: Assessment of the spatial relationships of the arterial trunks by four-dimensional echocardiography. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2008, 31, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Volpe, P.; Campobasso, G.; De Robertis, V.; Di Paolo, S.; Caruso, G.; Stanziano, A.; Volpe, N.; Gentile, M. Two- and four-dimensional echocardiography with B-flow imaging and spatiotemporal image correlation in prenatal diagnosis of isolated total anomalous pulmonary venous connection. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2007, 30, 830–837. [Google Scholar] [CrossRef]
- Pooh, R.K.; Korai, A. B-flow and B-flow spatio-temporal image correlation in visualizing fetal cardiac blood flow. Croat. Med. J. 2005, 46, 808–811. [Google Scholar] [PubMed]
- Volpe, P.; Campobasso, G.; Stanziano, A.; De Robertis, V.; Di Paolo, S.; Caruso, G.; Volpe, N.; Gentile, M. Novel application of 4D sonography with B-flow imaging and spatio-temporal image correlation (STIC) in the assessment of the anatomy of pulmonary arteries in fetuses with pulmonary atresia and ventricular septal defect. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2006, 28, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Dalla, P.R.; Römer, U.; Kozlik-Feldmann, R.; Netz, H. Real-time three-dimensional ultrasound—A valuable new tool in preoperative assessment of complex congenital cardiac disease. Images Paediatr. Cardiol. 2003, 5, 10–12. [Google Scholar]
- Yeo, L.; Romero, R. Fetal Intelligent Navigation Echocardiography (FINE): A novel method for rapid, simple, and automatic examination of the fetal heart. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2013, 42, 268–284. [Google Scholar] [CrossRef]
- Garcia, M.; Yeo, L.; Romero, R.; Haggerty, D.; Giardina, I.; Hassan, S.S.; Chaiworapongsa, T.; Hernandez-Andrade, E. Prospective evaluation of the fetal heart using Fetal Intelligent Navigation Echocardiography (FINE). Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2016, 47, 450–459. [Google Scholar] [CrossRef] [Green Version]
- Veronese, P.; Bogana, G.; Cerutti, A.; Yeo, L.; Romero, R.; Gervasi, M.T. A Prospective Study of the Use of Fetal Intelligent Navigation Echocardiography (FINE) to Obtain Standard Fetal Echocardiography Views. Fetal Diagn. Ther. 2017, 41, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, J.S.; Allan, L.D.; Chaoui, R.; Copel, J.A.; DeVore, G.R.; Hecher, K.; Lee, W.; Munoz, H.; Paladini, D.; International Society of Ultrasound in Obstetrics and Gynecology Null; et al. ISUOG Practice Guidelines (updated): Sonographic screening examination of the fetal heart. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2013, 41, 348–359. [Google Scholar] [CrossRef]
- AIUM Practice Guideline for the Performance of Obstetric Ultrasound Examinations. J. Ultrasound Med. 2013, 32, 1083–1101. [CrossRef]
- Mureşan, D.; Mărginean, C.; Zaharie, G.; Stamatian, F.; Rotar, I.C. Complete atrioventricular septal defect in the era of prenatal diagnosis. Med. Ultrason. 2016, 18, 500–507. [Google Scholar] [CrossRef] [Green Version]
- Yeo, L.; Luewan, S.; Romero, R. Fetal Intelligent Navigation Echocardiography (FINE) Detects 98% of Congenital Heart Disease. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2018, 37, 2577–2593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, L.; Luewan, S.; Markush, D.; Gill, N.; Romero, R. Prenatal Diagnosis of Dextrocardia with Complex Congenital Heart Disease Using Fetal Intelligent Navigation Echocardiography (FINE) and a Literature Review. Fetal Diagn. Ther. 2018, 43, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Li, Y.; Chen, R.; Huang, C.; Mao, Y.; Zhao, B. Diagnostic performance of fetal intelligent navigation echocardiography (FINE) in fetuses with double-outlet right ventricle (DORV). Int. J. Cardiovasc. Imaging 2020, 36, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Weichert, J.; Weichert, A. A “holistic” sonographic view on congenital heart disease: How automatic reconstruction using fetal intelligent navigation echocardiography eases unveiling of abnormal cardiac anatomy part II—Left heart anomalies. Echocardiography 2021, 38, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Weichert, J.; Weichert, A. A ‘holistic’ sonographic view on congenital heart disease—How automatic reconstruction using fetal intelligent navigation echocardiography (FINE) eases the unveiling of abnormal cardiac anatomy part I: Right heart anomalies. Echocardiography 2021, 38, 1430–1445. [Google Scholar] [CrossRef]
- Yeo, L.; Romero, R. Color and power Doppler combined with Fetal Intelligent Navigation Echocardiography (FINE) to evaluate the fetal heart. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2017, 50, 476–491. [Google Scholar] [CrossRef] [Green Version]
- Yıldırım, G.; Gungorduk, K.; Yazıcıoğlu, F.; Gul, A.; Cakar, F.; Celikkol, O.; Ceylan, Y. Prenatal diagnosis of complete atrioventricular septal defect: Perinatal and neonatal outcomes. Obstet. Gynecol. Int. 2009, 2009, 958496. [Google Scholar] [CrossRef]
- Kovalchin, J.P.; Silverman, N.H. The impact of fetal echocardiography. Pediatr. Cardiol. 2004, 25, 299–306. [Google Scholar] [CrossRef]
- Rasiah, S.V.; Ewer, A.K.; Miller, P.; Wright, J.G.; Tonks, A.; Kilby, M.D. Outcome following prenatal diagnosis of complete atrioventricular septal defect. Prenat. Diagn. 2008, 28, 95–101. [Google Scholar] [CrossRef]

| N | Maternal Age | Gestational Age at US | Cardiac Defect at 2D US | Chromosomal Abnormalities | Pregnancy Outcome | AVSD Confirmation |
|---|---|---|---|---|---|---|
| 1 | 30 | 20 | Complete AVSD | 47 XY + 21 | TOP | Autopsy: complete AVSD |
| 2 | 35 | 20 | Complete AVSD | 47 XX + 21 | TOP | Autopsy: complete AVSD |
| 3 | 28 | 18 | Complete AVSD | 47 XX + 21 | TOP | Autopsy: complete AVSD |
| 4 | 38 | 17 | Complete AVSD | 47 XX + 21 | TOP | Autopsy: complete AVSD |
| 2D echo | FINE | FINE | FINE | FINE | FINE | |
|---|---|---|---|---|---|---|
| N | Abnormal Finding | Abnormal Diagnostic plane | Abnormal Finding | Abnormal VISA Views | Abnormal VISA Finding | Findings in Systolic/Diastolic Phase |
| 1 | Complete AVSD | 4 CH | AVSD + AV valves in the same plane | 4 CH 5 CH | AVSD + common AV valve AVSD + common AV valve | Normal/AVSD + common AV valve |
| 2 | Complete AVSD | 4 CH | AVSD + Common AV valve | 4 CH 5 CH | AVSD + Common AV valve AVSD + Common AV valve | AV valves in the same plane + AVSD/Common AV valve + AVSD |
| 3 | Complete AVSD | 4 CH | AVSD + Common AV valve | 4 CH 5 CH | AVSD + Common AV valve AVSD Common AV valve | AV valves in the same plane + AVSD/Common AV valve + AVSD |
| 4 | Complete AVSD | 4 CH | AVSD + Common AV valve | 4 CH 5 CH | AVSD + Common AV valve AVSD + Common AV valve | AV valves in the same plane + AVSD/Common AV valve + AVSD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veronese, P.; Guariento, A.; Cattapan, C.; Fedrigo, M.; Gervasi, M.T.; Angelini, A.; Riva, A.; Vida, V. Prenatal Diagnosis and Fetopsy Validation of Complete Atrioventricular Septal Defects Using the Fetal Intelligent Navigation Echocardiography Method. Diagnostics 2023, 13, 456. https://doi.org/10.3390/diagnostics13030456
Veronese P, Guariento A, Cattapan C, Fedrigo M, Gervasi MT, Angelini A, Riva A, Vida V. Prenatal Diagnosis and Fetopsy Validation of Complete Atrioventricular Septal Defects Using the Fetal Intelligent Navigation Echocardiography Method. Diagnostics. 2023; 13(3):456. https://doi.org/10.3390/diagnostics13030456
Chicago/Turabian StyleVeronese, Paola, Alvise Guariento, Claudia Cattapan, Marny Fedrigo, Maria Teresa Gervasi, Annalisa Angelini, Arianna Riva, and Vladimiro Vida. 2023. "Prenatal Diagnosis and Fetopsy Validation of Complete Atrioventricular Septal Defects Using the Fetal Intelligent Navigation Echocardiography Method" Diagnostics 13, no. 3: 456. https://doi.org/10.3390/diagnostics13030456
APA StyleVeronese, P., Guariento, A., Cattapan, C., Fedrigo, M., Gervasi, M. T., Angelini, A., Riva, A., & Vida, V. (2023). Prenatal Diagnosis and Fetopsy Validation of Complete Atrioventricular Septal Defects Using the Fetal Intelligent Navigation Echocardiography Method. Diagnostics, 13(3), 456. https://doi.org/10.3390/diagnostics13030456






