Urine Biomarkers in the Management of Adult Neurogenic Lower Urinary Tract Dysfunction: A Systematic Review
Abstract
:1. Introduction
2. Material and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
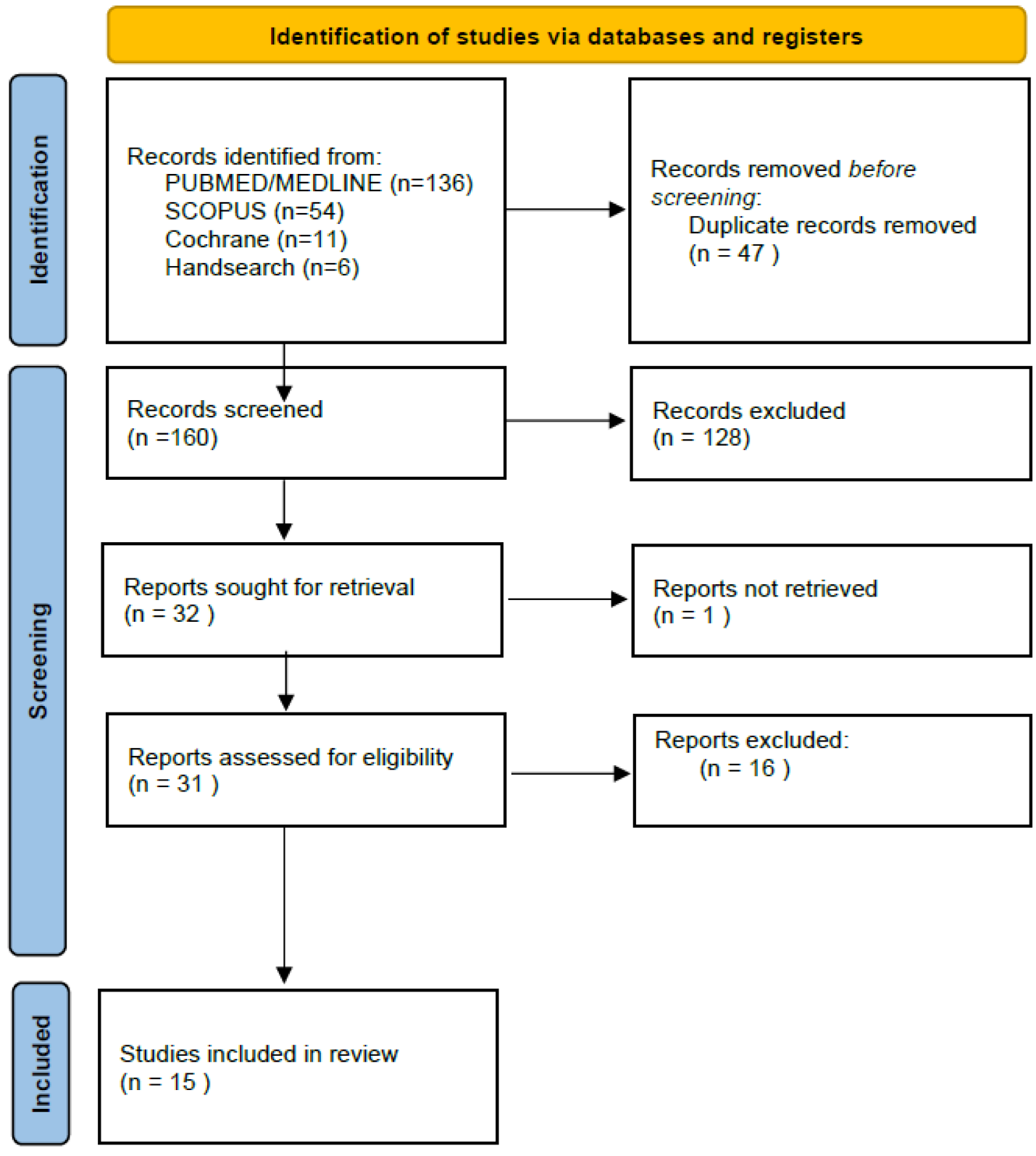
2.3. Study Selection Process
2.4. Quality Assessment
2.5. Data Extraction
3. Results
3.1. Study Characteristics
3.2. Methodologic Quality Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K.; Ferner, R.E. Biomarkers—A General Review. Curr. Protoc. Pharmacol. 2017, 76, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Harpole, M.; Davis, J.; Espina, V. Current state of the art for enhancing urine biomarker discovery. Expert Rev. Proteom. 2016, 13, 609–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, J. Urinalysis in Western culture: A brief history. Kidney Int. 2007, 71, 384–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, K.; Stenzl, A.; Sharma, A.; Vasdev, N. Urinary biomarkers in bladder cancer: A review of the current landscape and future directions. Urol Oncol. 2021, 39, 41–51. [Google Scholar] [CrossRef]
- Henning, G.M.; Barashi, N.S.; Smith, Z.L. Advances in Biomarkers for Detection, Surveillance, and Prognosis of Bladder Cancer. Clin. Genitourin. Cancer 2021, 19, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Nevo, A.; Navaratnam, A.; Andrews, P. Prostate cancer and the role of biomarkers. Abdom. Imaging 2019, 45, 2120–2132. [Google Scholar] [CrossRef]
- Sanda, M.G.; Feng, Z.; Howard, D.H.; Tomlins, S.A.; Sokoll, L.J.; Chan, D.W.; Regan, M.M.; Groskopf, J.; Chipman, J.; Patil, D.H.; et al. Association Between Combined TMPRSS2:ERG and PCA3 RNA Urinary Testing and Detection of Aggressive Prostate Cancer. JAMA Oncol. 2017, 3, 1085–1093. [Google Scholar] [CrossRef]
- Guzzi, F.; Cirillo, L.; Buti, E.; Becherucci, F.; Errichiello, C.; Roperto, R.M.; Hunter, J.P.; Romagnani, P. Urinary Biomarkers for Diagnosis and Prediction of Acute Kidney Allograft Rejection: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 6889. [Google Scholar] [CrossRef]
- Lim, J.-H.; Chung, B.H.; Lee, S.-H.; Jung, H.-Y.; Choi, J.-Y.; Cho, J.-H.; Park, S.-H.; Kim, Y.-L.; Kim, C.-D. Omics-based biomarkers for diagnosis and prediction of kidney allograft rejection. Korean J. Intern. Med. 2022, 37, 520–533. [Google Scholar] [CrossRef]
- Tsiapakidou, S.; Apostolidis, A.; Pantazis, K.; Grimbizis, G.F.; Mikos, T. The use of urinary biomarkers in the diagnosis of overactive bladder in female patients. A systematic review and meta-analysis. Int. Urogynecol. J. 2021, 32, 3143–3155. [Google Scholar] [CrossRef] [PubMed]
- Mangera, A.; Osman, N.I.; Chapple, C.R. Anatomy of the lower urinary tract. Surgery 2013, 31, 319–325. [Google Scholar]
- Chapple, C. Overview on the Lower Urinary Tract. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2011; Volume 202, pp. 1–14. [Google Scholar] [CrossRef]
- Gajewski, J.B.; Drake, M.J. Neurological lower urinary tract dysfunction essential terminology. Neurourol. Urodyn. 2018, 37, S25–S31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Averbeck, M.A.; Madersbacher, H. Follow-up of the neuro-urological patient: A systematic review. BJU Int. 2015, 115, 39–46. [Google Scholar] [CrossRef]
- Vince, R.A.; Klausner, A.P. Surveillance Strategies for Neurogenic Lower Urinary Tract Dysfunction. Urol. Clin. N. Am. 2017, 44, 367–375. [Google Scholar] [CrossRef]
- Amarenco, G.; Sheikh Ismaël, S.; Chesnel, C.; Charlanes, A.; Le Breton, F. Diagnosis and clinical evaluation of neurogenic bladder. Eur. J. Phys. Rehabil. Med. 2017, 53, 975–980. [Google Scholar] [CrossRef]
- Klingler, H.; Madersbacher, S.; Djavan, B.; Schatzl, G.; Marberger, M.; Schmidbauer, C. Morbidity of the Evaluation of the Lower Urinary Tract with Transurethral Multichannel Pressure-Flow Studies. J. Urol. 1998, 159, 191–194. [Google Scholar] [CrossRef]
- Yokoyama, T.; Kumon, H.; Nagai, A. Correlation of urinary nerve growth factor level with pathogenesis of overactive bladder. Neurourol. Urodyn. 2007, 27, 417–420. [Google Scholar] [CrossRef]
- Liu, H.-T.; Chancellor, M.B.; Kuo, H.-C. Urinary Nerve Growth Factor Levels are Elevated in Patients with Detrusor Overactivity and Decreased in Responders to Detrusor Botulinum Toxin—A Injection. Eur. Urol. 2009, 56, 700–707. [Google Scholar] [CrossRef]
- Liu, H.-T.; Liu, A.-B.; Chancellor, M.B.; Kuo, H.-C. Urinary nerve growth factor level is correlated with the severity of neurological impairment in patients with cerebrovascular accident. BJU Int. 2009, 104, 1158–1162. [Google Scholar] [CrossRef]
- Yamauchi, H.; Akino, H.; Ito, H.; Aoki, Y.; Nomura, T.; Yokoyama, O. Urinary prostaglandin E₂ was increased in patients with suprapontine brain diseases, and associated with overactive bladder syndrome. Urology 2010, 76, e13–e1267. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J.; Pavlicek, D.; Stoyanov, J.; Pannek, J.; Wöllner, J. Nerve growth factor does not seem to be a biomarker for neurogenic lower urinary tract dysfunction after spinal cord injury. Neurourol. Urodyn. 2016, 36, 659–662. [Google Scholar] [CrossRef]
- Richard, C.; Bendavid, C.; Hascoet, J.; Alimi, Q.; Khene, Z.-E.; Kerdraon, J.; Voiry, C.; Brochard, C.; Bouguen, G.; Siproudhis, L.; et al. Urinary biomarkers profiles in patients with neurogenic detrusor overactivity according to their neurological condition. World J. Urol. 2019, 38, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Peyronnet, B.; Richard, C.; Bendavid, C.; Naudet, F.; Hascoet, J.; Brochard, C.; Senal, N.; Jezequel, M.; Alimi, Q.; Khene, Z.; et al. Urinary TIMP-2 and MMP-2 are significantly associated with poor bladder compliance in adult patients with spina bifida. Neurourol. Urodyn. 2019, 38, 2151–2158. [Google Scholar] [CrossRef]
- Li, J.; Cai, S.; Zeng, C.; Chen, L.; Zhao, C.; Huang, Y.; Cai, W. Urinary exosomal vitronectin predicts vesicoureteral reflux in patients with neurogenic bladders and spinal cord injuries. Exp. Ther. Med. 2021, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- von Siebenthal, M.; Besic, M.; Gheinani, A.H.; Akshay, A.; Lizun-Platoni, S.; Kunz, N. Urinary miRNA profiles discriminate between obstruction-induced bladder dysfunction and healthy controls. Sci Rep. 2021, 11, 10204. [Google Scholar] [CrossRef] [PubMed]
- Forster, C.S.; Lamanna, O.K.; Rounds, A.; Sprague, B.M.; Ljungberg, I.; Groah, S.L. The association between urine neutrophil gelatinase-associated lipocalin and UTI in people with neurogenic lower urinary tract dysfunction. Spinal Cord 2020, 59, 959–966. [Google Scholar] [CrossRef]
- Sundén, F.; Butler, D.; Wullt, B. Triggered Urine Interleukin-6 Correlates to Severity of Symptoms in Nonfebrile Lower Urinary Tract Infections. J. Urol. 2017, 198, 107–115. [Google Scholar] [CrossRef]
- Stonehill, W.H.; Goldman, H.B.; Dmochowski, R.R. The use of urine cytology for diagnosing bladder cancer in spinal cord injured patients. J Urol. 1997, 157, 2112–2114. [Google Scholar] [CrossRef]
- Hess, M.J.; Zhan, E.H.; Foo, D.K.; Yalla, S.V. Bladder Cancer in Patients with Spinal Cord Injury. J. Spinal Cord Med. 2003, 26, 335–338. [Google Scholar] [CrossRef]
- Davies, B.; Chen, J.J.; McMurry, T.; Landsittel, D.; Lewis, N.; Brenes, G.; Getzenberg, R.H. Efficacy of BTA stat, cytology, and survivin in bladder cancer surveillance over 5 years in patients with spinal cord injury. Urology 2005, 66, 908–911. [Google Scholar] [CrossRef]
- Pannek, J.; Rademacher, F.; Wöllner, J. Clinical usefulness of urine cytology in the detection of bladder tumors in patients with neurogenic lower urinary tract dysfunction. Res. Rep. Urol. 2017, 9, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Brooke, B.S.; Schwartz, T.A.; Pawlik, T.M. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021, 156, 787–788. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; Robertson, J.; Peterson, J.; Welch, V.; Losos, M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analysis. Available online: http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf (accessed on 10 November 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 29, 372. [Google Scholar]
- Abrams, P.; Andersson, K.E.; Apostolidis, A.; Birder, L.; Bliss, D.; Brubaker, L. 6th International Consultation on Incontinence. Recommendations of the International Scientific Committee: Evaluation and Treatment of Urinary Incontinence, Pelvic Organ Prolapse and Faecal Incontinence. Neurourol. Urodyn. 2018, 37, 2271–2282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, A.; Oliveira, R.; Antunes-Lopes, T.; Cruz, C.D. Partners in Crime: NGF and BDNF in Visceral Dysfunction. Curr. Neuropharmacol. 2019, 17, 1021–1038. [Google Scholar] [CrossRef]
- Ochodnický, P.; Cruz, C.D.; Yoshimura, N.; Michel, M.C. Nerve growth factor in bladder dysfunction: Contributing factor, biomarker, and therapeutic target. Neurourol. Urodyn. 2011, 30, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Zhang, H.; Ruth, K.-H. Could urinary nerve growth factor be a biomarker for overactive bladder? A meta-analysis. Neurourol. Urodyn. 2017, 36, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Seth, J.H.; Sahai, A.; Khan, M.S.; van der Aa, F.; de Ridder, D.; Panicker, J.N. Nerve growth factor (NGF): A potential urinary biomarker for overactive bladder syndrome (OAB)? BJU Int. 2013, 111, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.C.; Yan, S.; Zhang, X.L.; Zhu, X.W.; Liu, Y.L.; Wang, P. Urinary nerve growth factor levels could be a biomarker for overactive bladder symptom: A meta-analysis. Genet. Mol. Res. 2014, 13, 8609–8619. [Google Scholar] [CrossRef]
- Siddiqui, N.Y.; Helfand, B.T.; Andreev, V.P.; Kowalski, J.T.; Bradley, M.S.; Lai, H.H.; Berger, M.B.; Mueller, M.G.; Bickhaus, J.A.; Packiam, V.T.; et al. Biomarkers Implicated in Lower Urinary Tract Symptoms: Systematic Review and Pathway Analyses. J. Urol. 2019, 202, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Malerba, F.; Paoletti, F.; Cattaneo, A. NGF and proNGF Reciprocal Interference in Immunoassays: Open Questions, Criticalities, and Ways Forward. Front. Mol. Neurosci. 2016, 9, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamper, M.; Moser, R.; Viereck, V. Have we been led astray by the NGF biomarker data? Neurourol. Urodyn. 2017, 36, 203–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krueger, F.; Pardini, M.; Huey, E.D.; Raymont, V.; Solomon, J.; Lipsky, R.H.; Hodgkinson, C.A.; Goldman, D.; Grafman, J. The Role of the Met66 Brain-Derived Neurotrophic Factor Allele in the Recovery of Executive Functioning after Combat-Related Traumatic Brain Injury. J. Neurosci. 2011, 31, 598–606. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Zhang, Z.; Sun, D.; Xu, Z.; Yuan, Y.; Zhang, X.; Li, L. Low serum BDNF may indicate the development of PSD in patients with acute ischemic stroke. Int. J. Geriatr. Psychiatry 2010, 26, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Morichi, S.; Kashiwagi, Y.; Takekuma, K.; Hoshika, A.; Kawashima, H. Expressions of Brain-Derived Neurotrophic Factor (BDNF) in Cerebrospinal Fluid and Plasma of Children with Meningitis and Encephalitis/Encephalopathy. Int. J. Neurosci. 2012, 123, 17–23. [Google Scholar] [CrossRef]
- Şekerci, Ç.A.; Işbilen, B.; Işman, F.; Akbal, C.; Şimşek, F.; Tarcan, T. Urinary NGF, TGF-β1, TIMP-2 and bladder wall thickness predict neurourological findings in children with myelodysplasia. J. Urol. 2014, 191, 199–205. [Google Scholar] [CrossRef]
- Nayak, S.; Bawa, M.; Kanojia, R.P.; Pal, A.; Jain, A.; Samujh, R. TIMP-2 as a noninvasive urinary marker for predicting neurogenic bladder in patients under follow-up for spina bifida. Neurourol. Urodyn. 2020, 40, 168–175. [Google Scholar] [CrossRef]
- Kuwabara, Y.; Kobayashi, T.; D’Alessandro-Gabazza, C.N.; Toda, M.; Yasuma, T.; Nishihama, K.; Takeshita, A.; Fujimoto, H.; Nagao, M.; Fujisawa, T.; et al. Role of Matrix Metalloproteinase-2 in Eosinophil-Mediated Airway Remodeling. Front. Immunol. 2018, 9, 2163. [Google Scholar] [CrossRef]
- Hayashida, M.; Hashimoto, K.; Ishikawa, T.; Miyamoto, Y. Vitronectin deficiency attenuates hepatic fibrosis in a non-alcoholic steatohepatitis-induced mouse model. Int. J. Exp. Pathol. 2019, 100, 72–82. [Google Scholar] [CrossRef]
- Shen, T.L.; Liu, M.N.; Zhang, Q.; Feng, W.; Yu, W.; Fu, X.L. The positive role of vitronectin in radiation induced lung toxicity: The in vitro and in vivo mechanism study. J. Transl. Med. 2018, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lendínez-Cano, G.; Ojeda-Claro, A.V.; Gómez-Gómez, E.; Jimenez, P.M.; Martin, J.F.; Dominguez, J.F.; Amores, J.; Cozar, J.M.; Bachiller, J.; Juárez, A.; et al. Prospective study of diagnostic accuracy in the detection of high-grade prostate cancer in biopsy-naïve patients with clinical suspicion of prostate cancer who underwent the Select MDx test. Prostate 2021, 81, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Abramovic, I.; Ulamec, M.; Bojanac, A.K.; Bulic-Jakus, F.; Jezek, D.; Sincic, N. miRNA in prostate cancer: Challenges toward translation. Epigenomics 2020, 12, 543–558. [Google Scholar] [CrossRef]
- Auprich, M.; Bjartell, A.; Chun, F.K.-H.; de la Taille, A.; Freedland, S.J.; Haese, A.; Schalken, J.; Stenzl, A.; Tombal, B.; van der Poel, H. Contemporary Role of Prostate Cancer Antigen 3 in the Management of Prostate Cancer. Eur. Urol. 2011, 60, 1045–1054. [Google Scholar] [CrossRef]
- Deras, I.L.; Aubin, S.M.; Blase, A.; Day, J.R.; Koo, S.; Partin, A.W.; Ellis, W.J.; Marks, L.S.; Fradet, Y.; Rittenhouse, H.; et al. PCA3: A Molecular Urine Assay for Predicting Prostate Biopsy Outcome. J. Urol. 2008, 179, 1587–1592. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Rhodes, D.R.; Perner, S.; Dhanasekaran, S.M.; Mehra, R.; Sun, X.-W. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005, 310, 644–648. [Google Scholar] [CrossRef]
- Grimaldi, A.M.; Lapucci, C.; Salvatore, M.; Incoronato, M.; Ferrari, M. Urinary miRNAs as a Diagnostic Tool for Bladder Cancer: A Systematic Review. Biomedicines 2022, 10, 2766. [Google Scholar] [CrossRef] [PubMed]
- Kutwin, P.; Konecki, T.; Borkowska, E.M.; Traczyk-Borszyńska, M.; Jabłonowski, Z. Urine miRNA as a potential biomarker for bladder cancer detection—A meta-analysis. Central Eur. J. Urol. 2018, 71, 177–185. [Google Scholar] [CrossRef]
- López-Beltrán, A.; Cheng, L.; Gevaert, T.; Blanca, A.; Cimadamore, A.; Santoni, M.; Massari, F.; Scarpelli, M.; Raspollini, M.R.; Montironi, R. Current and emerging bladder cancer biomarkers with an emphasis on urine biomarkers. Expert Rev. Mol. Diagn. 2019, 20, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, J.I.; Moreira, D.M.; Hartman, C.; Elsamra, S.E.; Smith, A.D.; Okeke, Z. Age-Related Changes in 24-Hour Urine Composition Must be Considered in the Medical Management of Nephrolithiasis. J. Endourol. 2014, 28, 871–876. [Google Scholar] [CrossRef]
- Pannek, J. Prevention of Recurrent Urinary Tract Infections in Neurourology. Eur. Urol. Focus 2020, 6, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Dinh, A.; Davido, B.; Duran, C.; Bouchand, F.; Gaillard, J.-L.; Even, A.; Denys, P.; Chartier-Kastler, E.; Bernard, L. Urinary tract infections in patients with neurogenic bladder. Med. Mal. Infect. 2019, 49, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Hooton, T.M.; Bradley, S.F.; Cardenas, D.D.; Colgan, R.; Geerlings, S.E.; Rice, J.C.; Saint, S.; Schaeffer, A.J.; Tambayh, P.A.; Tenke, P.; et al. Diagnosis, Prevention, and Treatment of Catheter-Associated Urinary Tract Infection in Adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 625–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.-H.; Jhang, J.-F.; Hsu, Y.-H.; Kuo, H.-C. Usefulness of Urinary Biomarkers for Assessing Bladder Condition and Histopathology in Patients with Interstitial Cystitis/Bladder Pain Syndrome. Int. J. Mol. Sci. 2022, 23, 12044. [Google Scholar] [CrossRef]
- Forster, C.S.; Jackson, E.; Ma, Q.; Bennett, M.; Shah, S.S.; Goldstein, S.L. Predictive ability of NGAL in identifying urinary tract infection in children with neurogenic bladders. Pediatr. Nephrol. 2018, 33, 1365–1374. [Google Scholar] [CrossRef]
- Gupta, S.; Preece, J.; Haynes, R.A.; Becknell, B.; Ching, C. Differentiating Asymptomatic Bacteriuria from Urinary Tract Infection in the Pediatric Neurogenic Bladder Population: NGAL As a Promising Biomarker. Top. Spinal Cord Inj. Rehabil. 2019, 25, 214–221. [Google Scholar] [CrossRef]
- Nickolas, T.L.; O’Rourke, M.J.; Yang, J.; Sise, M.E.; Canetta, P.A.; Barasch, N. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann. Intern. Med. 2008, 148, 810–819. [Google Scholar] [CrossRef]
- Hatipoglu, S.; Sevketoglu, E.; Gedikbasi, A.; Yilmaz, A.; Kiyak, A.; Mulazimoglu, M. Urinary MMP-9/NGAL complex in children with acute cystitis. Pediatr. Nephrol. 2011, 26, 1263–1268. [Google Scholar] [CrossRef]
- Lee, H.-E.; Kim, D.K.; Kang, H.K.; Park, K. The diagnosis of febrile urinary tract infection in children may be facilitated by urinary biomarkers. Pediatr. Nephrol. 2014, 30, 123–130. [Google Scholar] [CrossRef]
- Pannek, J. Transitional cell carcinoma in patients with spinal cord injury: A high risk malignancy? Urology 2002, 59, 240–244. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Sun, L.-M.; Lin, C.-L.; Liang, J.-A.; Chang, Y.-J.; Sung, F.-C.; Kao, C.-H. Risk of prostate and bladder cancers in patients with spinal cord injury: A population-based cohort study1. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 51.e1–51.e7. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.; Karsenty, G.; Chartier-Kastler, E.; Cussenot, O.; Compérat, E.; Rouprêt, M.; Phé, V. Prevalence, management, and prognosis of bladder cancer in patients with neurogenic bladder: A systematic review. Neurourol. Urodyn. 2017, 37, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Nahm, L.S.; Chen, Y.; DeVivo, M.J.; Lloyd, L.K. Bladder Cancer Mortality after Spinal Cord Injury over 4 Decades. J. Urol. 2015, 193, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Groah, S.L.; Weitzenkamp, D.A.; Lammertse, D.; Whiteneck, G.G.; Lezotte, D.C.; Hamman, R.F. Excess risk of bladder cancer in spinal cord injury: Evidence for an association between indwelling catheter use and bladder cancer. Arch. Phys. Med. Rehabil. 2002, 83, 346–351. [Google Scholar] [CrossRef] [Green Version]
- Sammer, U.; Walter, M.; Knüpfer, S.C.; Mehnert, U.; Bode-Lesniewska, B.; Kessler, T.M. Do we need surveillance urethro-cystoscopy in patients with neurogenic lower urinary tract dysfunction? PLoS ONE 2015, 10, e0140970. [Google Scholar] [CrossRef] [Green Version]
- Raitanen, M.-P.; Marttila, T.; Nurmi, M.; Ala-Opas, M.; Nieminen, P.; Aine, R.; Tammela, T.L. Finnbladder Group Human Complement Factor H Related Protein Test for Monitoring Bladder Cancer. J. Urol. 2001, 165, 374–377. [Google Scholar] [CrossRef]
- Guo, A.; Wang, X.; Shi, J.; Sun, C.; Wan, Z. Bladder tumour antigen (BTA stat) test compared to the urine cytology in the diagnosis of bladder cancer: A meta-analysis. Can. Urol. Assoc. J. 2014, 8, 347–352. [Google Scholar] [CrossRef] [Green Version]
- Nasuti, J.F.; Gomella, L.G.; Ismial, M.; Bibbo, M. Utility of the BTA stat test kit for bladder cancer screening. Diagn. Cytopathol. 1999, 21, 27–29. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, T.; Li, X.; Yu, F.; Yu, H.; Shao, S. Urine Biomarkers for the Diagnosis of Bladder Cancer: A Network Meta-Analysis. Urol. J. 2021, 18, 623–632. [Google Scholar]
| Author | Type Of Study | Population | Patients (N) | Subgroups | Biomarker |
|---|---|---|---|---|---|
| Stonehill et al. [30]. (1997) | Single-Center Retrospective | NLUTD | 208 | SCI | Cytology |
| Hess et al. [31]. (2003) | Single-Center Retrospective | NLUTD | 16 | SCI | Cytology |
| Davies et al. [32]. (2005) | Single-Center Prospective | NLUTD | 457 | SCI | Cytology BTA Survivin |
| Yokoyama et al. [19]. (2008) | Single-Center Prospective | Mixed (1) NLUTD (2) nNLUTD (3) Healthy Controls | 51 + 32 | NLUTD (n = 16) - SCI (n = 10) - CVD (n = 6) nNLUTD (n = 35) - Idiopathic (n= 19) - BOO (n = 16) Controls (n = 32) | NGF |
| Liu et al. [20]. (2008) | Single-Center (Prospective) Cross-Sectional | Mixed | 281 | NLUTD (n = 100) nNLUTD (n = 143) Controls (n = 38) | NGF |
| Liu et al. [21]. (2009) | Single-Center Prospective | Mixed | NLUTD (n = 93) -CVA Controls (n = 40) | NGF | |
| Yamauchi et al. [22]. (2010) | Single-Center Prospective | Mixed | 141 | NLUTD–SL (n = 114) Controls (n = 27) | PGE2, PGF2a NGF Substance P |
| Krebs et al. [23]. (2016) | Single-center Prospective | Mixed | 47 | NLUTD -SCI (n = 37) Controls (n = 10) | NGF |
| Pannek et al. [33]. (2017) | Single-Center Retrospective | NLUTD | 79 | SCI (n = 65) MS (n = 8) Other (n = 6) | Cytology |
| Sunden et al. [29]. (2017) | Single-Center Prospective | NLUTD | 23 | DI (n = 12) SCI (n = 11) | IL-6, IL-8 |
| Richard et al. [24]. (2019) | Single-Center Prospective | NLUTD | 41 | MS (n = 6) SCI (n = 20) SB (n = 15) | NGF. BDNF TGFβ-1, PGE2 TIMP-2 |
| Peyronnet et al. [25]. (2019) | Single-Center Prospective | NLUTD | 40 | SB | NFG, BDNF, PGE2, MMP-2, TIMP-2, TGF-B1 |
| Forster et al. [28]. (2020) | Multicenter Prospective | NLUTD | 27 | SCI (n = 24) SB (n = 1) MS (n = 2) | uNGAL |
| von Siebenthal et al. [27]. (2021) | Single-Center Prospective | Mixed (1) NLUTD (2) BLUTD (3) Healthy Controls | 50 | NLUTD (n = 12) BLUTD (n= 26) Controls (n = 12) | miRNA |
| Li et al. [26]. (2021) | Multicenter Prospective | NLUTD | 60 | SCI | Exosomal vitronectin |
| Study | Case Definition | Representativeness of Cases | Selection of Controls | Definition of Controls | Cohort Representativeness | Selection of Non-Exposed Cohort | Ascertainment of Exposure | Outcomes of Interest Not Present | Sample Representativeness | Sample Size | Non-Respondents | Ascertainment of The Exposure | Matched Controls | Additional Factors | Ascertainment of Exposure | Ascertainment Of Participants | Non-Response Rate | Assessment of Outcome | Follow-Up Long Enough | Adequacy of Follow-Up | Assessment of The Outcome | Statistical Test | Total Score | Quality of Study |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stonehill et al. [30]. (1997) | * | * | * | * | * | * | 6 | Medium | ||||||||||||||||
| Hess et al. [31]. (2003) | * | * | * | * | * | * | ‘6 | Medium | ||||||||||||||||
| Davies et al. [32]. (2005) | * | * | * | * | * | 5 | Low | |||||||||||||||||
| Yokoyama et al. [19]. (2008) | * | * | * | * | * | * | * | 7 | Medium | |||||||||||||||
| Liu et al. [20]. (2008) | * | * | * | ** | * | 6 | Medium | |||||||||||||||||
| Liu et al. [21]. (2009) | * | * | * | * | * | * | * | * | * | 9 | High | |||||||||||||
| Yamauchi et al. [22]. (2010) | * | * | * | * | * | * | * | * | 8 | High | ||||||||||||||
| Krebs et al. [23]. (2016) | * | * | * | * | * | * | * | * | 8 | High | ||||||||||||||
| Pannek et al. [33]. (2017) | * | * | * | * | 4 | Low | ||||||||||||||||||
| Sunden et al. [29]. (2017) | * | * | * | * | * | 5 | Low | |||||||||||||||||
| Richard et al. [24]. (2019) | * | * | * | * | * | * | 6 | Medium | ||||||||||||||||
| Peyronnet et al. [25]. (2019) | * | * | ** | * | 5 | Low | ||||||||||||||||||
| Forster et al. [28]. (2020) | * | * | * | * | * | * | 6 | Medium | ||||||||||||||||
| von Siebenthal et al. [27]. (2021) | * | * | * | * | * | * | * | * | 8 | High | ||||||||||||||
| Li et al. [26]. (2021) | * | * | * | * | * | * | 6 | Medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koukourikis, P.; Papaioannou, M.; Papanikolaou, D.; Apostolidis, A. Urine Biomarkers in the Management of Adult Neurogenic Lower Urinary Tract Dysfunction: A Systematic Review. Diagnostics 2023, 13, 468. https://doi.org/10.3390/diagnostics13030468
Koukourikis P, Papaioannou M, Papanikolaou D, Apostolidis A. Urine Biomarkers in the Management of Adult Neurogenic Lower Urinary Tract Dysfunction: A Systematic Review. Diagnostics. 2023; 13(3):468. https://doi.org/10.3390/diagnostics13030468
Chicago/Turabian StyleKoukourikis, Periklis, Maria Papaioannou, Dimitrios Papanikolaou, and Apostolos Apostolidis. 2023. "Urine Biomarkers in the Management of Adult Neurogenic Lower Urinary Tract Dysfunction: A Systematic Review" Diagnostics 13, no. 3: 468. https://doi.org/10.3390/diagnostics13030468





