Effects of Acidic Challenge on Demineralized Root Surface Treated with Silver Diamine Fluoride and Potassium Iodide
Abstract
1. Introduction
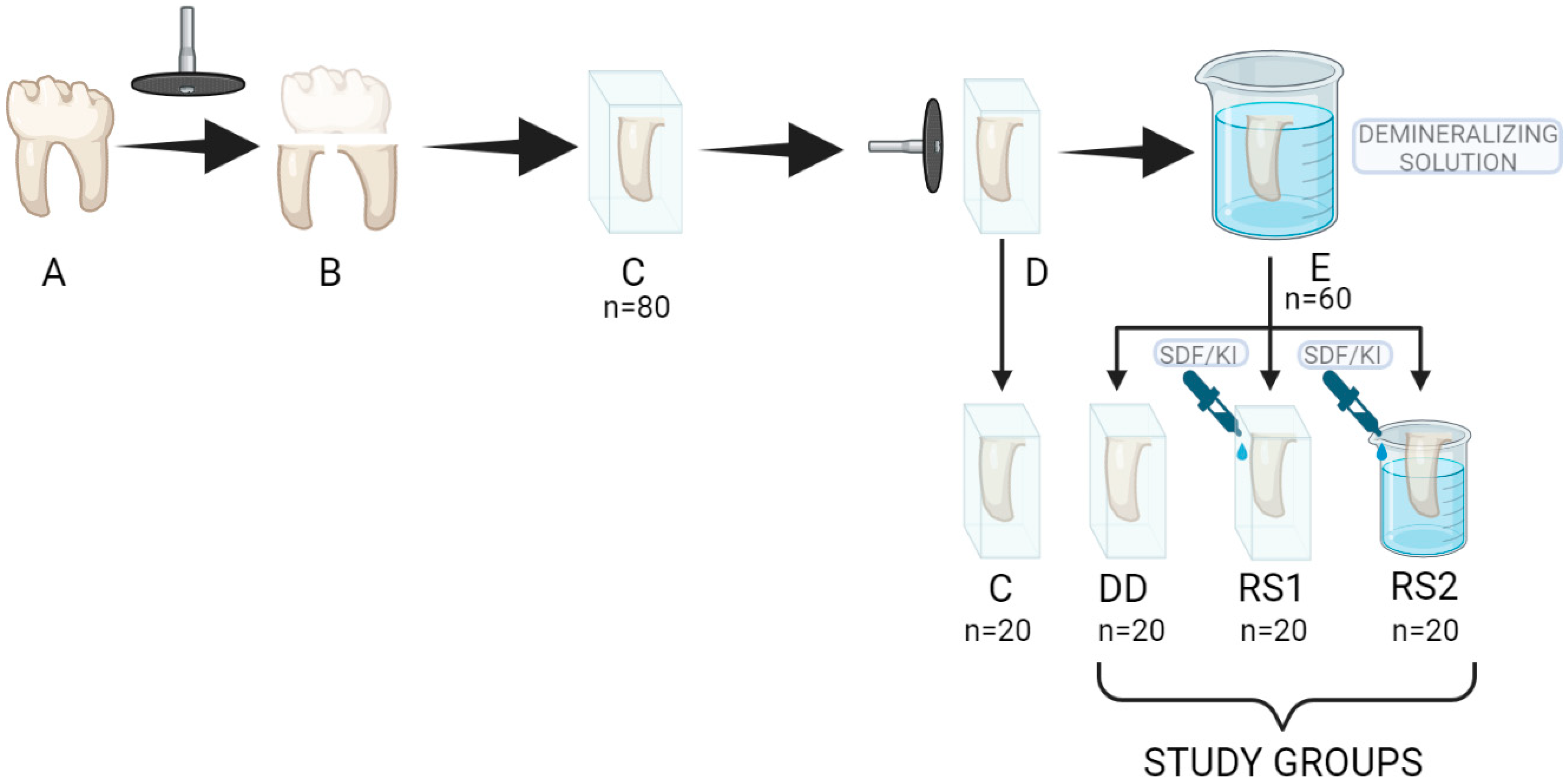
2. Materials and Methods
2.1. Sample Preparation
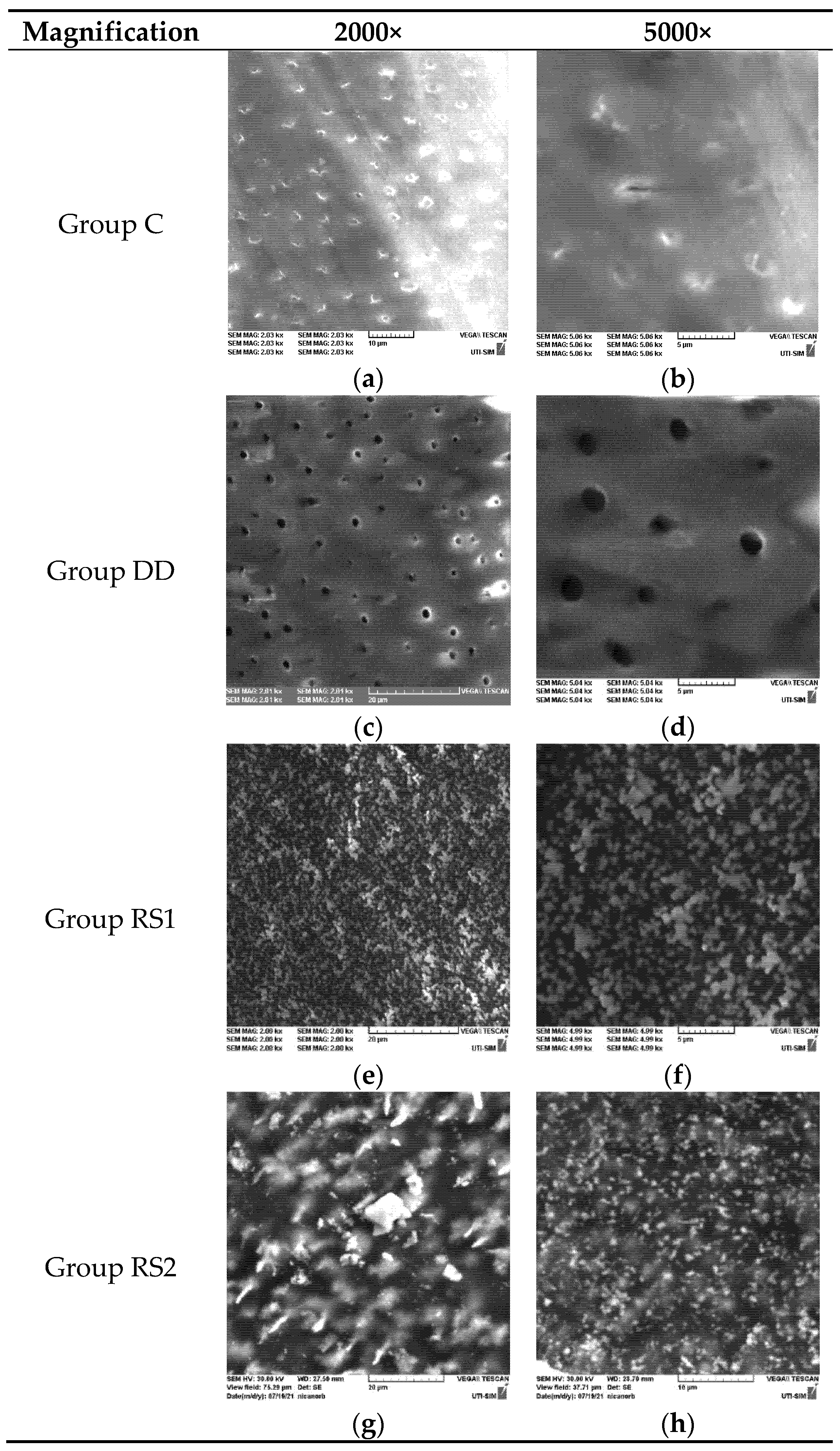
2.2. SEM Evaluation
2.3. EDX Evaluation
2.4. Microhardness Test
2.5. Statistical Analysis
3. Results
3.1. SEM Evaluation Results
3.2. EDX Evaluation Results
3.3. Microhardness Test Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Potârnichie, O.; Solomon, S.; Păsărin, L.; Mârţu, A.; Niţescu, D.C.; Mârţu, S. Statistical study on the prevalence of gingival recession in young adults. Int. J. Med. Dent. 2013, 3, 219–224. [Google Scholar]
- Sufaru, I.G.; Martu, M.A.; Solomon, S.M. Advances in Periodontal Pathogens. Microorganisms 2022, 10, 1439. [Google Scholar] [CrossRef] [PubMed]
- Al Qranei, M.S.; Balhaddad, A.A.; Melo, M.A.S. The burden of root caries: Updated perspectives and advances on management strategies. Gerodontology 2021, 38, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Paris, S.; Banerjee, A.; Bottenberg, P.; Breschi, L.; Campus, G.; Doméjean, S.; Ekstrand, K.; Giacaman, R.A.; Haak, R.; Hannig, M.; et al. How to intervene in the caries process in older adults: A joint ORCA and EFCD expert Delphi consensus statement. Caries Res. 2020, 54, 459–465. [Google Scholar] [CrossRef]
- Eden, E.; Frencken, J.; Gao, S.; Horst, J.A.; Innes, N. Managing dental caries against the backdrop of COVID-19: Approaches to reduce aerosol generation. Br. Dent. J. 2020, 229, 411–416. [Google Scholar] [CrossRef] [PubMed]
- van Strijp, G.; van Loveren, C. No Removal and Inactivation of Carious Tissue: Non-Restorative Cavity Control. In Caries Excavation: Evolution of Treating Cavitated Carious Lesions; Karger Publishers: Basel, Switzerland, 2018; Volume 27, pp. 124–136. [Google Scholar]
- Santamaria, R.M.; Innes, N.P.; Machiulskiene, V.; Schmoeckel, J.; Alkilzy, M.; Splieth, C.H. Alternative caries management options for primary molars: 2.5-year outcomes of a randomised clinical trial. Caries Res. 2017, 51, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Mijan, M.; de Amorim, R.G.; Leal, S.C.; Mulder, J.; Oliveira, L.; Creugers, N.H.; Frencken, J.E. The 3.5-year survival rates of primary molars treated according to three treatment protocols: A controlled clinical trial. Clin. Oral Investig. 2014, 18, 1061–1069. [Google Scholar]
- Grandjean, M.L.; Maccarone-Ruetsche, N.; McKenna, G.; Muller, F.; Srinivasan, M. Silver Diamine Fluoride (SDF) in the management of root caries in elders: A systematic review and meta-analysis. Swiss Dent. J. 2021, 131, 417–424. [Google Scholar]
- Castelo, R.; Attik, N.; Catirse, A.B.; Pradelle-Plasse, N.; Tirapelli, C.; Grosgogeat, B. Is there a preferable management for root caries in middle-aged and older adults? A systematic review. Br. Dent. J. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Knight, G.M.; McIntyre, J.M.; Craig, G.G.; Mulyani Zilm, P.S.; Gully, N.J. Inability to form a biofilm of Streptococcus mutans on silver fluoride- and potassium iodide-treated demineralized dentin. Quintessence Int. 2009, 40, 155–161. [Google Scholar]
- Srisomboon, S.; Kettratad, M.; Stray, A.; Pakawanit, P.; Rojviriya, C.; Patntirapong, S.; Panpisut, P. Effects of silver diamine nitrate and silver diamine fluoride on dentin remineralization and cytotoxicity to dental pulp cells: An in vitro study. J. Funct. Biomater. 2022, 13, 16. [Google Scholar] [CrossRef]
- Yagi, K.; Yamamoto, H.; Uemura, R.; Matsuda, Y.; Okuyama, K.; Ishimoto, T.; Nakano, T.; Hayashi, M. Use of PIXE/PIGE for sequential Ca and F measurements in root carious model. Sci. Rep. 2017, 7, 13450. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Komatsu, H.; Murata, Y.; Tanaka, T.; Sano, H. A newly designed automatic pH-cycling system to stimulate daily pH fluctuation. Dent. Mater. J. 2006, 25, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Peterson, I.M.; Pajares, A.; Lawn, B.R.; Thompson, V.P.; Rekow, E.D. Mechanical characterization of dental ceramics by Hertzian contacts. J. Dent. Res. 1998, 77, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Burrow, M.F.; Manton, D.J.; Tsuda, Y.; Sobh, E.G.; Palamara, J.E.A. Effects of silver diamine fluoride/potassium iodide on artificial root caries lesions with adjunctive application of proanthocyanidin. Acta Biomater. 2019, 88, 491–502. [Google Scholar] [CrossRef]
- Ganss, C.; Hardt, M.; Lussi, A.; Cocks, A.K.; Klimek, J.; Schlueter, N. Mechanism of action of tin-containing fluoride solutions as anti-erosive agents in dentine—An in vitro tin-uptake, tissue loss, and scanning electron microscopy study. Eur. J. Oral Sci. 2010, 118, 376–384. [Google Scholar] [CrossRef]
- Iovan, A.; Stoleriu, S.; Ghiorghe, C.A.; Pancu, G.; Topoliceanu, C.; Moldovanu, A.; Iovan, G.; Andrian, S. Effect of silver diamine fluoride and potassium iodide on artificial root caries subjected to demineralization. Rom. J. Oral Rehabil. 2021, 13, 82–91. [Google Scholar]
- Seifo, N.; Robertson, M.; Maclean, J.; Blain, K.; Grosse, S.; Milne, R.; Seeballuck, C.; Innes, N. The use of silver diamine fluoride (SDF) in dental practice. Br. Dent. J. 2020, 228, 75–81. [Google Scholar] [CrossRef]
- Mei, M.L.; Nudelman, F.; Marzec, B.; Walker, J.M.; Lo, E.C.M.; Walls, A.W.; Chu, C.H. Formation of fluorohydroxyapatite with silver diamine fluoride. J. Dent. Res. 2017, 96, 1122–1128. [Google Scholar] [CrossRef]
- Zhao, I.S.; Gao, S.S.; Hiraishi, N.; Burrow, M.F.; Duangthip, D.; Mei, M.L.; Lo, E.C.; Chu, C.H. Mechanisms of silver diamine fluoride on arresting caries: A literature review. Int. Dent. J. 2018, 68, 67–76. [Google Scholar] [CrossRef]
- Firouzmandi, M.; Vasei, F.; Giti, R.; Sadeghi, H. Effect of silver diamine fluorid and proanthocyanidin on resistance of carious dentin to acid challenge. PLoS ONE 2020, 15, e0238590. [Google Scholar] [CrossRef]
- Srisomboon, S.; Kettratad, M.; Pakawanit, P.; Rojviriya, C.; Phantumvanit, P.; Panpisut, P. Effects of Different Application Times of Silver Diamine Fluoride on Mineral Precipitation in Demineralized Dentin. Dent. J. 2021, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Barrera Ortega, C.C.; Araiza Tellez, M.A.; Garcia Perez, A.L. Assessment of Enamel Surface Microhardness with Different Fluorinated Compounds under pH Cycling Conditions: An in Vitro Study. J. Clin. Diagn. Res. 2019, 13, ZC05–ZC10. [Google Scholar]
- Mohanty, S.; Satyarup, D.; Nagarajappa, R.; Mahapatra, I.; Dalai, R.P.; Sahu, S. Silver Diamine Fluoride: Game Changer in Dental Public Health: A Review. Indian J. Forensic Med. Toxicol. 2020, 14, 8655–8659. [Google Scholar]
- Sayed, M.; Matsui, N.; Hiraishi, N.; Inoue, G.; Nikaido, T.; Burrow, M.F. Evaluation of discoloration of sound/demineralized root dentin with silver diamine fluoride: In-vitro study. Dent. Mater. J. 2019, 38, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.L.; Chu, C.H.; Lo, E.C.; Samaranayake, L.P. Fluoride and silver concentrations of silver diammine fluoride solutions for dental use. Int. J. Paediatr. Dent. 2013, 23, 279–285. [Google Scholar] [CrossRef]
- ten Cate, J.M.; Damen, J.J.M.; Buijs, M.J. Inhibition of dentin demineralization by fluoride in vitro. Caries Res. 1998, 32, 141–147. [Google Scholar] [CrossRef]
- Mei, M.L.; Ito, L.; Cao, Y.; Li, Q.; Chu, C.H.; Lo, E.C. The inhibitory effects of silver diamine fluoride on cysteine cathepsins. J. Dent. 2014, 42, 329–335. [Google Scholar] [CrossRef]
- Yu, O.Y.; Mei, M.L.; Zhao, I.S.; Li, Q.L.; Lo, E.C.M.; Chu, C.H. Remineralisation of enamel with silver diamine fluoride and sodium fluoride. Dent. Mater. 2018, 34, 344–352. [Google Scholar] [CrossRef]
- Mei, M.L.; Ito, L.; Cao, Y.; Li, Q.; Lo, E.C.; Chu, C.H. Inhibitory effect of silver diamine fluoride on dentine demineralisation and collagen degradation. J. Dent. 2013, 41, 809–817. [Google Scholar] [CrossRef]
- Zhao, I.S.; Mei, M.L.; Burrow, M.F.; Lo, E.C.; Chu, C.H. Prevention of secondary caries using silver diamine fluoride treatment and casein phosphopeptide-amorphous calcium phosphate modified glass-ionomer cement. J. Dent. 2017, 57, 38–44. [Google Scholar] [CrossRef]
- Sayed, M.; Matsui, N.; Nikaido, T.; Oikawa, M.; Burrow, M.F.; Tagami, J. Morphological and elemental analysis of silver penetration into sound/demineralized dentin after SDF application. Dent. Mater. 2019, 35, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Bertassoni, L.E.; Habelitz, S.; Kinney, J.H.; Marshall, S.J.; Marshall, G.W., Jr. Biomechanical perspective on the remineralization of dentin. Caries Res. 2009, 43, 70–77. [Google Scholar] [CrossRef]
- Kinney, J.H.; Marshall, S.J.; Marshall, G.W. The mechanical properties of human dentin: A critical review and re-evaluation of the dental literature. Crit. Rev. Oral Biol. Med. 2003, 14, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Kanarakis, I.; Sandu, D.; Solomon, S.M.; Pasarin, L.; Sufaru, I.G.; Martu, M.A.; Sioustis, I.A.; Kappenberg-Nitescu, D.C.; Luchian, I. Contemporary aspects regarding the etiology of gingival recessions. A review. Rom. J. Oral Rehabil. 2021, 13, 78–86. [Google Scholar]
- Ferreira, A.C.; de Lima Oliveira, R.F.; Amorim, A.A.; Geng-Vivanco, R.; de Carvalho Panzeri Pires-de-Souza, F. Remineralization of caries-affected dentin and color stability of teeth restored after treatment with silver diamine fluoride and bioactive glass–ceramic. Clin. Oral Investig. 2022, 26, 4805–4816. [Google Scholar] [CrossRef] [PubMed]
- Tiba, A.A.; Tiba, A.; Horvath, F.; Huh, E.Y.; Ford, A.A.; Arens, D.K.; Sarwar, T.; Hwang, Y. Effects of a Two-Step Silver Diamine Fluoride Varnish on Shear Bond Strength of Restorations, Dentin and Enamel Hardness, and Biofilm Formation. Mil. Med. 2022, 1–11. [Google Scholar] [CrossRef]
- Wierichs, R.J.; Stausberg, S.; Lausch, J.; Meyer-Lueckel, H.; Esteves-Oliveira, M. Caries-Preventive Effect of NaF, NaF plus TCP, NaFplus CPP-ACP, and SDF Varnishes on Sound Dentinand Artificial Dentin Caries in vitro. Caries Res. 2018, 52, 199–211. [Google Scholar] [CrossRef]
- Firouzmandi, M.; Shafiei, F.; Jowkar, Z.; Nazemi, F. Effect of Silver Diamine Fluoride and Proanthocyanidin on Mechanical Properties of Caries-Affected Dentin. Eur. J. Dent. 2019, 13, 255–260. [Google Scholar] [CrossRef]
- Vieira, A.; Ruben, J.L.; Huysmans, M.C. Effect of titanium tetrafluoride, amine fluoride and fluoride varnish on enamel erosion in vitro. Caries Res. 2005, 39, 371–379. [Google Scholar] [CrossRef]
 ) show statistical differences between groups.
) show statistical differences between groups.
 ) show statistical differences between groups.
) show statistical differences between groups.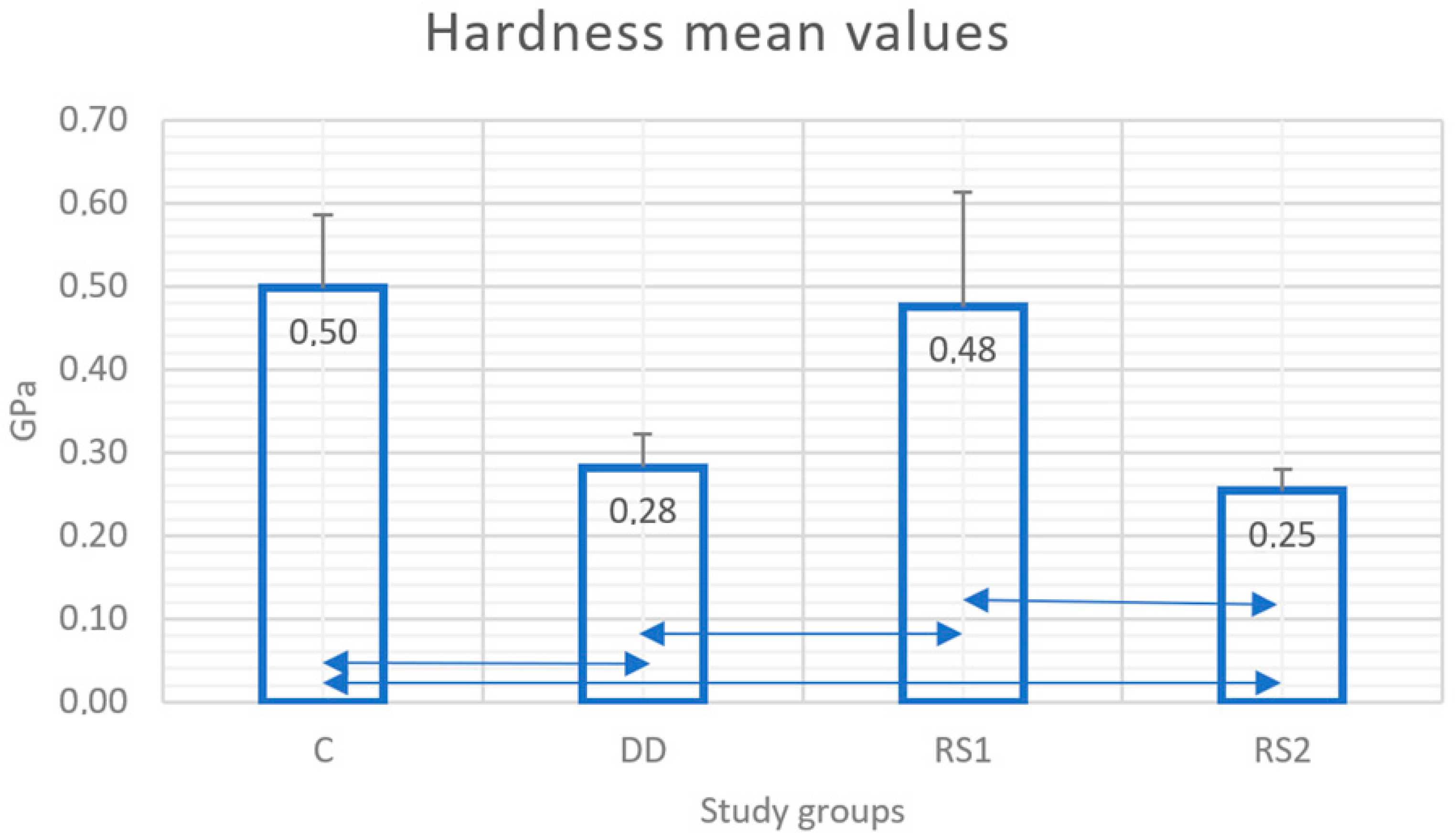
| Study Group | Study Protocol |
|---|---|
| C (n = 20) | Control group: Samples were submersed in distilled water |
| DD (n = 20) | Samples were submersed in demineralizing solution for 3 days |
| RS 1 (n = 20) | Samples were submersed in demineralizing solution for 3 days Riva Star product (RS) (SDI limited, Bayswater, Australia) was then applied on the exposed dentin surface |
| RS 2 (n = 20) | Samples were submersed in demineralizing solution for 3 days Riva Star product (RS) (SDI limited, Bayswater, Australia) was then applied on the exposed dentin surface Samples were stored in distilled water for 24 h Samples were submersed in demineralizing solution for 3 days |
| Product | Composition | Batch No. |
|---|---|---|
| Riva Star Capsule Kit (SDI Limited Australia) | Riva Star Step 1: 38% silver diaminofluoride (SDF) Riva Star Step 2: potassium iodide (KI) | 1164696 |
| Protocol |
|---|
| Riva Star Solution Step 1 was applied on the exposed dentin surface Riva Star Solution Step 2 was immediately applied to the treated surface until the creamy-white precipitate became clear. |
| Element | Mass Norm. (%) | Atom (%) | abs. Error (%) (1 Sigma) |
|---|---|---|---|
| Oxygen | 46.12745 | 53.05732 | 7.906657 |
| Calcium | 24.93783 | 11.45097 | 0.931029 |
| Carbon | 19.5091 | 29.89149 | 4.159093 |
| Phosphorus | 9.425618 | 5.600225 | 0.495739 |
| Sum | 100 | 100 |
| Element | Mass Norm. (%) | Atom (%) | abs. Error (%) (1 Sigma) |
|---|---|---|---|
| Carbon | 46.2307 | 54.4655 | 6.85517 |
| Oxygen | 34.19054 | 30.23935 | 5.822515 |
| Nitrogen | 12.40779 | 12.53513 | 3.712765 |
| Calcium | 4.972625 | 1.755698 | 0.185945 |
| Phosphorus | 2.198341 | 1.004318 | 0.123339 |
| Sum | 100 | 100 |
| Element | Mass Norm. (%) | Atom (%) | abs. Error (%) (1 Sigma) |
|---|---|---|---|
| Iodine | 46.34 | 20.72 | 3.90 |
| Silver | 35.93 | 18.90 | 2.30 |
| Oxygen | 16.38 | 58.10 | 3.70 |
| Potassium | 1.13 | 1.64 | 0.57 |
| Fluorine | 0.22 | 0.64 | 0.73 |
| Sum | 100 | 100 |
| Element | Mass Norm. (%) | Atom (%) | abs. Error (%) (1 Sigma) |
|---|---|---|---|
| Oxygen | 59.03 | 90.55 | 3.012 |
| Iodine | 23.05 | 4.46 | 0.31 |
| Silver | 15.67 | 3.56 | 0.23 |
| Calcium | 1.96 | 1.20 | 0.10 |
| Phosphorus | 0.29 | 0.23 | 0.08 |
| Sum | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iovan, A.; Benchea, M.; Stoleriu, S.; Tărăboanță, I.; Cimpoeșu, N.; Nica, I.; Andrian, S. Effects of Acidic Challenge on Demineralized Root Surface Treated with Silver Diamine Fluoride and Potassium Iodide. Diagnostics 2023, 13, 530. https://doi.org/10.3390/diagnostics13030530
Iovan A, Benchea M, Stoleriu S, Tărăboanță I, Cimpoeșu N, Nica I, Andrian S. Effects of Acidic Challenge on Demineralized Root Surface Treated with Silver Diamine Fluoride and Potassium Iodide. Diagnostics. 2023; 13(3):530. https://doi.org/10.3390/diagnostics13030530
Chicago/Turabian StyleIovan, Alexandru, Marcelin Benchea, Simona Stoleriu, Ionuț Tărăboanță, Nicanor Cimpoeșu, Irina Nica, and Sorin Andrian. 2023. "Effects of Acidic Challenge on Demineralized Root Surface Treated with Silver Diamine Fluoride and Potassium Iodide" Diagnostics 13, no. 3: 530. https://doi.org/10.3390/diagnostics13030530
APA StyleIovan, A., Benchea, M., Stoleriu, S., Tărăboanță, I., Cimpoeșu, N., Nica, I., & Andrian, S. (2023). Effects of Acidic Challenge on Demineralized Root Surface Treated with Silver Diamine Fluoride and Potassium Iodide. Diagnostics, 13(3), 530. https://doi.org/10.3390/diagnostics13030530








