Photon-Counting Computed Tomography (PCCT): Technical Background and Cardio-Vascular Applications
Abstract
:1. Introduction
2. Search Strategy
3. Photon-Counting Detector Technology
3.1. Comparison between Conventional and Photon Counting Detectors
3.2. Technical Challenges of PCDs
4. Benefits of PCDs
4.1. Reduction of Electronic Noise
4.2. Improvement in Spatial Resolution
4.3. Contrast Improvement
4.4. Reduction of Beam-Hardening
4.5. Multienergy Acquisitions and K-Edge Imaging
4.6. Dose Efficiency
5. Pre-Clinical and Clinical Studies
5.1. Coronary Imaging
5.2. Neuroimaging
5.3. Abdominal Imaging
| Reference | Type of Study | Main Finding |
|---|---|---|
| Coronary imaging | ||
| Soschynski et al. 2022 [72] | Clinical (92 patients with chronic coronary syndrome). | Excellent imaging quality, very high CNR, and good assessability of coronary segments and vessels, even in cases of calcified plaques and stents, provided by PCCT. |
| Li et al. 2020 [77] |
| Accuracy and precision of stenosis severity measurements higher in four-threshold PCCT images than DECT and two-threshold PCCT images. |
| Boussel et al. 2014 [80] | Ex vivo (10 calcified and 13 lipid-rich non-calcified plaques from post-mortem human coronary arteries). | Capability of PCCT to discriminate between calcifications and iodine-infused regions of human coronary artery atherosclerotic plaque samples, by detecting differences in spectral attenuation and iodine-based contrast agent concentration. |
| Si-Mohamed et al. 2022 [81] |
|
|
| Mergen et al. 2022 [82] | Clinical (20 patients with atherosclerotic plaques in proximal coronary arteries). | Reduced blooming artifacts with consequent improved visualization of fibrotic and lipid-rich plaque components obtained with the ultra-high-resolution mode of PCCT (slice thickness of 0.6 mm used as reference standard for comparison). |
| Sandstedt et al. 2021 [86] | Ex vivo (excised coronary specimens). | More accurate quantification of coronary calcifications and lower image noise achievable with high-resolution PCD-CT compared to conventional EID-CT. |
| van der Werf et al. 2022 [84] | In vitro phantom (anthropomorphic thorax phantom with inside CAC containing cylindrical inserts). | Improved CAC detection, even at 50% radiation dose reduction, and more accurate physical volume estimation, especially at reduced slice thickness and for high-density CAC, with PCCT compared to conventional CT. |
| van der Werf et al. 2022 [87] | In vitro phantom (anthropomorphic thorax phantom with artificial CAC with three densities). | Potential dose reduction of 50% for CAC scoring in medium- and high-density calcifications allowed by PCCT using low mono-energetic reconstructions. |
| Symons et al. 2019 [85] |
| Significant improvement in CAC score image quality or reduction of CAC score radiation, without a negative impact on diagnostic image quality, achievable with PCD compared to conventional EID CT. |
| Cormode et al. 2010 [63] |
| Capability of PCCT to accurately differentiate gold-based contrast agent, iodinated contrast agent, tissue, and calcium-rich matter, which may allow for the simultaneous detection of macrophages in atherosclerosis and the imaging the vasculature and calcified tissue. |
| Si-Mohamed et al. 2021 [22] | In vivo animal (7 atherosclerotic and 4 control New Zealand white rabbits imaged before and after injection of gold nanoparticles). |
|
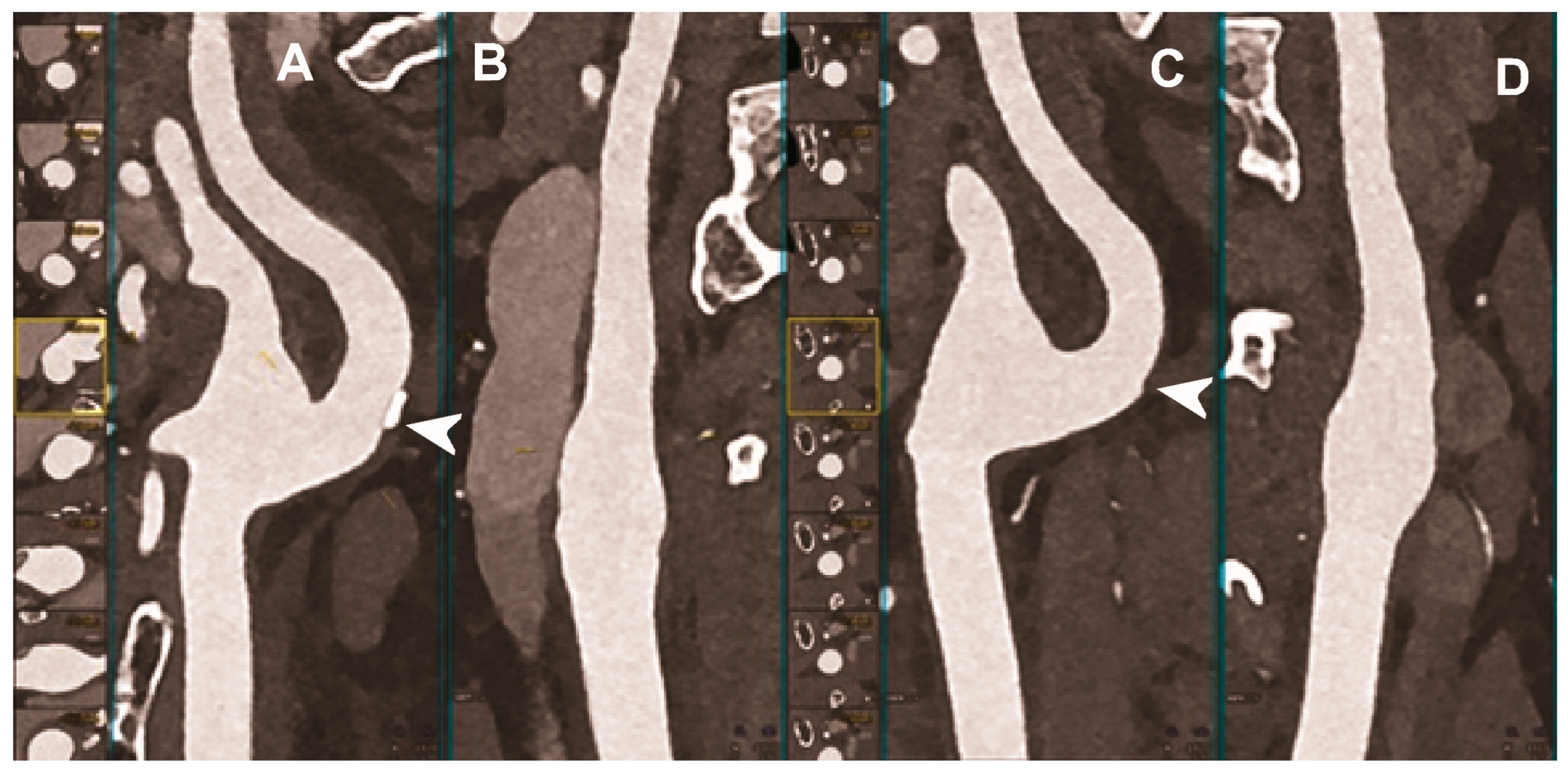
| Mannil et al. 2018 [94] | In vitro phantom (18 different coronary artery stents with different material composition, expanded in a plastic tube simulating the coronary artery). | Improved delineation of lumen, lower image noise, reduced blooming effect, and improved overall image quality with PCCT compared to conventional CT, despite the matched CT scan protocol settings and the identical image reconstruction parameters. |
| Symons et al. 2018 #110 [95] | In vitro phantom (18 coronary stents with different diameters implanted into a coronary artery phantom consisting of plastic tubes filled with contrast material). |
|
| von Spiczak et al. 2018 [96] | In vitro phantom (18 different coronary stents expanded in plastic tubes of 3 mm diameter, filled with diluted contrast agent, sealed, and immersed in oil). | Improved coronary in-stent lumen visualization with PCCT obtained thanks to the application of a sharp convolution kernel adapted to the intrinsic higher spatial resolution of the PCDs. |
| Rajagopal et al. 2021 [97] | In vitro (coronary artery phantom containing cylindrical probes simulating plaques with different composition and stenosis, imaged with and without coronary stents). | Improved visualization with less blooming artifacts and more accurate quantitative assessment of coronary plaques and stents with HR-PCCT compared to either photon-counting or energy-integrating CT. |
| Feuerlein et al. 2008. [98] | In vitro phantom (polymethylmethacrylate phantom with simulated low-density calcified plaque in a coronary metal stent). | Capability of gadolinium k-edge imaging performed with a multiple threshold–level PCCT to clearly separate the calcified plaque and the intra-vascular gadolinium and to effectively suppress the beam-hardening artifacts, for an accurate characterization of the perfused vessel lumen. |
| Bratke et al. 2020 [99] | In vitro phantom (10 different coronary stents placed in the middle of plastic tubes, used as a coronary artery phantoms and filled with a contrast agent). |
|
| Head and neck imaging | ||
| Symons et al. 2018 [49] | Clinical (16 asymptomatic subjects). |
|
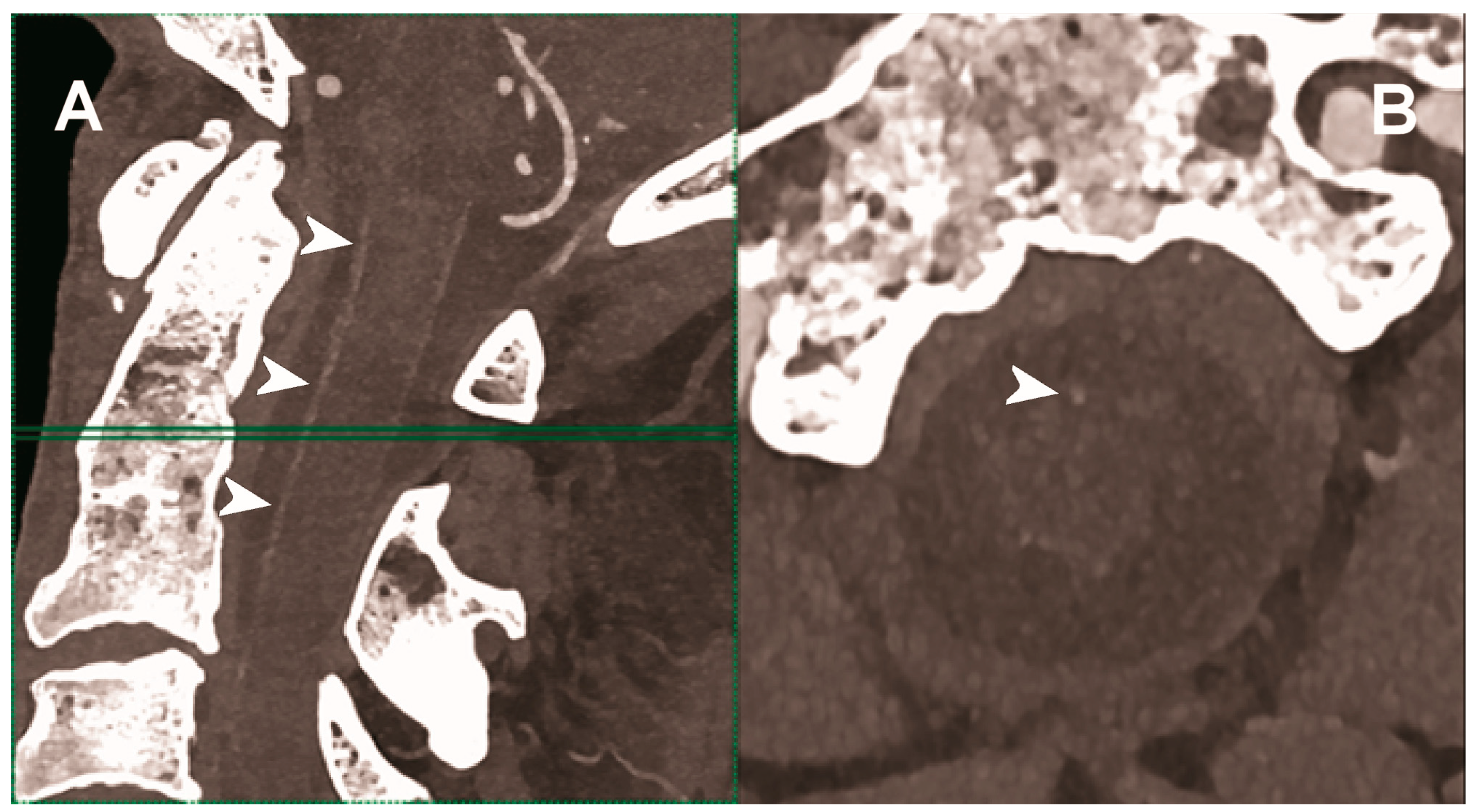
| Hetterich et al. 2014 [103] | Ex vivo (7 postmortem human carotid artery specimens). |
|
| Sartoretti et al. 2020 [104] | Ex vivo (carotid artery specimen of deceased male donor). | Improved lumen and plaque visualization and image noise with PCCT employing the multi-energy bin option in combination with tungsten as contrast media compared with the standard iodine. |
| Abdominal imaging | ||
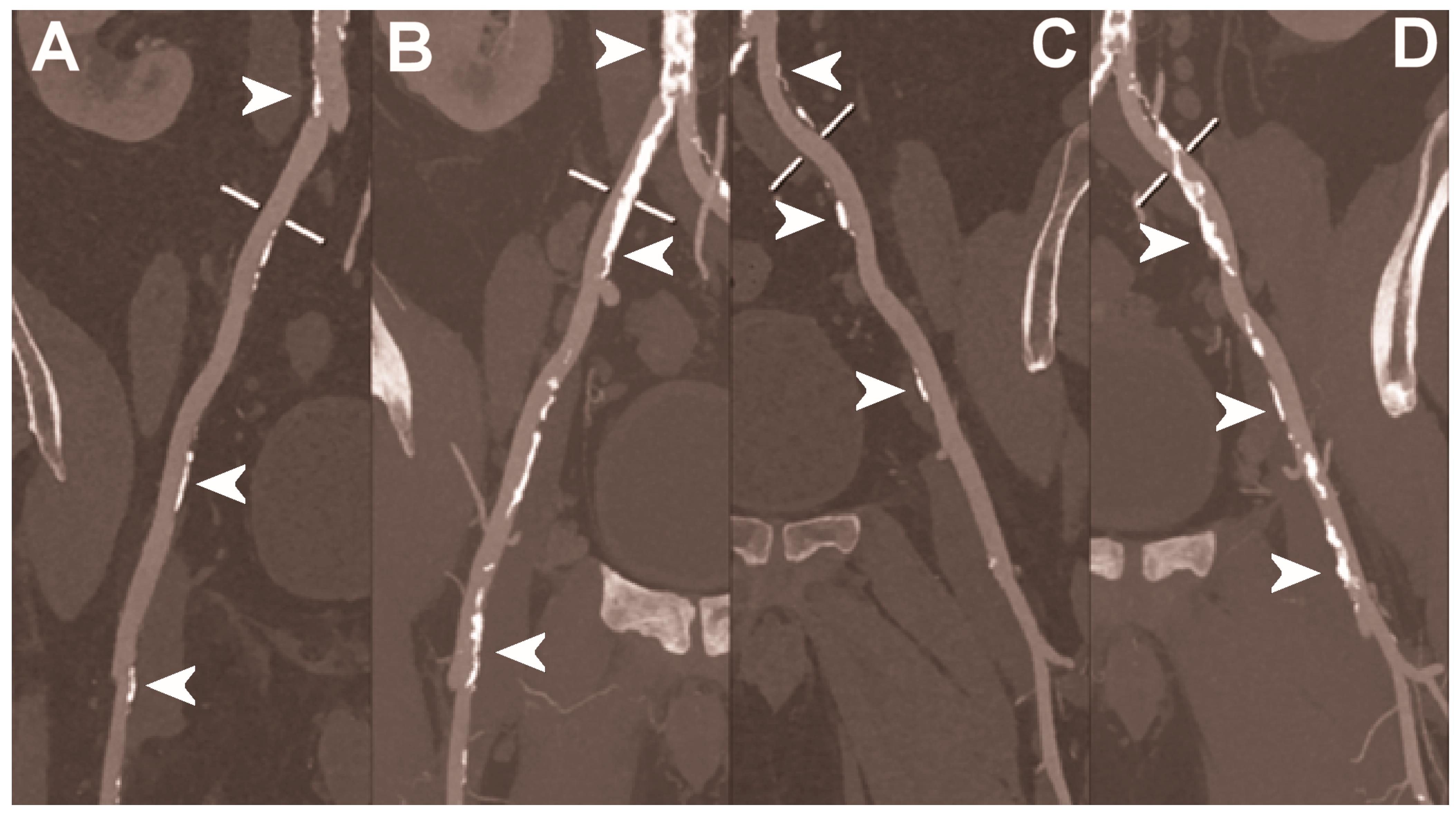
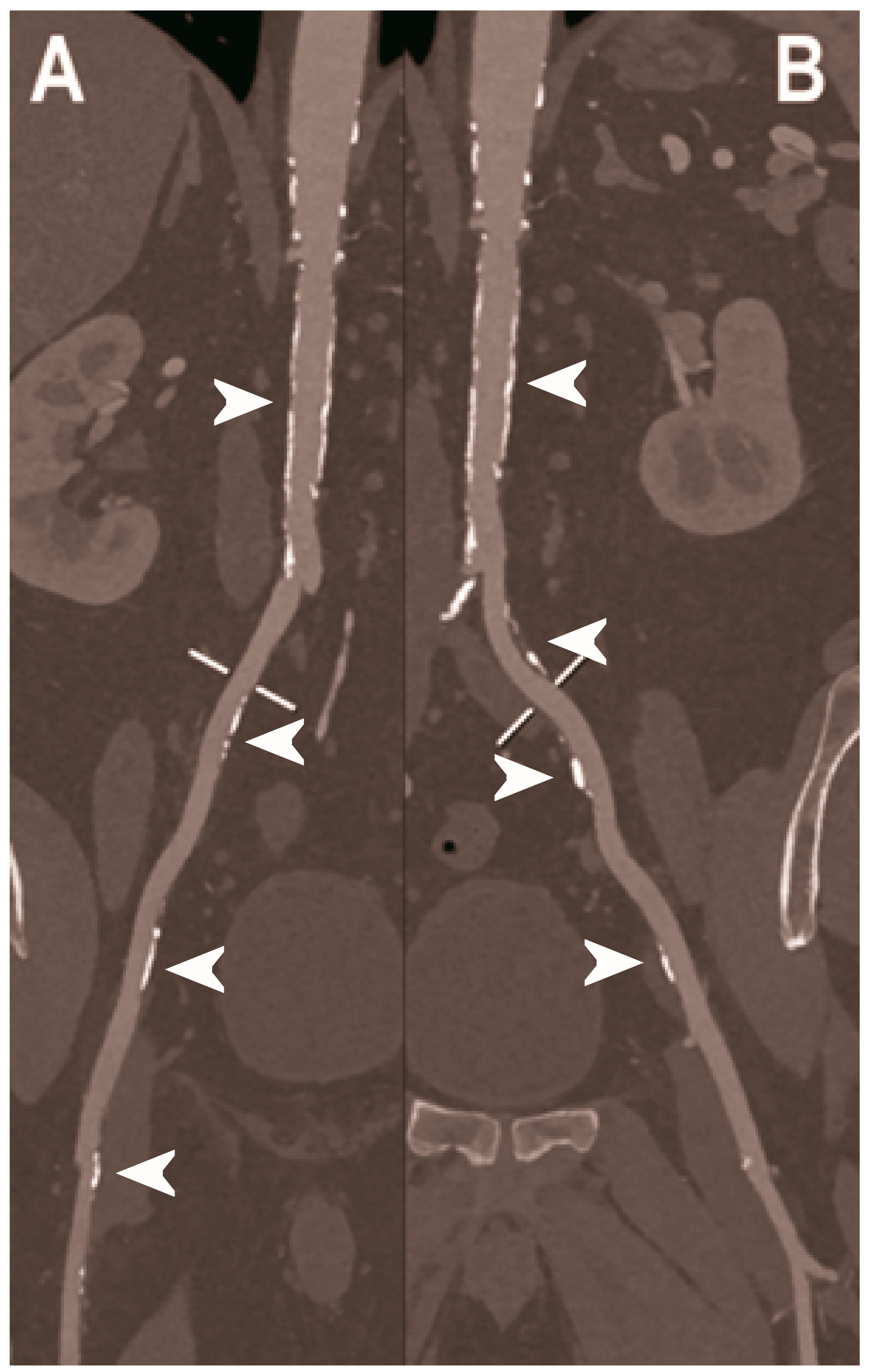
| Dangelmaier et al. 2018 [111] | In vitro phantom (abdominal aortic aneurysm phantom filled with iodine, gadolinium, or calcium). | Ability of PCCT in combination with a dual contrast agent injection to capture endoleak dynamics and effectively distinct leaking contrast media from intra-aneurysmatic calcifications, thereby allowing for a significant reduction of radiation exposure. |
| Sigovan et al. 2019 [112] |
|
|
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Libby, P.; Ridker, P.M. Inflammation and atherosclerosis: Role of C-reactive protein in risk assessment. Am. J. Med. 2004, 116, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Yadav, K. Pathogenesis of atherosclerosis a review. Med. Clin. Rev. 2016, 2, 1–6. [Google Scholar]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Andreini, D.; Martuscelli, E.; Guaricci, A.I.; Carrabba, N.; Magnoni, M.; Tedeschi, C.; Pelliccia, A.; Pontone, G. Clinical recommendations on Cardiac-CT in 2015: A position paper of the Working Group on Cardiac-CT and Nuclear Cardiology of the Italian Society of Cardiology. J. Cardiovasc. Med. 2016, 17, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Heseltine, T.D.; Murray, S.W.; Ruzsics, B.; Fisher, M. Latest Advances in Cardiac CT. Eur. Cardiol. 2020, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Met, R.; Bipat, S.; Legemate, D.A.; Reekers, J.A.; Koelemay, M.J. Diagnostic performance of computed tomography angiography in peripheral arterial disease: A systematic review and meta-analysis. JAMA 2009, 301, 415–424. [Google Scholar] [CrossRef]
- Rajiah, P. Updates in Vascular Computed Tomography. Radiol. Clin. N. Am. 2020, 58, 671–691. [Google Scholar] [CrossRef]
- Green, B.N.; Johnson, C.D.; Adams, A. Writing narrative literature reviews for peer-reviewed journals: Secrets of the trade. J. Chiropr. Med. 2006, 5, 101–117. [Google Scholar] [CrossRef]
- Kreisler, B. Photon counting Detectors: Concept, technical Challenges, and clinical outlook. Eur. J. Radiol. 2022, 149, 110229. [Google Scholar] [CrossRef] [PubMed]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-counting CT: Technical Principles and Clinical Prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef]
- Gnatyuk, V.; Maslyanchuk, O.; Solovan, M.; Brus, V.; Aoki, T. CdTe X/γ-ray Detectors with Different Contact Materials. Sensors 2021, 21, 3518. [Google Scholar] [CrossRef] [PubMed]
- Tortora, M.; Gemini, L.; D’Iglio, I.; Ugga, L.; Spadarella, G.; Cuocolo, R. Spectral Photon-Counting Computed Tomography: A Review on Technical Principles and Clinical Applications. J. Imaging 2022, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Iwanczyk, J.S. Vision 20/20: Single photon counting x-ray detectors in medical imaging. Med. Phys. 2013, 40, 100901. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yveborg, M.; Grönberg, F.; Xu, C.; Su, Q.; Danielsson, M.; Persson, M. Robustness of optimal energy thresholds in photon-counting spectral CT. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2020, 953, 163132. [Google Scholar] [CrossRef]
- Persson, M.; Bujila, R.; Nowik, P.; Andersson, H.; Kull, L.; Andersson, J.; Bornefalk, H.; Danielsson, M. Upper limits of the photon fluence rate on CT detectors: Case study on a commercial scanner. Med. Phys. 2016, 43, 4398–4411. [Google Scholar] [CrossRef]
- Danielsson, M.; Persson, M.; Sjölin, M. Photon-counting x-ray detectors for CT. Phys. Med. Biol. 2021, 66, 03TR01. [Google Scholar] [CrossRef]
- Hsieh, S.S.; Sjolin, M. Digital count summing vs analog charge summing for photon counting detectors: A performance simulation study. Med. Phys. 2018, 45, 4085–4093. [Google Scholar] [CrossRef]
- Tanguay, J.; Cunningham, I.A. Cascaded systems analysis of charge sharing in cadmium telluride photon-counting x-ray detectors. Med. Phys. 2018, 45, 1926–1941. [Google Scholar] [CrossRef]
- Procz, S.; Wartig, K.A.; Fauler, A.; Zwerger, A.; Luebke, J.; Ballabriga, R.; Blaj, G.; Campbell, M.; Mix, M.; Fiederle, M. Medipix3 CT for material sciences. J. Instrum. 2013, 8, C01025. [Google Scholar] [CrossRef]
- Yu, Z.; Leng, S.; Kappler, S.; Hahn, K.; Li, Z.; Halaweish, A.F.; Henning, A.; McCollough, C.H. Noise performance of low-dose CT: Comparison between an energy integrating detector and a photon counting detector using a whole-body research photon counting CT scanner. J. Med. Imaging 2016, 3, 043503. [Google Scholar] [CrossRef]
- Symons, R.; Cork, T.E.; Sahbaee, P.; Fuld, M.K.; Kappler, S.; Folio, L.R.; Bluemke, D.A.; Pourmorteza, A. Low-dose lung cancer screening with photon-counting CT: A feasibility study. Phys. Med. Biol. 2017, 62, 202–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si-Mohamed, S.A.; Sigovan, M.; Hsu, J.C.; Tatard-Leitman, V.; Chalabreysse, L.; Naha, P.C.; Garrivier, T.; Dessouky, R.; Carnaru, M.; Boussel, L.; et al. In Vivo Molecular K-Edge Imaging of Atherosclerotic Plaque Using Photon-counting CT. Radiology 2021, 300, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.; Rajendran, K.; Gong, H.; Zhou, W.; Halaweish, A.F.; Henning, A.; Kappler, S.; Baer, M.; Fletcher, J.G.; McCollough, C.H. 150-μm Spatial Resolution Using Photon-Counting Detector Computed Tomography Technology: Technical Performance and First Patient Images. Invest. Radiol. 2018, 53, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Ferda, J.; Vendiš, T.; Flohr, T.; Schmidt, B.; Henning, A.; Ulzheimer, S.; Pecen, L.; Ferdová, E.; Baxa, J.; Mírka, H. Computed tomography with a full FOV photon-counting detector in a clinical setting, the first experience. Eur. J. Radiol. 2021, 137, 109614. [Google Scholar] [CrossRef]
- Rajendran, K.; Petersilka, M.; Henning, A.; Shanblatt, E.R.; Schmidt, B.; Flohr, T.G.; Ferrero, A.; Baffour, F.; Diehn, F.E.; Yu, L.; et al. First Clinical Photon-counting Detector CT System: Technical Evaluation. Radiology 2022, 303, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Flohr, T.G.; Stierstorfer, K.; Süss, C.; Schmidt, B.; Primak, A.N.; McCollough, C.H. Novel ultrahigh resolution data acquisition and image reconstruction for multi-detector row CT. Med. Phys. 2007, 34, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Oostveen, L.J.; Boedeker, K.L.; Brink, M.; Prokop, M.; de Lange, F.; Sechopoulos, I. Physical evaluation of an ultra-high-resolution CT scanner. Eur. Radiol. 2020, 30, 2552–2560. [Google Scholar] [CrossRef]
- Leng, S.; Yu, Z.; Halaweish, A.; Kappler, S.; Hahn, K.; Henning, A.; Li, Z.; Lane, J.; Levin, D.L.; Jorgensen, S.; et al. Dose-efficient ultrahigh-resolution scan mode using a photon counting detector computed tomography system. J. Med. Imaging 2016, 3, 043504. [Google Scholar] [CrossRef]
- Swank, R.K. Absorption and noise in x-ray phosphors. J. Appl. Phys. 1973, 44, 4199–4203. [Google Scholar] [CrossRef]
- Iwanczyk, J.S.; Nygård, E.; Meirav, O.; Arenson, J.; Barber, W.C.; Hartsough, N.E.; Malakhov, N.; Wessel, J.C. Photon Counting Energy Dispersive Detector Arrays for X-ray Imaging. IEEE Trans. Nucl. Sci. 2009, 56, 535–542. [Google Scholar] [CrossRef]
- Shikhaliev, P.M. Energy-resolved computed tomography: First experimental results. Phys. Med. Biol. 2008, 53, 5595–5613. [Google Scholar] [CrossRef] [PubMed]
- Shikhaliev, P.M.; Fritz, S.G. Photon counting spectral CT versus conventional CT: Comparative evaluation for breast imaging application. Phys. Med. Biol. 2011, 56, 1905–1930. [Google Scholar] [CrossRef] [PubMed]
- Silkwood, J.D.; Matthews, K.L.; Shikhaliev, P.M. Photon counting spectral breast CT: Effect of adaptive filtration on CT numbers, noise, and contrast to noise ratio. Med. Phys. 2013, 40, 051905. [Google Scholar] [CrossRef] [PubMed]
- Giersch, J.; Niederlöhner, D.; Anton, G. The influence of energy weighting on X-ray imaging quality. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2004, 531, 68–74. [Google Scholar] [CrossRef]
- Schmidt, T.G. Optimal “image-based” weighting for energy-resolved CT. Med. Phys. 2009, 36, 3018–3027. [Google Scholar] [CrossRef]
- Brooks, R.A.; Chiro, G.D. Beam hardening in X-ray reconstructive tomography. Phys. Med. Biol. 1976, 21, 390–398. [Google Scholar] [CrossRef]
- Barrett, J.F.; Keat, N. Artifacts in CT: Recognition and avoidance. Radiographics 2004, 24, 1679–1691. [Google Scholar] [CrossRef]
- Shikhaliev, P.M. Beam hardening artefacts in computed tomography with photon counting, charge integrating and energy weighting detectors: A simulation study. Phys. Med. Biol. 2005, 50, 5813–5827. [Google Scholar] [CrossRef]
- Lee, C.-L.; Park, J.; Nam, S.; Choi, J.; Choi, Y.; Lee, S.; Lee, K.-Y.; Cho, M. Metal artifact reduction and tumor detection using photon-counting multi-energy computed tomography. PLoS ONE 2021, 16, e0247355. [Google Scholar] [CrossRef]
- Gutjahr, R.; Halaweish, A.F.; Yu, Z.; Leng, S.; Yu, L.; Li, Z.; Jorgensen, S.M.; Ritman, E.L.; Kappler, S.; McCollough, C.H. Human Imaging With Photon Counting-Based Computed Tomography at Clinical Dose Levels: Contrast-to-Noise Ratio and Cadaver Studies. Invest. Radiol. 2016, 51, 421–429. [Google Scholar] [CrossRef]
- Yeh, B.M.; FitzGerald, P.F.; Edic, P.M.; Lambert, J.W.; Colborn, R.E.; Marino, M.E.; Evans, P.M.; Roberts, J.C.; Wang, Z.J.; Wong, M.J.; et al. Opportunities for new CT contrast agents to maximize the diagnostic potential of emerging spectral CT technologies. Adv. Drug Deliv. Rev. 2017, 113, 201–222. [Google Scholar] [CrossRef] [Green Version]
- Hounsfield, G.N. Computerized transverse axial scanning (tomography): Part 1. Description of system. Br. J. Radiol. 1973, 46, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Coursey, C.A.; Nelson, R.C.; Boll, D.T.; Paulson, E.K.; Ho, L.M.; Neville, A.M.; Marin, D.; Gupta, R.T.; Schindera, S.T. Dual-Energy Multidetector CT: How Does It Work, What Can It Tell Us, and When Can We Use It in Abdominopelvic Imaging? RadioGraphics 2010, 30, 1037–1055. [Google Scholar] [CrossRef]
- Xue, Y.; Jiang, Y.; Yang, C.; Lyu, Q.; Wang, J.; Luo, C.; Zhang, L.; Desrosiers, C.; Feng, K.; Sun, X.; et al. Accurate Multi-Material Decomposition in Dual-Energy CT: A Phantom Study. IEEE Trans. Comput. Imaging 2019, 5, 515–529. [Google Scholar] [CrossRef]
- Liu, X.; Yu, L.; Primak, A.N.; McCollough, C.H. Quantitative imaging of element composition and mass fraction using dual-energy CT: Three-material decomposition. Med. Phys. 2009, 36, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Kappler, S.; Henning, A.; Kreisler, B.; Schoeck, F.; Stierstorfer, K.; Flohr, T. Photon Counting CT at Elevated X-ray Tube Currents: Contrast Stability, Image Noise and Multi-Energy Performance. In Proceedings of the Medical Imaging 2014: Physics of Medical Imaging, San Diego, CA, USA, 15–20 February 2014; SPIE: Bellingham, WA, USA, 2014; Volume 9033. [Google Scholar]
- Le, H.Q.; Molloi, S. Segmentation and quantification of materials with energy discriminating computed tomography: A phantom study. Med. Phys. 2011, 38, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Symons, R.; Reich, D.S.; Bagheri, M.; Cork, T.E.; Krauss, B.; Ulzheimer, S.; Kappler, S.; Bluemke, D.A.; Pourmorteza, A. Photon-Counting Computed Tomography for Vascular Imaging of the Head and Neck: First In Vivo Human Results. Invest. Radiol. 2018, 53, 135–142. [Google Scholar] [CrossRef]
- Leng, S.; Zhou, W.; Yu, Z.; Halaweish, A.; Krauss, B.; Schmidt, B.; Yu, L.; Kappler, S.; McCollough, C. Spectral performance of a whole-body research photon counting detector CT: Quantitative accuracy in derived image sets. Phys. Med. Biol. 2017, 62, 7216–7232. [Google Scholar] [CrossRef] [PubMed]
- Laukamp, K.R.; Lennartz, S.; Neuhaus, V.F.; Große Hokamp, N.; Rau, R.; Le Blanc, M.; Abdullayev, N.; Mpotsaris, A.; Maintz, D.; Borggrefe, J. CT metal artifacts in patients with total hip replacements: For artifact reduction monoenergetic reconstructions and post-processing algorithms are both efficient but not similar. Eur. Radiol. 2018, 28, 4524–4533. [Google Scholar] [CrossRef]
- Mergen, V.; Racine, D.; Jungblut, L.; Sartoretti, T.; Bickel, S.; Monnin, P.; Higashigaito, K.; Martini, K.; Alkadhi, H.; Euler, A. Virtual Noncontrast Abdominal Imaging with Photon-counting Detector CT. Radiology 2022, 305, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Symons, R.; Krauss, B.; Sahbaee, P.; Cork, T.E.; Lakshmanan, M.N.; Bluemke, D.A.; Pourmorteza, A. Photon-counting CT for simultaneous imaging of multiple contrast agents in the abdomen: An in vivo study. Med. Phys. 2017, 44, 5120–5127. [Google Scholar] [CrossRef] [PubMed]
- Faby, S.; Kuchenbecker, S.; Sawall, S.; Simons, D.; Schlemmer, H.P.; Lell, M.; Kachelrieß, M. Performance of today’s dual energy CT and future multi energy CT in virtual non-contrast imaging and in iodine quantification: A simulation study. Med. Phys. 2015, 42, 4349–4366. [Google Scholar] [CrossRef] [PubMed]
- Yveborg, M.; Danielsson, M.; Bornefalk, H. Theoretical comparison of a dual energy system and photon counting silicon detector used for material quantification in spectral CT. IEEE Trans. Med. Imaging 2015, 34, 796–806. [Google Scholar] [CrossRef]
- Roessl, E.; Proksa, R. K-edge imaging in x-ray computed tomography using multi-bin photon counting detectors. Phys. Med. Biol. 2007, 52, 4679–4696. [Google Scholar] [CrossRef] [PubMed]
- Schlomka, J.P.; Roessl, E.; Dorscheid, R.; Dill, S.; Martens, G.; Istel, T.; Bäumer, C.; Herrmann, C.; Steadman, R.; Zeitler, G.; et al. Experimental feasibility of multi-energy photon-counting K-edge imaging in pre-clinical computed tomography. Phys. Med. Biol. 2008, 53, 4031–4047. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Schirra, C.O.; Senpan, A.; Schmieder, A.H.; Stacy, A.J.; Roessl, E.; Thran, A.; Wickline, S.A.; Proska, R.; Lanza, G.M. An early investigation of ytterbium nanocolloids for selective and quantitative “multicolor” spectral CT imaging. ACS Nano. 2012, 6, 3364–3370. [Google Scholar] [CrossRef]
- Si-Mohamed, S.; Cormode, D.P.; Bar-Ness, D.; Sigovan, M.; Naha, P.C.; Langlois, J.-B.; Chalabreysse, L.; Coulon, P.; Blevis, I.; Roessl, E.; et al. Evaluation of spectral photon counting computed tomography K-edge imaging for determination of gold nanoparticle biodistribution in vivo. Nanoscale 2017, 9, 18246–18257. [Google Scholar] [CrossRef]
- Schirra, C.O.; Brendel, B.; Anastasio, M.A.; Roessl, E. Spectral CT: A technology primer for contrast agent development. Contrast. Media. Mol. Imaging 2014, 9, 62–70. [Google Scholar] [CrossRef]
- Müllner, M.; Schlattl, H.; Hoeschen, C.; Dietrich, O. Feasibility of spectral CT imaging for the detection of liver lesions with gold-based contrast agents—A simulation study. Phys. Med. 2015, 31, 875–881. [Google Scholar] [CrossRef]
- Kim, J.; Bar-Ness, D.; Si-Mohamed, S.; Coulon, P.; Blevis, I.; Douek, P.; Cormode, D.P. Assessment of candidate elements for development of spectral photon-counting CT specific contrast agents. Sci. Rep. 2018, 8, 12119. [Google Scholar] [CrossRef] [PubMed]
- Cormode, D.P.; Roessl, E.; Thran, A.; Skajaa, T.; Gordon, R.E.; Schlomka, J.P.; Fuster, V.; Fisher, E.A.; Mulder, W.J.; Proksa, R.; et al. Atherosclerotic plaque composition: Analysis with multicolor CT and targeted gold nanoparticles. Radiology 2010, 256, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Balegamire, J.; Vandamme, M.; Chereul, E.; Si-Mohamed, S.; Azzouz Maache, S.; Almouazen, E.; Ettouati, L.; Fessi, H.; Boussel, L.; Douek, P.; et al. Iodinated polymer nanoparticles as contrast agent for spectral photon counting computed tomography. Biomater. Sci. 2020, 8, 5715–5728. [Google Scholar] [CrossRef]
- Dong, Y.C.; Kumar, A.; Rosario-Berríos, D.N.; Si-Mohamed, S.; Hsu, J.C.; Nieves, L.M.; Douek, P.; Noël, P.B.; Cormode, D.P. Ytterbium Nanoparticle Contrast Agents for Conventional and Spectral Photon-Counting CT and Their Applications for Hydrogel Imaging. ACS Appl. Mater. Interfaces 2022, 14, 39274–39284. [Google Scholar] [CrossRef] [PubMed]
- Muenzel, D.; Daerr, H.; Proksa, R.; Fingerle, A.A.; Kopp, F.K.; Douek, P.; Herzen, J.; Pfeiffer, F.; Rummeny, E.J.; Noël, P.B. Simultaneous dual-contrast multi-phase liver imaging using spectral photon-counting computed tomography: A proof-of-concept study. Eur. Radiol. Exp. 2017, 1, 25. [Google Scholar] [CrossRef] [PubMed]
- Symons, R.; Cork, T.E.; Lakshmanan, M.N.; Evers, R.; Davies-Venn, C.; Rice, K.A.; Thomas, M.L.; Liu, C.Y.; Kappler, S.; Ulzheimer, S.; et al. Dual-contrast agent photon-counting computed tomography of the heart: Initial experience. Int. J. Cardiovasc. Imaging 2017, 33, 1253–1261. [Google Scholar] [CrossRef]
- Cormode, D.P.; Si-Mohamed, S.; Bar-Ness, D.; Sigovan, M.; Naha, P.C.; Balegamire, J.; Lavenne, F.; Coulon, P.; Roessl, E.; Bartels, M.; et al. Multicolor spectral photon-counting computed tomography: In vivo dual contrast imaging with a high count rate scanner. Sci. Rep. 2017, 7, 4784. [Google Scholar] [CrossRef]
- Rajagopal, J.R.; Farhadi, F.; Solomon, J.; Sahbaee, P.; Saboury, B.; Pritchard, W.F.; Jones, E.C.; Samei, E. Comparison of Low Dose Performance of Photon-Counting and Energy Integrating CT. Acad. Radiol. 2021, 28, 1754–1760. [Google Scholar] [CrossRef]
- Channon, K.M.; Newby, D.E.; Nicol, E.D.; Deanfield, J. Cardiovascular computed tomography imaging for coronary artery disease risk: Plaque, flow and fat. Heart 2022, 108, 1510–1515. [Google Scholar] [CrossRef]
- Schuijf, J.D.; Lima, J.A.C.; Boedeker, K.L.; Takagi, H.; Tanaka, R.; Yoshioka, K.; Arbab-Zadeh, A. CT imaging with ultra-high-resolution: Opportunities for cardiovascular imaging in clinical practice. J. Cardiovasc. Comput. Tomogr. 2022, 16, 388–396. [Google Scholar] [CrossRef]
- Soschynski, M.; Hagen, F.; Baumann, S.; Hagar, M.T.; Weiss, J.; Krauss, T.; Schlett, C.L.; von zur Mühlen, C.; Bamberg, F.; Nikolaou, K.; et al. High Temporal Resolution Dual-Source Photon-Counting CT for Coronary Artery Disease: Initial Multicenter Clinical Experience. J. Clin. Med. 2022, 11, 6003. [Google Scholar] [CrossRef]
- Saba, L.; Anzidei, M.; Sanfilippo, R.; Montisci, R.; Lucatelli, P.; Catalano, C.; Passariello, R.; Mallarini, G. Imaging of the carotid artery. Atherosclerosis 2012, 220, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, E.S.; Symons, S.P.; Fox, A.J. Correlation of carotid stenosis diameter and cross-sectional areas with CT angiography. AJNR Am. J. Neuroradiol. 2006, 27, 638–642. [Google Scholar] [PubMed]
- Kruk, M.; Noll, D.; Achenbach, S.; Mintz, G.S.; Pręgowski, J.; Kaczmarska, E.; Kryczka, K.; Pracoń, R.; Dzielińska, Z.; Sleszycka, J.; et al. Impact of coronary artery calcium characteristics on accuracy of CT angiography. JACC Cardiovasc. Imaging 2014, 7, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Levin, D.C.; Halpern, E.J.; Fischman, D.; Savage, M.; Walinsky, P. Accuracy of MDCT in assessing the degree of stenosis caused by calcified coronary artery plaques. AJR Am. J. Roentgenol. 2008, 191, 1676–1683. [Google Scholar] [CrossRef]
- Li, Z.; Leng, S.; Halaweish, A.F.; Yu, Z.; Yu, L.; Ritman, E.L.; McCollough, C.H. Overcoming calcium blooming and improving the quantification accuracy of percent area luminal stenosis by material decomposition of multi-energy computed tomography datasets. J. Med. Imaging 2020, 7, 053501. [Google Scholar] [CrossRef] [PubMed]
- Bittner, D.O.; Mayrhofer, T.; Budoff, M.; Szilveszter, B.; Foldyna, B.; Hallett, T.R.; Ivanov, A.; Janjua, S.; Meyersohn, N.M.; Staziaki, P.V.; et al. Prognostic Value of Coronary CTA in Stable Chest Pain: CAD-RADS, CAC, and Cardiovascular Events in PROMISE. JACC Cardiovasc. Imaging 2020, 13, 1534–1545. [Google Scholar] [CrossRef]
- Williams, M.C.; Kwiecinski, J.; Doris, M.; McElhinney, P.; D’Souza, M.S.; Cadet, S.; Adamson, P.D.; Moss, A.J.; Alam, S.; Hunter, A.; et al. Low-Attenuation Noncalcified Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction: Results From the Multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART). Circulation 2020, 141, 1452–1462. [Google Scholar] [CrossRef]
- Boussel, L.; Coulon, P.; Thran, A.; Roessl, E.; Martens, G.; Sigovan, M.; Douek, P. Photon counting spectral CT component analysis of coronary artery atherosclerotic plaque samples. Br. J. Radiol. 2014, 87, 20130798. [Google Scholar] [CrossRef]
- Si-Mohamed, S.A.; Boccalini, S.; Lacombe, H.; Diaw, A.; Varasteh, M.; Rodesch, P.-A.; Dessouky, R.; Villien, M.; Tatard-Leitman, V.; Bochaton, T.; et al. Coronary CT Angiography with Photon-counting CT: First-In-Human Results. Radiology 2022, 303, 303–313. [Google Scholar] [CrossRef]
- Mergen, V.; Eberhard, M.; Manka, R.; Euler, A.; Alkadhi, H. First in-human quantitative plaque characterization with ultra-high resolution coronary photon-counting CT angiography. Front. Cardiovasc. Med. 2022, 9, 981012. [Google Scholar] [CrossRef]
- Hecht, H.; Blaha, M.J.; Berman, D.S.; Nasir, K.; Budoff, M.; Leipsic, J.; Blankstein, R.; Narula, J.; Rumberger, J.; Shaw, L.J. Clinical indications for coronary artery calcium scoring in asymptomatic patients: Expert consensus statement from the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2017, 11, 157–168. [Google Scholar] [CrossRef] [PubMed]
- van der Werf, N.R.; Rodesch, P.A.; Si-Mohamed, S.; van Hamersvelt, R.W.; Greuter, M.J.W.; Leiner, T.; Boussel, L.; Willemink, M.J.; Douek, P. Improved coronary calcium detection and quantification with low-dose full field-of-view photon-counting CT: A phantom study. Eur. Radiol. 2022, 32, 3447–3457. [Google Scholar] [CrossRef] [PubMed]
- Symons, R.; Sandfort, V.; Mallek, M.; Ulzheimer, S.; Pourmorteza, A. Coronary artery calcium scoring with photon-counting CT: First in vivo human experience. Int. J. Cardiovasc. Imaging 2019, 35, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Sandstedt, M.; Marsh, J.; Rajendran, K.; Gong, H.; Tao, S.; Persson, A.; Leng, S.; McCollough, C. Improved coronary calcification quantification using photon-counting-detector CT: An ex vivo study in cadaveric specimens. Eur. Radiol. 2021, 31, 6621–6630. [Google Scholar] [CrossRef]
- van der Werf, N.R.; Greuter, M.J.W.; Booij, R.; van der Lugt, A.; Budde, R.P.J.; van Straten, M. Coronary calcium scores on dual-source photon-counting computed tomography: An adapted Agatston methodology aimed at radiation dose reduction. Eur. Radiol. 2022, 32, 5201–5209. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006, 47, C13–C18. [Google Scholar] [CrossRef]
- Koenig, W.; Khuseyinova, N. Biomarkers of Atherosclerotic Plaque Instability and Rupture. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 15–26. [Google Scholar] [CrossRef]
- Hyafil, F.; Cornily, J.C.; Feig, J.E.; Gordon, R.; Vucic, E.; Amirbekian, V.; Fisher, E.A.; Fuster, V.; Feldman, L.J.; Fayad, Z.A. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat. Med. 2007, 13, 636–641. [Google Scholar] [CrossRef]
- Sandfort, V.; Persson, M.; Pourmorteza, A.; Noël, P.B.; Fleischmann, D.; Willemink, M.J. Spectral photon-counting CT in cardiovascular imaging. J. Cardiovasc. Comput. Tomogr. 2021, 15, 218–225. [Google Scholar] [CrossRef]
- Dangas, G.D.; Claessen, B.E.; Caixeta, A.; Sanidas, E.A.; Mintz, G.S.; Mehran, R. In-stent restenosis in the drug-eluting stent era. J. Am. Coll. Cardiol. 2010, 56, 1897–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, S.; Achenbach, S.; Bengel, F.; Burgstahler, C.; Cademartiri, F.; de Feyter, P.; George, R.; Kaufmann, P.; Kopp, A.F.; Knuuti, J.; et al. Cardiac computed tomography: Indications, applications, limitations, and training requirements: Report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur. Heart J. 2008, 29, 531–556. [Google Scholar] [CrossRef] [PubMed]
- Mannil, M.; Hickethier, T.; von Spiczak, J.; Baer, M.; Henning, A.; Hertel, M.; Schmidt, B.; Flohr, T.; Maintz, D.; Alkadhi, H. Photon-Counting CT: High-Resolution Imaging of Coronary Stents. Invest. Radiol. 2018, 53, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Symons, R.; De Bruecker, Y.; Roosen, J.; Van Camp, L.; Cork, T.E.; Kappler, S.; Ulzheimer, S.; Sandfort, V.; Bluemke, D.A.; Pourmorteza, A. Quarter-millimeter spectral coronary stent imaging with photon-counting CT: Initial experience. J. Cardiovasc. Comput. Tomogr. 2018, 12, 509–515. [Google Scholar] [CrossRef] [PubMed]
- von Spiczak, J.; Mannil, M.; Peters, B.; Hickethier, T.; Baer, M.; Henning, A.; Schmidt, B.; Flohr, T.; Manka, R.; Maintz, D.; et al. Photon Counting Computed Tomography With Dedicated Sharp Convolution Kernels: Tapping the Potential of a New Technology for Stent Imaging. Invest. Radiol. 2018, 53, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, J.R.; Farhadi, F.; Richards, T.; Nikpanah, M.; Sahbaee, P.; Shanbhag, S.M.; Bandettini, W.P.; Saboury, B.; Malayeri, A.A.; Pritchard, W.F.; et al. Evaluation of Coronary Plaques and Stents with Conventional and Photon-counting CT: Benefits of High-Resolution Photon-counting CT. Radiol. Cardiothorac. Imaging 2021, 3, e210102. [Google Scholar] [CrossRef]
- Feuerlein, S.; Roessl, E.; Proksa, R.; Martens, G.; Klass, O.; Jeltsch, M.; Rasche, V.; Brambs, H.J.; Hoffmann, M.H.; Schlomka, J.P. Multienergy photon-counting K-edge imaging: Potential for improved luminal depiction in vascular imaging. Radiology 2008, 249, 1010–1016. [Google Scholar] [CrossRef]
- Bratke, G.; Hickethier, T.; Bar-Ness, D.; Bunck, A.C.; Maintz, D.; Pahn, G.; Coulon, P.; Si-Mohamed, S.; Douek, P.; Sigovan, M. Spectral Photon-Counting Computed Tomography for Coronary Stent Imaging: Evaluation of the Potential Clinical Impact for the Delineation of In-Stent Restenosis. Invest. Radiol. 2020, 55, 61–67. [Google Scholar] [CrossRef]
- Mathiesen, E.B.; Johnsen, S.H.; Wilsgaard, T.; Bønaa, K.H.; Løchen, M.-L.; Njølstad, I. Carotid Plaque Area and Intima-Media Thickness in Prediction of First-Ever Ischemic Stroke. Stroke 2011, 42, 972–978. [Google Scholar] [CrossRef]
- Brinjikji, W.; Huston, J., 3rd; Rabinstein, A.A.; Kim, G.M.; Lerman, A.; Lanzino, G. Contemporary carotid imaging: From degree of stenosis to plaque vulnerability. J. Neurosurg. 2016, 124, 27–42. [Google Scholar] [CrossRef]
- Weir-McCall, J.R.; Wang, R.; Halankar, J.; Hsieh, J.; Hague, C.J.; Rosenblatt, S.; Fan, Z.; Sellers, S.L.; Murphy, D.T.; Blanke, P.; et al. Effect of a calcium deblooming algorithm on accuracy of coronary computed tomography angiography. J. Cardiovasc. Comput. Tomogr. 2020, 14, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Hetterich, H.; Willner, M.; Fill, S.; Herzen, J.; Bamberg, F.; Hipp, A.; Schüller, U.; Adam-Neumair, S.; Wirth, S.; Reiser, M.; et al. Phase-contrast CT: Qualitative and quantitative evaluation of atherosclerotic carotid artery plaque. Radiology 2014, 271, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Sartoretti, T.; Eberhard, M.; Rüschoff, J.H.; Pietsch, H.; Jost, G.; Nowak, T.; Schmidt, B.; Flohr, T.; Euler, A.; Alkadhi, H. Photon-counting CT with tungsten as contrast medium: Experimental evidence of vessel lumen and plaque visualization. Atherosclerosis 2020, 310, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Parodi, J.C.; Palmaz, J.C.; Barone, H.D. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann. Vasc. Surg. 1991, 5, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Prinssen, M.; Verhoeven, E.L.; Buth, J.; Cuypers, P.W.; van Sambeek, M.R.; Balm, R.; Buskens, E.; Grobbee, D.E.; Blankensteijn, J.D. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N. Engl. J. Med. 2004, 351, 1607–1618. [Google Scholar] [CrossRef]
- Chaikof, E.L.; Brewster, D.C.; Dalman, R.L.; Makaroun, M.S.; Illig, K.A.; Sicard, G.A.; Timaran, C.H.; Upchurch, G.R., Jr.; Veith, F.J. SVS practice guidelines for the care of patients with an abdominal aortic aneurysm: Executive summary. J. Vasc. Surg. 2009, 50, 880–896. [Google Scholar] [CrossRef]
- White, S.B.; Stavropoulos, S.W. Management of Endoleaks following Endovascular Aneurysm Repair. Semin. Interv. Radiol. 2009, 26, 33–38. [Google Scholar] [CrossRef]
- Kalef-Ezra, J.A.; Karavasilis, S.; Ziogas, D.; Dristiliaris, D.; Michalis, L.K.; Matsagas, M. Radiation burden of patients undergoing endovascular abdominal aortic aneurysm repair. J. Vasc. Surg. 2009, 49, 283–287; discussion 287. [Google Scholar] [CrossRef]
- Javor, D.; Wressnegger, A.; Unterhumer, S.; Kollndorfer, K.; Nolz, R.; Beitzke, D.; Loewe, C. Endoleak detection using single-acquisition split-bolus dual-energy computer tomography (DECT). Eur. Radiol. 2017, 27, 1622–1630. [Google Scholar] [CrossRef]
- Dangelmaier, J.; Bar-Ness, D.; Daerr, H.; Muenzel, D.; Si-Mohamed, S.; Ehn, S.; Fingerle, A.A.; Kimm, M.A.; Kopp, F.K.; Boussel, L.; et al. Experimental feasibility of spectral photon-counting computed tomography with two contrast agents for the detection of endoleaks following endovascular aortic repair. Eur. Radiol. 2018, 28, 3318–3325. [Google Scholar] [CrossRef]
- Sigovan, M.; Si-Mohamed, S.; Bar-Ness, D.; Mitchell, J.; Langlois, J.B.; Coulon, P.; Roessl, E.; Blevis, I.; Rokni, M.; Rioufol, G.; et al. Feasibility of improving vascular imaging in the presence of metallic stents using spectral photon counting CT and K-edge imaging. Sci. Rep. 2019, 9, 19850. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meloni, A.; Frijia, F.; Panetta, D.; Degiorgi, G.; De Gori, C.; Maffei, E.; Clemente, A.; Positano, V.; Cademartiri, F. Photon-Counting Computed Tomography (PCCT): Technical Background and Cardio-Vascular Applications. Diagnostics 2023, 13, 645. https://doi.org/10.3390/diagnostics13040645
Meloni A, Frijia F, Panetta D, Degiorgi G, De Gori C, Maffei E, Clemente A, Positano V, Cademartiri F. Photon-Counting Computed Tomography (PCCT): Technical Background and Cardio-Vascular Applications. Diagnostics. 2023; 13(4):645. https://doi.org/10.3390/diagnostics13040645
Chicago/Turabian StyleMeloni, Antonella, Francesca Frijia, Daniele Panetta, Giulia Degiorgi, Carmelo De Gori, Erica Maffei, Alberto Clemente, Vincenzo Positano, and Filippo Cademartiri. 2023. "Photon-Counting Computed Tomography (PCCT): Technical Background and Cardio-Vascular Applications" Diagnostics 13, no. 4: 645. https://doi.org/10.3390/diagnostics13040645
APA StyleMeloni, A., Frijia, F., Panetta, D., Degiorgi, G., De Gori, C., Maffei, E., Clemente, A., Positano, V., & Cademartiri, F. (2023). Photon-Counting Computed Tomography (PCCT): Technical Background and Cardio-Vascular Applications. Diagnostics, 13(4), 645. https://doi.org/10.3390/diagnostics13040645









