Combined 3D Endoanal Ultrasound and Transperineal Ultrasound Improves the Detection of Anal Sphincter Defects †
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Ultrasound Assessment of the Anal Sphincter
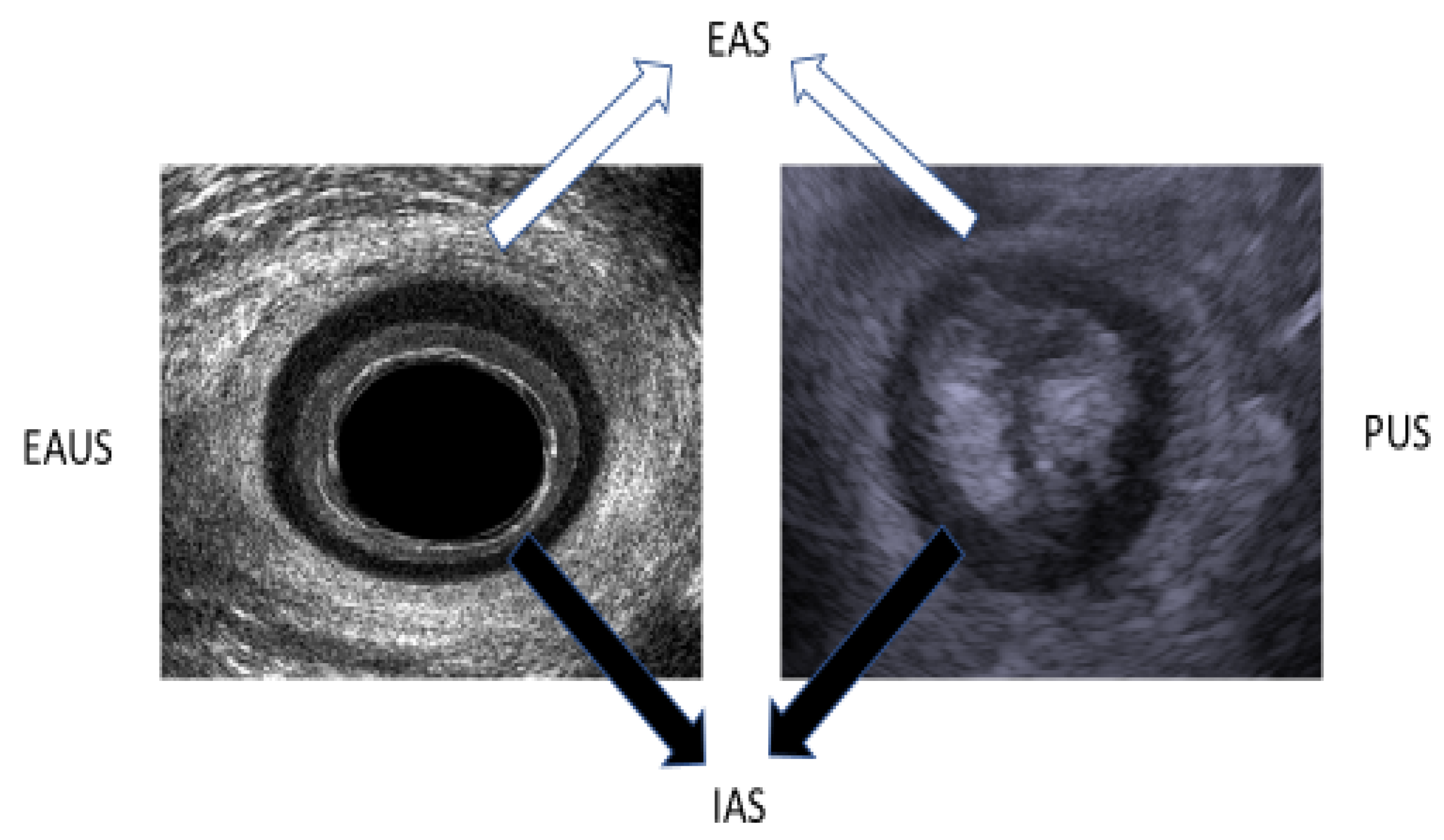
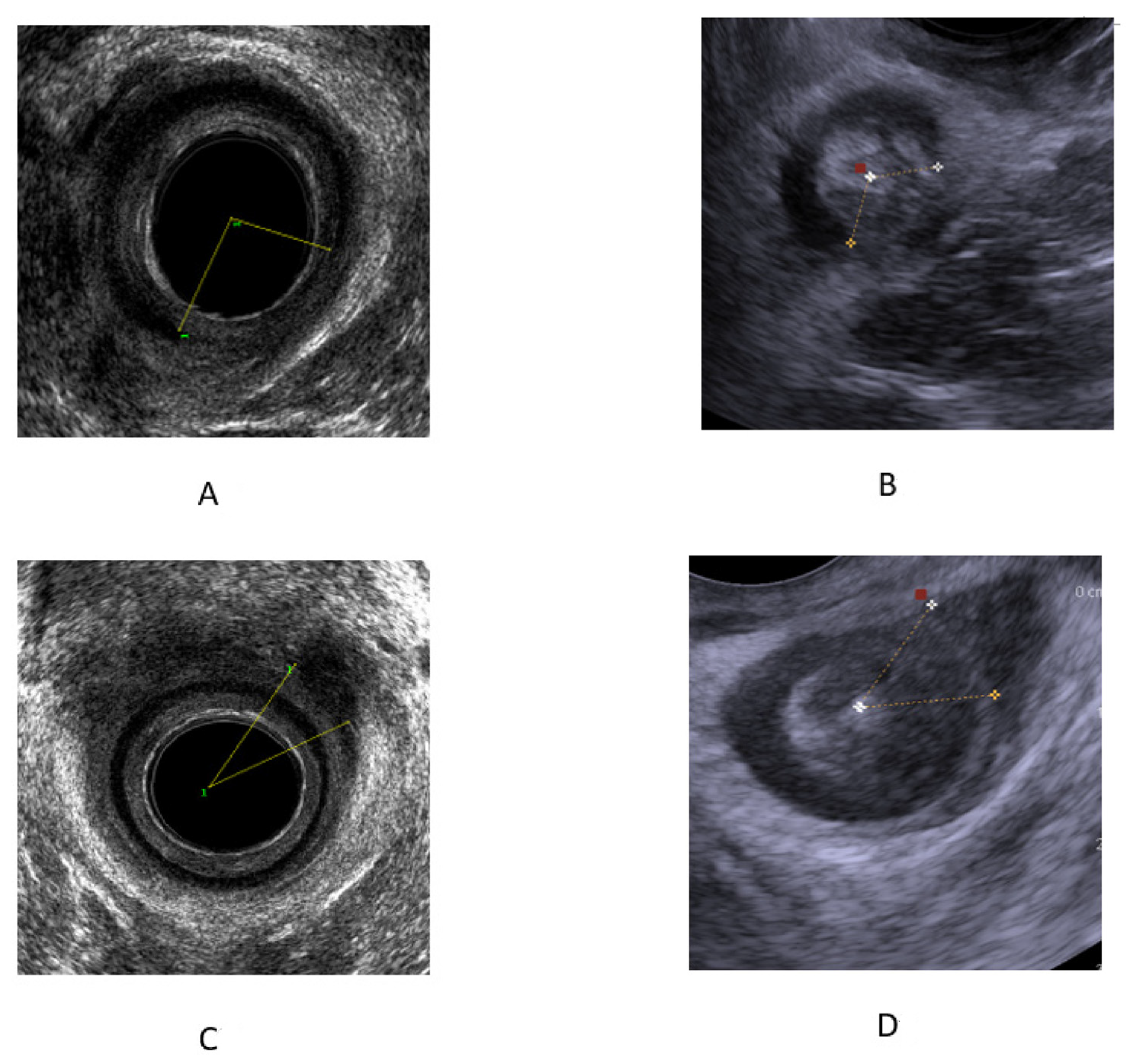
2.3. 3D EAUS
2.4. TPUS
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bharucha, A.E.; Zinsmeister, A.R.; Locke, G.R.; Seide, B.M.; McKeon, K.; Schleck, C.D.; Melton, L.J. Prevalence and burden of fecal incontinence: A population-based study in women. Gastroenterology 2005, 129, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, W.E.; Borrud, L.; Goode, P.S.; Meikle, S.; Mueller, E.R.; Tuteja, A.; Weidner, A.; Weinstein, M.; Ye, W.; Pelvic Floor Disorders Network. Fecal incontinence in US adults: Epidemiology and risk factors. Gastroenterology 2009, 137, 512–517. [Google Scholar] [CrossRef]
- Cotterill, N.; Norton, C.; Avery, K.N.; Abrams, P.; Donovan, J.L. Psychometric evaluation of a new patient-completed questionnaire for evaluating anal incontinence symptoms and impact on quality of life: The ICIQ-B. Dis. Colon. Rectum. 2011, 54, 1235–1250. [Google Scholar] [CrossRef]
- Perry, S.; Shaw, C.; McGrother, C.; Matthews, R.J.; Assassa, R.P.; Dallosso, H.; Williams, K.; Brittain, K.R.; Azam, U.; Clarke, M.; et al. The prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut 2002, 50, 480–484. [Google Scholar] [CrossRef]
- Jorge, J.M.; Wexner, S.D. Etiology and management of fecal incontinence. Dis. Colon. Rectum. 1993, 36, 77–97. [Google Scholar] [CrossRef]
- Zbar, A.P.; Beer-Gabel, M.; Chiappa, A.C.; Aslam, M. Fecal incontinence after minor anorectal surgery. Dis. Colon. Rectum. 2001, 44, 1610–1619. [Google Scholar] [CrossRef]
- Nevler, A. The epidemiology of anal incontinence and symptom severity scoring. Gastroenterol. Rep. 2014, 2, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Thiagamoorthy, G.; Johnson, A.; Thakar, R.; Sultan, A.H. National survey of perineal trauma 471and its subsequent management in the United Kingdom. Int. Urogynecol. J. 2014, 25, 1621–1627. [Google Scholar] [CrossRef]
- Landy, H.J.; Laughon, S.K.; Bailit, J.L.; Kominiarek, M.A.; Gonzalez-Quintero, V.H.; Ramirez, M.; Haberman, S.; Hibbard, J.; Wilkins, I.; Branch, D.W.; et al. Characteristics Associated with Severe Perineal and Cervical Lacerations During Vaginal Delivery. Obstet. Gynecol. 2011, 117, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Gurol-Urganci, I.; Cromwell, D.A.; Edozien, L.C.; Mahmood, T.A.; Adams, E.J.; Richmond, D.H.; Templeton, A.; van der Meulen, J.H. Third-and fourth-degree perineal tears among primiparous women in England between 2000 and 2012: Time trends and risk factors. BJOG Int. J. Obstet. Gy. 2013, 120, 1516–1525. [Google Scholar] [CrossRef]
- LaCross, A.; Groff, M.; Smaldone, A. Obstetric Anal Sphincter Injury and Anal Incontinence Following Vaginal Birth: A Systematic Review and Meta-Analysis. J. Midwifery Women’s Health 2015, 60, 37–47. [Google Scholar] [CrossRef]
- Hayden, D.M.; Weiss, E.G. Fecal incontinence. Fecal incontinence: Etiology, evaluation, and treatment. Clin. Colon. Rectal. Surg. 2011, 24, 64–70. [Google Scholar] [CrossRef]
- Bliss, D.J.; Mimura, T.; Berghmans, B. Assessment and Conservative Management of Fecal Incontinence and Quality of Life in Adults; Abrams, P., Cardozo, L., Wagg, A., Wein, A., Eds.; Incontinence. 6th ed.; ICI-ICS. International Continence Society: Bristol, UK, 2017; pp. 1993–2085. [Google Scholar]
- Nuernberg, D.; Saftoiu, A.; Barreiros, A.P.; Burmester, E.; Ivan, E.T.; Clevert, D.A.; Dietrich, C.F.; Gilja, O.H.; Lorentzen, T.; Maconi, G.; et al. EFSUMB Recommendations for Gastrointestinal Ultrasound Part 3: Endorectal, Endoanal and Perineal Ultrasound. Ultrasound Int. Open 2019, 5, E34–E51. [Google Scholar] [CrossRef]
- Valsky, D.V.; Messing, B.; Petkova, R.; Savchev, S.; Rosenak, D.; Hochner-Celnikier, D.; Yagel, S. Postpartum evaluation of the anal sphincter by transperineal three-dimensional ultrasound in primiparous women after vaginal delivery and following surgical repair of third-degree tears by the overlapping technique. Ultrasound Obstet. Gynecol. 2007, 29, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Ros, C.; Martínez-Franco, E.; Wozniak, M.M.; Cassado, J.; Santoro, G.A.; Elías, N.; López, M.; Palacio, M.; Wieczorek, A.P.; Espuña-Pons, M. Postpartum 2D and 3D ultrasound evaluation of the anal sphincter complex in women with obstetric anal sphincter injury. Ultrasound Obstet. Gynecol. 2017, 49, 508–514. [Google Scholar] [CrossRef]
- Bartram, C.I.; Sultan, A.H. Anal endosonography in faecal incontinence. Gut 1995, 37, 4–6. [Google Scholar] [CrossRef]
- Rostaminia, G.; White, D.; Quiroz, L.H.; Shobeiri, S.A. 3D pelvic floor ultrasound findings and severity of anal incontinence. Int. Urogynecol. J. 2014, 25, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Speksnijder, L.; Oom, D.M.J.; DELeeuw, J.W.; Steensma, A.B. Which factors are associated with anal incontinence after obstetric anal sphincter injury? Ultrasound Obstet. Gynecol. 2021, 58, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Taithongchai, A.; van Gruting, I.M.; Volløyhaug, I.; Arendsen, L.P.; Sultan, A.H.; Thakar, R. Comparing the diagnostic accuracy of 3 ultrasound modalities for diagnosing obstetric anal sphincter injuries. Am. J. Obstet. Gynecol. 2019, 221, 134. [Google Scholar] [CrossRef] [PubMed]
- Roche, B.; Deléaval, J.; Fransioli, A.; Marti, M.C. Comparison of transanal and external perineal ultrasonography. Eur. Radiol. 2001, 11, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Roos, A.M.; Abdool, Z.; Sultan, A.H.; Thakar, R. The diagnostic accuracy of endovaginal and transperineal ultrasound for detecting anal sphincter defects: The PREDICT study. Clin. Radiol. 2001, 66, 597–604. [Google Scholar] [CrossRef]
- Cornelia, L.; Stephan, B.; Michel, B.; Antoine, W.; Felix, K. Trans-perineal versus endo-anal ultrasound in the detection of anal sphincter tears. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 103, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Oom, D.M.; West, R.L.; Schouten, W.R.; Steensma, A.B. Detection of anal sphincter defects in female patients with fecal incontinence: A comparison of 3-dimensional transperineal ultrasound and 2-dimensional endoanal ultrasound. Dis. Colon. Rectum. 2012, 55, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.M.; Pretorius, D.H.; Jung, S.A.; Nager, C.W.; Mittal, R.K. Transperineal three-dimensional ultrasound imaging for detection of anatomic defects in the anal sphincter complex muscles. Clin. Gastroenterol. Hepatol. 2009, 7, 205–211. [Google Scholar] [CrossRef]
- Stuart, A.; Ignell, C.; Örnö, A.K. Comparison of transperineal and endoanal ultrasound in detecting residual obstetric anal sphincter injury. Acta Obstet. Gynecol. Scand. 2019, 98, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Zbar, A.P.; Horesh, N.; Bucholtz, V.; Zmora, O.; Beer-Gabel, M.; Carter, D. Are there specific endosonographic features in Crohn’s patients with perianal fistulae? J. Crohns. Colitis 2013, 7, 490–496. [Google Scholar] [CrossRef]
- Martinez Franco, E.; Ros, C.; Santoro, G.A.; Cassadó Garriga, J.; Amat Tardiu, L.; Cuadras, D.; Espuña, M. Transperineal anal sphincter complex evaluation after obstetric anal sphincter injuries: With or without tomographic ultrasound imaging technique? Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 257, 70–75. [Google Scholar] [CrossRef]
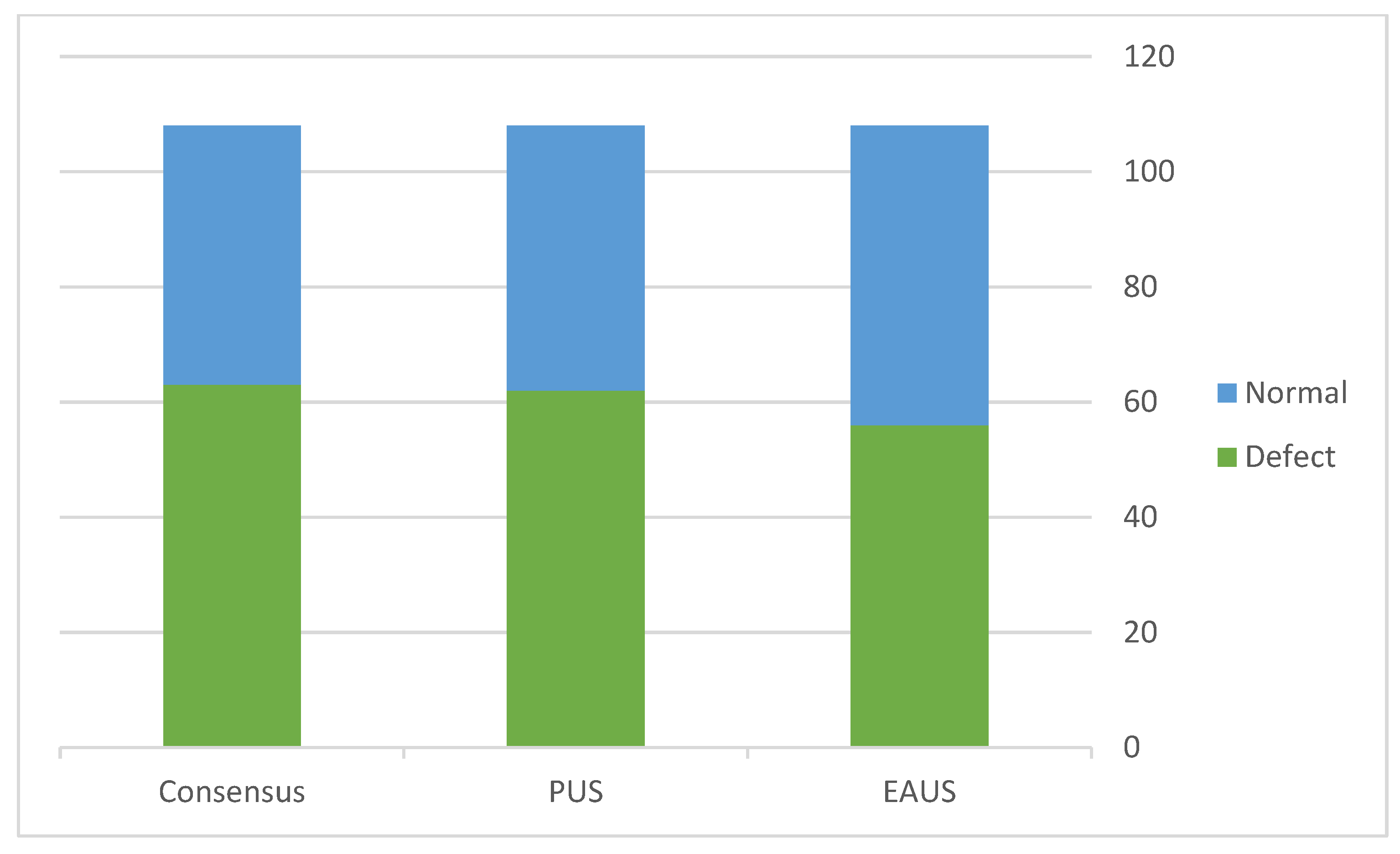
| STD | ||
|---|---|---|
| Age (years) (Mean) | 69 | 13 |
| Female/male | 106/2 | |
| Ethnicity White Caucasians | 100% | |
| Deliveries (mean) | 2.6 | 1.7 |
| Cesarean section (% of all deliveries) | 14 (5%) | |
| Assisted deliveries (% of all deliveries) | 10 (3.6%) | |
| Previous anorectal surgery (% of the study cohort) | 28 (26%) |
| Consensus | 3-D EAUS | TPUS | |
|---|---|---|---|
| Defects | 61 | 53 | 59 |
| EAS | 38 | 36 | 38 |
| IAS | 19 | 13 | 18 |
| EAS + IAS | 4 | 4 | 4 |
| Normal | 47 | 56 | 49 |
| Sensitivity | 0.86 | 0.96 | |
| Specificity | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carter, D.; Ram, E.; Engel, T. Combined 3D Endoanal Ultrasound and Transperineal Ultrasound Improves the Detection of Anal Sphincter Defects. Diagnostics 2023, 13, 682. https://doi.org/10.3390/diagnostics13040682
Carter D, Ram E, Engel T. Combined 3D Endoanal Ultrasound and Transperineal Ultrasound Improves the Detection of Anal Sphincter Defects. Diagnostics. 2023; 13(4):682. https://doi.org/10.3390/diagnostics13040682
Chicago/Turabian StyleCarter, Dan, Edward Ram, and Tal Engel. 2023. "Combined 3D Endoanal Ultrasound and Transperineal Ultrasound Improves the Detection of Anal Sphincter Defects" Diagnostics 13, no. 4: 682. https://doi.org/10.3390/diagnostics13040682






