Assessment of Arterial Transit Time and Cerebrovascular Reactivity in Moyamoya Disease by Simultaneous PET/MRI
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MR Imaging
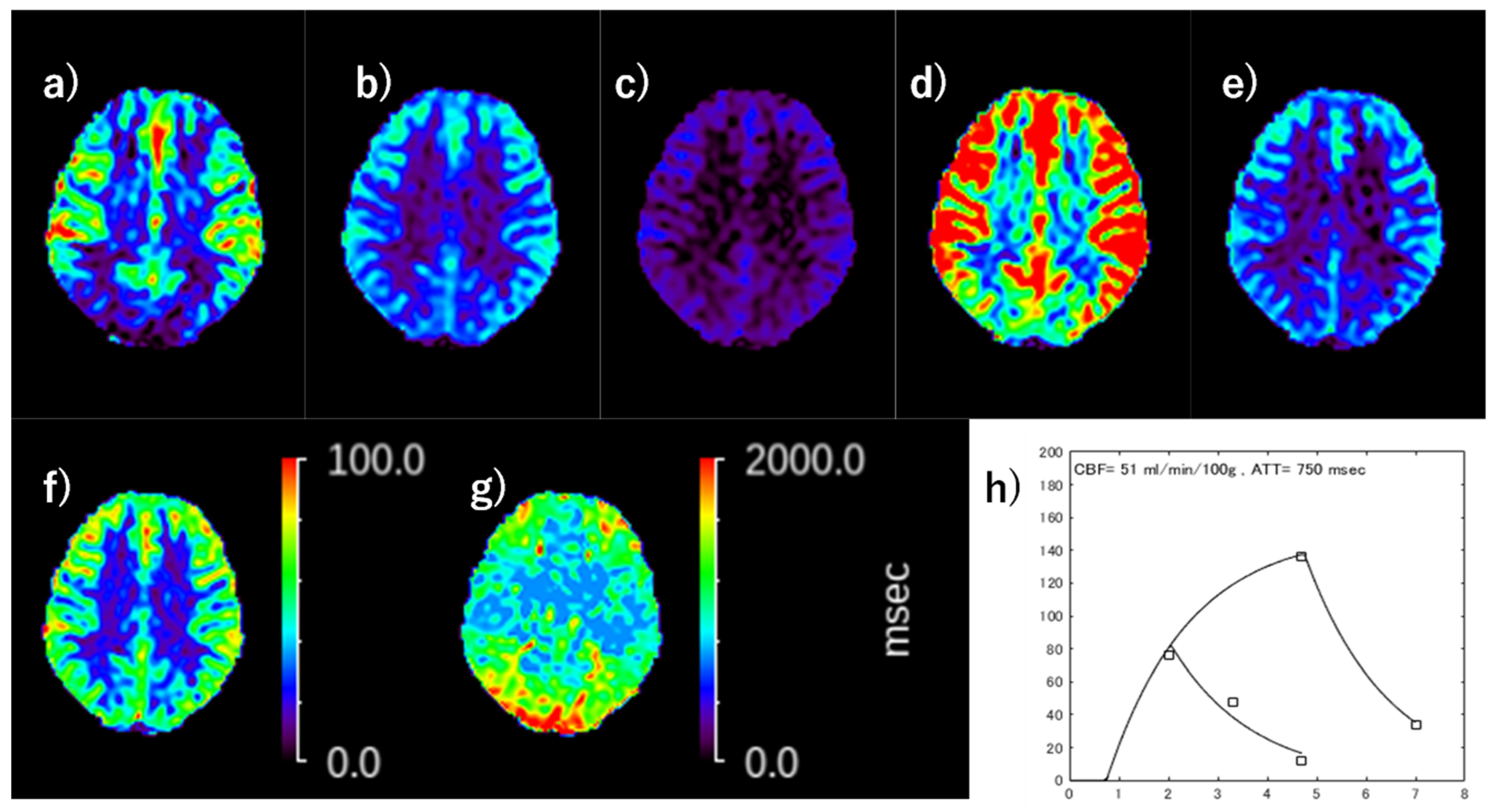
2.3. ATT and CBF Calculation on ASL
2.4. PET Acquisition and CBF Calculation
2.5. Date Analysis
2.6. Statistical Analysis
3. Results
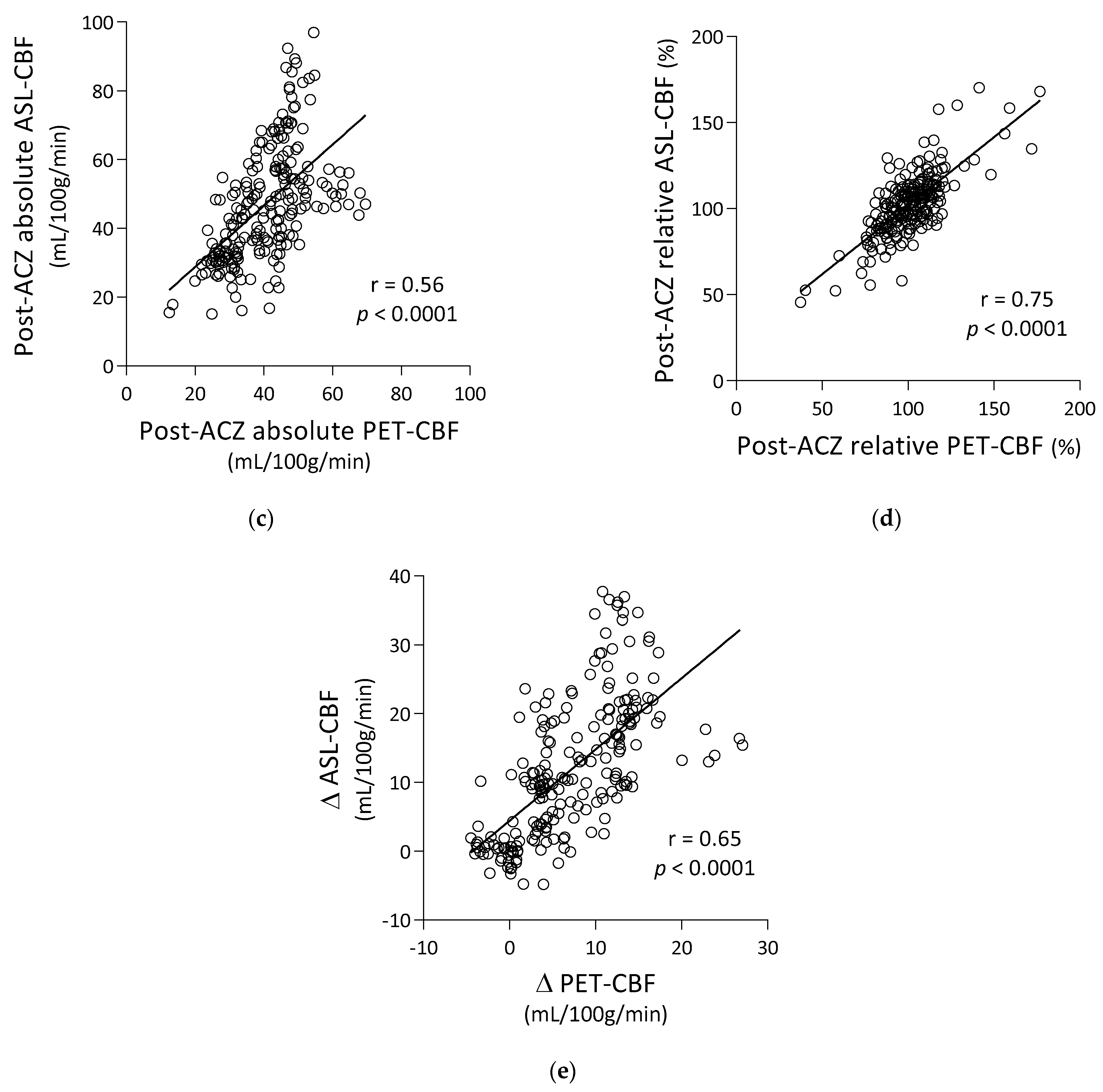
3.1. Correlation of ASL-CBF with PET-CBF
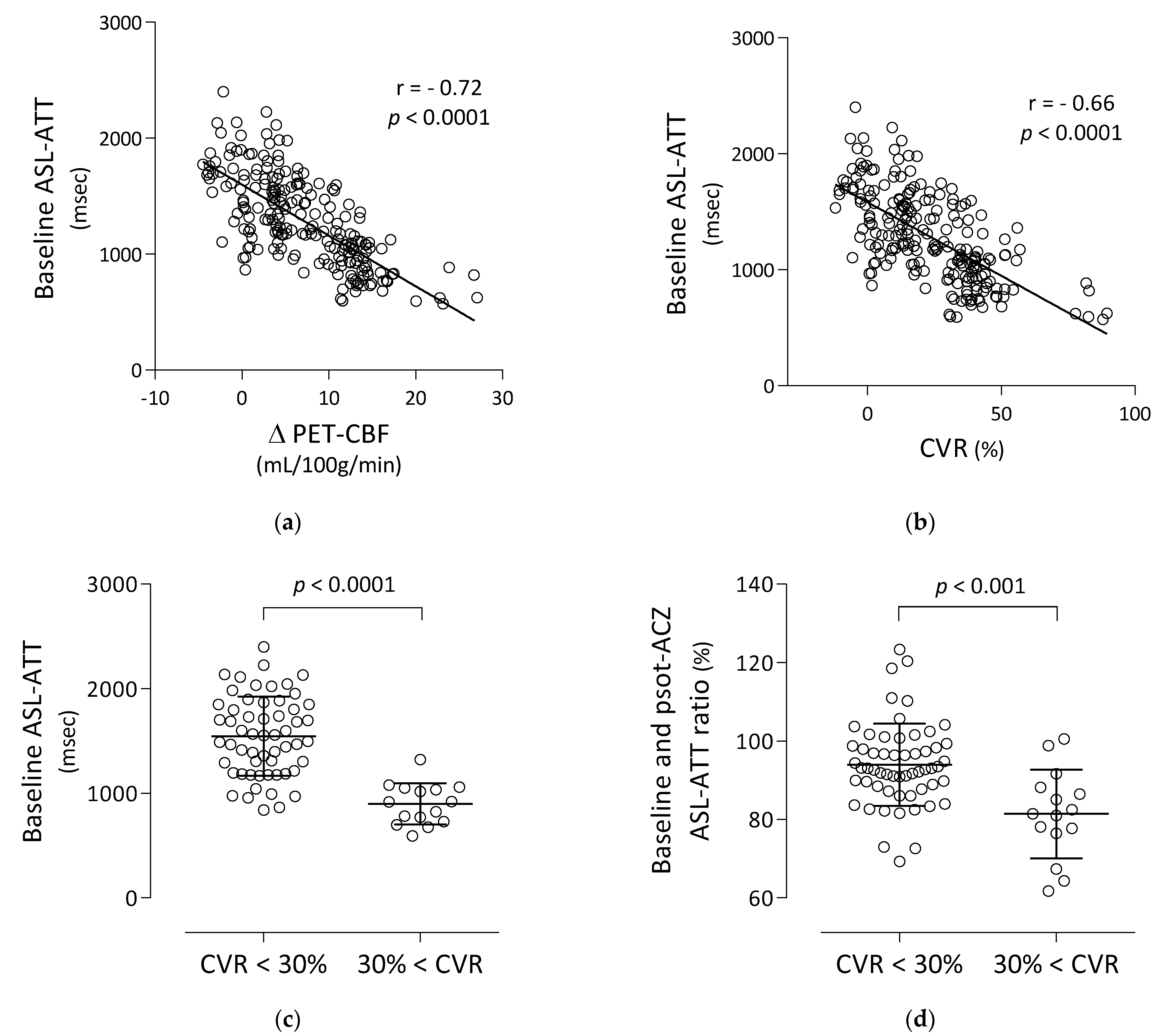
3.2. Correlation and Comparison of Baseline ASL-ATT with PET-CBF Changes
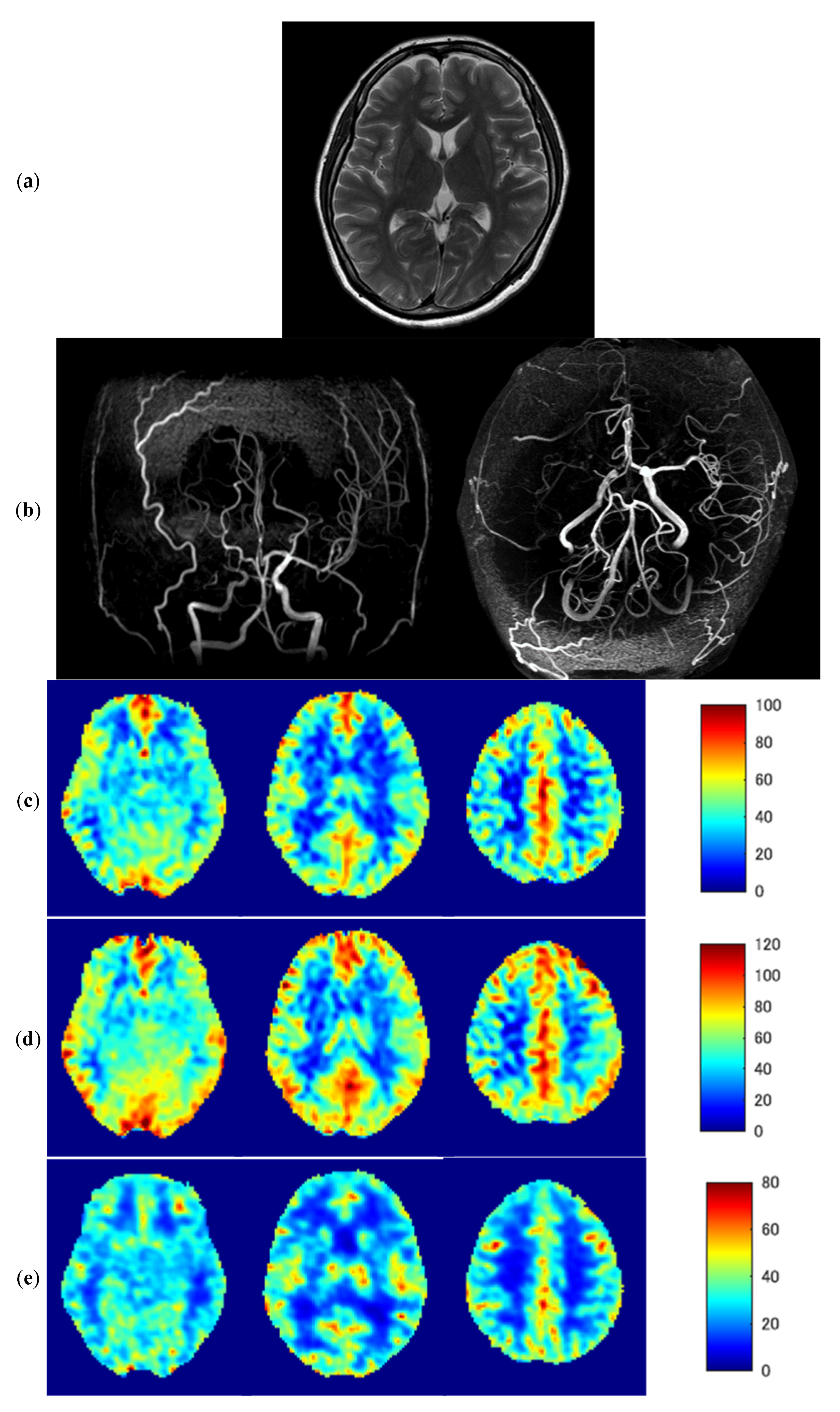
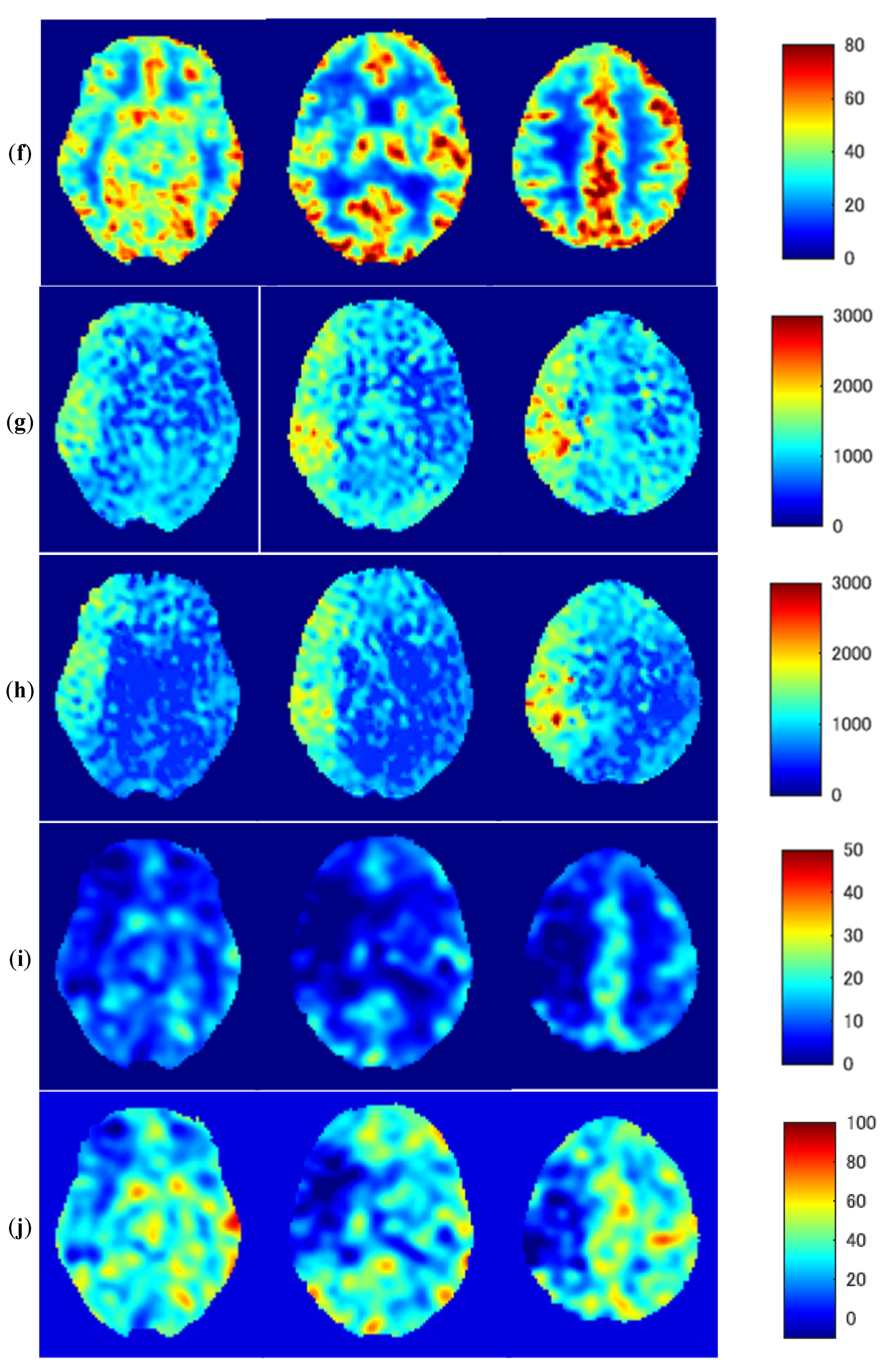
3.3. Representative Images
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grubb, R.L., Jr.; Derdeyn, C.P.; Fritsch, S.M.; Carpenter, D.A.; Yundt, K.D.; Videen, T.O.; Spitznagel, E.L.; Powers, W.J. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 1998, 280, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, H.; Fukuyama, H.; Nagahama, Y.; Nabatame, H.; Ueno, M.; Nishizawa, S.; Konishi, J.; Shio, H. Significance of increased oxygen extraction fraction in five-year prognosis of major cerebral arterial occlusive diseases. J. Nucl. Med. 1999, 40, 1992–1998. [Google Scholar]
- Derdeyn, C.P.; Grubb, R.L., Jr.; Powers, W.J. Cerebral hemodynamic impairment: Methods of measurement and association with stroke risk. Neurology 1999, 53, 251–259. [Google Scholar] [CrossRef]
- Gupta, A.; Chazen, J.L.; Hartman, M.; Delgado, D.; Anumula, N.; Shao, H.; Mazumdar, M.; Segal, A.Z.; Kamel, H.; Leifer, D.; et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: A systematic review and meta-analysis. Stroke 2012, 43, 2884–2891. [Google Scholar] [CrossRef]
- Wintermark, M.; Sesay, M.; Barbier, E.; Borbely, K.; Dillon, W.P.; Eastwood, J.D.; Glenn, T.C.; Grandin, C.B.; Pedraza, S.; Soustiel, J.F.; et al. Comparative overview of brain perfusion imaging techniques. Stroke 2005, 36, e83–e99. [Google Scholar] [CrossRef]
- Kim, E.; Sohn, C.H.; Na, D.G.; Kim, J.E.; Chang, K.H.; Kim, J.H.; Jeon, S.J. Perfusion computed tomography evaluation of cerebral hemodynamic impairment in patients with unilateral chronic steno-occlusive disease: A comparison with the acetazolamide challenge 99mTc-hexamethylpropyleneamine oxime single-photon emission computed tomography. J. Comput. Assist. Tomogr. 2009, 33, 546–551. [Google Scholar] [CrossRef]
- Williams, D.S.; Detre, J.A.; Leigh, J.S.; Koretsky, A.P. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc. Natl. Acad. Sci. USA 1992, 89, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Detre, J.A.; Leigh, J.S.; Williams, D.S.; Koretsky, A.P. Perfusion imaging. Magn. Reson. Med. 1992, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Alsop, D.C.; Detre, J.A. Multisection cerebral blood flow MR imaging with continuous arterial spin labeling. Radiology 1998, 208, 410–416. [Google Scholar] [CrossRef]
- Ye, F.Q.; Berman, K.F.; Ellmore, T.; Esposito, G.; van Horn, J.D.; Yang, Y.; Duyn, J.; Smith, A.M.; Frank, J.A.; Weinberger, D.R.; et al. H(2)(15)O PET validation of steady-state arterial spin tagging cerebral blood flow measurements in humans. Magn. Reson. Med. 2000, 44, 450–456. [Google Scholar] [CrossRef]
- Fan, A.P.; Jahanian, H.; Holdsworth, S.J.; Zaharchuk, G. Comparison of cerebral blood flow measurement with [15O]-water positron emission tomography and arterial spin labeling magnetic resonance imaging: A systematic review. J. Cereb. Blood Flow Metab. 2016, 36, 842–861. [Google Scholar] [CrossRef] [PubMed]
- Parkes, L.M.; Tofts, P.S. Improved accuracy of human cerebral blood perfusion measurements using arterial spin labeling: Accounting for capillary water permeability. Magn. Reson. Med. 2002, 48, 27–41. [Google Scholar] [CrossRef]
- Scott, R.M.; Smith, E.R. Moyamoya disease and moyamoya syndrome. N. Engl. J. Med. 2009, 360, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Tyagi, A.; Romo, M.; Amoroso, K.C.; Sonia, F. Moyamoya Disease: A Review of Current Literature. Cureus 2020, 12, e10141. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Kado, H.; Koshimoto, Y.; Tsuchida, T.; Yonekura, Y.; Itoh, H. Multislice continuous arterial spin-labeled perfusion MRI in patients with chronic occlusive cerebrovascular disease: A correlative study with CO2 PET validation. J. Magn. Reason. Imaging 2005, 22, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, T.; Kimura, H.; Matsuda, T.; Fujiwara, Y.; Isozaki, M.; Kikuta, K.; Okazawa, H. Arterial Transit Time Mapping Obtained by Pulsed Continuous 3D ASL Imaging with Multiple Post-Label Delay Acquisitions: Comparative Study with PET-CBF in Patients with Chronic Occlusive Cerebrovascular Disease. PLoS ONE 2016, 11, e0156005. [Google Scholar] [CrossRef]
- Kamano, H.; Yoshiura, T.; Hiwatashi, A.; Abe, K.; Togao, O.; Yamashita, K.; Honda, H. Arterial spin labeling in patients with chronic cerebral artery steno-occlusive disease: Correlation with 15O-PET. Acta Radiol. 2013, 54, 99–106. [Google Scholar] [CrossRef]
- Dai, W.; Robson, P.M.; Shankaranarayanan, A.; Alsop, D.C. Reduced resolution transit delay prescan for quantitative continuous arterial spin labeling perfusion imaging. Magn. Reson. Med. 2012, 67, 1252–1265. [Google Scholar] [CrossRef]
- Ishida, S.; Kimura, H.; Isozaki, M.; Takei, N.; Fujiwara, Y.; Kanamoto, M.; Kosaka, N.; Matsuda, T.; Kidoya, E. Robust arterial transit time and cerebral blood flow estimation using combined acquisition of Hadamard-encoded multi-delay and long-labeled long-delay pseudo-continuous arterial spin labeling: A simulation and in vivo study. NMR Biomed. 2020, 33, e4319. [Google Scholar] [CrossRef]
- Powers, W.J.; Press, G.A.; Grubb, R.L., Jr.; Gado, M.; Raichle, M.E. The effect of hemodynamically significant carotid artery disease on the hemodynamic status of the cerebral circulation. Ann. Intern. Med. 1987, 106, 27–34. [Google Scholar] [CrossRef]
- Mangla, R.; Kolar, B.; Almast, J.; Ekholm, S.E. Border zone infarcts: Pathophysiologic and imaging characteristics. Radiographics 2011, 31, 1201–1214. [Google Scholar] [CrossRef]
- Hendrikse, J.; Petersen, E.T.; van Laar, P.J.; Golay, X. Cerebral border zones between distal end branches of intracranial arteries: MR imaging. Radiology 2008, 246, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Levin, C.S.; Maramraju, S.H.; Khalighi, M.M.; Deller, T.W.; Delso, G.; Jansen, F. Design Features and Mutual Compatibility Studies of the Time-of-Flight PET Capable GE SIGNA PET/MR System. IEEE Trans. Med. Imaging 2016, 35, 1907–1914. [Google Scholar] [CrossRef]
- Okazawa, H.; Higashino, Y.; Tsujikawa, T.; Arishima, H.; Mori, T.; Kiyono, Y.; Kimura, H.; Kikuta, K.I. Noninvasive method for measurement of cerebral blood flow using O-15 water PET/MRI with ASL correlation. Eur. J. Radiol. 2018, 105, 102–109. [Google Scholar] [CrossRef]
- Okazawa, H.; Tsujikawa, T.; Higashino, Y.; Kikuta, K.I.; Mori, T.; Makino, A.; Kiyono, Y. No significant difference found in PET/MRI CBF values reconstructed with CT-atlas-based and ZTE MR attenuation correction. EJNMMI Res. 2019, 9, 26. [Google Scholar] [CrossRef]
- Mutsaerts, H.J.; van Dalen, J.W.; Heijtel, D.F.; Groot, P.F.; Majoie, C.B.; Petersen, E.T.; Richard, E.; Nederveen, A.J. Cerebral Perfusion Measurements in Elderly with Hypertension Using Arterial Spin Labeling. PLoS ONE 2015, 10, e0133717. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar] [PubMed]
- Okazawa, H.; Yamauchi, H.; Toyoda, H.; Sugimoto, K.; Fujibayashi, Y.; Yonekura, Y. Relationship between vasodilatation and cerebral blood flow increase in impaired hemodynamics: A PET study with the acetazolamide test in cerebrovascular disease. J. Nucl. Med. 2003, 44, 1875–1883. [Google Scholar] [PubMed]
- Yamauchi, H.; Okazawa, H.; Kishibe, Y.; Sugimoto, K.; Takahashi, M. Oxygen extraction fraction and acetazolamide reactivity in symptomatic carotid artery disease. J. Neurol. Neurosurg. Psychiatry 2004, 75, 33–37. [Google Scholar]
- Nemoto, E.M.; Yonas, H.; Kuwabara, H.; Pindzola, R.R.; Sashin, D.; Meltzer, C.C.; Price, J.C.; Chang, Y.; Johnson, D.W. Identification of hemodynamic compromise by cerebrovascular reserve and oxygen extraction fraction in occlusive vascular disease. J. Cereb. Blood Flow Metab. 2004, 24, 1081–1089. [Google Scholar] [CrossRef]
- Okazawa, H.; Tsuchida, T.; Kobayashi, M.; Arai, Y.; Pagani, M.; Isozaki, M.; Yonekura, Y. Can the detection of misery perfusion in chronic cerebrovascular disease be based on reductions in baseline CBF and vasoreactivity? Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 121–129. [Google Scholar] [CrossRef]
- Takeuchi, K.; Isozaki, M.; Higashino, Y.; Kosaka, N.; Kikuta, K.I.; Ishida, S.; Kanamoto, M.; Takei, N.; Okazawa, H.; Kimura, H. The Utility of Arterial Transit Time Measurement for Evaluating the Hemodynamic Perfusion State of Patients with Chronic Cerebrovascular Stenosis or Occlusive Disease: Correlative Study between MR Imaging and 15O-labeled H2O Positron Emission Tomography. Magn. Reson. Med. Sci. 2022. [Google Scholar] [CrossRef]
- Saito, H.; Ogasawara, K.; Suzuki, T.; Kuroda, H.; Kobayashi, M.; Yoshida, K.; Kubo, Y.; Ogawa, A. Adverse effects of intravenous acetazolamide administration for evaluation of cerebrovascular reactivity using brain perfusion single-photon emission computed tomography in patients with major cerebral artery steno-occlusive diseases. Neurol. Med. Chir. 2011, 51, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Detre, J.A.; Alsop, D.C.; Vives, L.R.; Maccotta, L.; Teener, J.W.; Raps, E.C. Noninvasive MRI evaluation of cerebral blood flow in cerebrovascular disease. Neurology 1998, 50, 633–641. [Google Scholar] [CrossRef]
- Chalela, J.A.; Alsop, D.C.; Gonzalez-Atavales, J.B.; Maldjian, J.A.; Kasner, S.E.; Detre, J.A. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke 2000, 31, 680–687. [Google Scholar] [CrossRef]
- Zaharchuk, G.; Do, H.M.; Marks, M.P.; Rosenberg, J.; Moseley, M.E.; Steinberg, G.K. Arterial spin-labeling MRI can identify the presence and intensity of collateral perfusion in patients with moyamoya disease. Stroke 2011, 42, 2485–2491. [Google Scholar] [CrossRef]
- Ye, F.Q.; Mattay, V.S.; Jezzard, P.; Frank, J.A.; Weinberger, D.R.; McLaughlin, A.C. Correction for vascular artifacts in cerebral blood flow values measured by using arterial spin tagging techniques. Magn. Reson. Med. 1997, 37, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, S.; Watanabe, Y.; Takei, N.; Ueyama, T.; Miyawaki, S.; Koizumi, S.; Kato, S.; Takao, H.; Abe, O.; Saito, N. Arterial Transit Time-Based Multidelay Combination Strategy Improves Arterial Spin Labeling Cerebral Blood Flow Measurement Accuracy in Severe Steno-Occlusive Diseases. J. Magn. Reason. Imaging 2022, 55, 178–187. [Google Scholar] [CrossRef] [PubMed]


| Patient | Age | Sex | Stenosis/Occlusion | Severity | Symptom |
|---|---|---|---|---|---|
| 1 | 44 | F | R MCA | severe | asymptomatic |
| 2 | 85 | M | L ICA | severe | hemiparesis |
| 3 | 44 | F | R ICA, MCA, A1. L ICA top | severe | hemiparesis |
| 4 | 38 | F | R ICA top, MCA, A1 | severe | hemiparesis |
| 5 | 51 | M | R ICA, MCA. R/L ACA | severe | hemiparesis, dysarthria |
| 6 | 46 | F | R ICA top, MCA, A1 | severe | TIA |
| 7 | 46 | M | R ICA top, MCA, A1 | severe | cerebral hemorrhage |
| 8 | 45 | F | R/L ICA top, MCA | severe | higher brain dysfunction |
| 9 | 42 | F | R/L ICA top | severe | TIA |
| 10 | 42 | M | R ICA top, MCA | severe | asymptomatic |
| 11 | 15 | F | R/L ICA top, MCA | severe | headache |
| 12 | 11 | F | R/L ICA top, MCA | severe | headache |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takata, K.; Kimura, H.; Ishida, S.; Isozaki, M.; Higashino, Y.; Kikuta, K.-I.; Okazawa, H.; Tsujikawa, T. Assessment of Arterial Transit Time and Cerebrovascular Reactivity in Moyamoya Disease by Simultaneous PET/MRI. Diagnostics 2023, 13, 756. https://doi.org/10.3390/diagnostics13040756
Takata K, Kimura H, Ishida S, Isozaki M, Higashino Y, Kikuta K-I, Okazawa H, Tsujikawa T. Assessment of Arterial Transit Time and Cerebrovascular Reactivity in Moyamoya Disease by Simultaneous PET/MRI. Diagnostics. 2023; 13(4):756. https://doi.org/10.3390/diagnostics13040756
Chicago/Turabian StyleTakata, Kenji, Hirohiko Kimura, Shota Ishida, Makoto Isozaki, Yoshifumi Higashino, Ken-Ichiro Kikuta, Hidehiko Okazawa, and Tetsuya Tsujikawa. 2023. "Assessment of Arterial Transit Time and Cerebrovascular Reactivity in Moyamoya Disease by Simultaneous PET/MRI" Diagnostics 13, no. 4: 756. https://doi.org/10.3390/diagnostics13040756
APA StyleTakata, K., Kimura, H., Ishida, S., Isozaki, M., Higashino, Y., Kikuta, K.-I., Okazawa, H., & Tsujikawa, T. (2023). Assessment of Arterial Transit Time and Cerebrovascular Reactivity in Moyamoya Disease by Simultaneous PET/MRI. Diagnostics, 13(4), 756. https://doi.org/10.3390/diagnostics13040756







