DTBV: A Deep Transfer-Based Bone Cancer Diagnosis System Using VGG16 Feature Extraction
Abstract
1. Introduction
- Developing a DTBV system that can assist doctors by providing a second opinion to improve diagnostic efficiency by comparing malignant and healthy bone images in real-time.
- Prediction of bone cancer at an early stage using the VGG16 model for feature extraction, mutual information statistics for feature selection, and the SVM model for classification with X-ray images as a modality.
- A comparative study on the performance of various CNN models for feature extraction and ML models for classification, with further consideration to the performance of the proposed DTBV system with other existing systems.
2. Materials and Methods
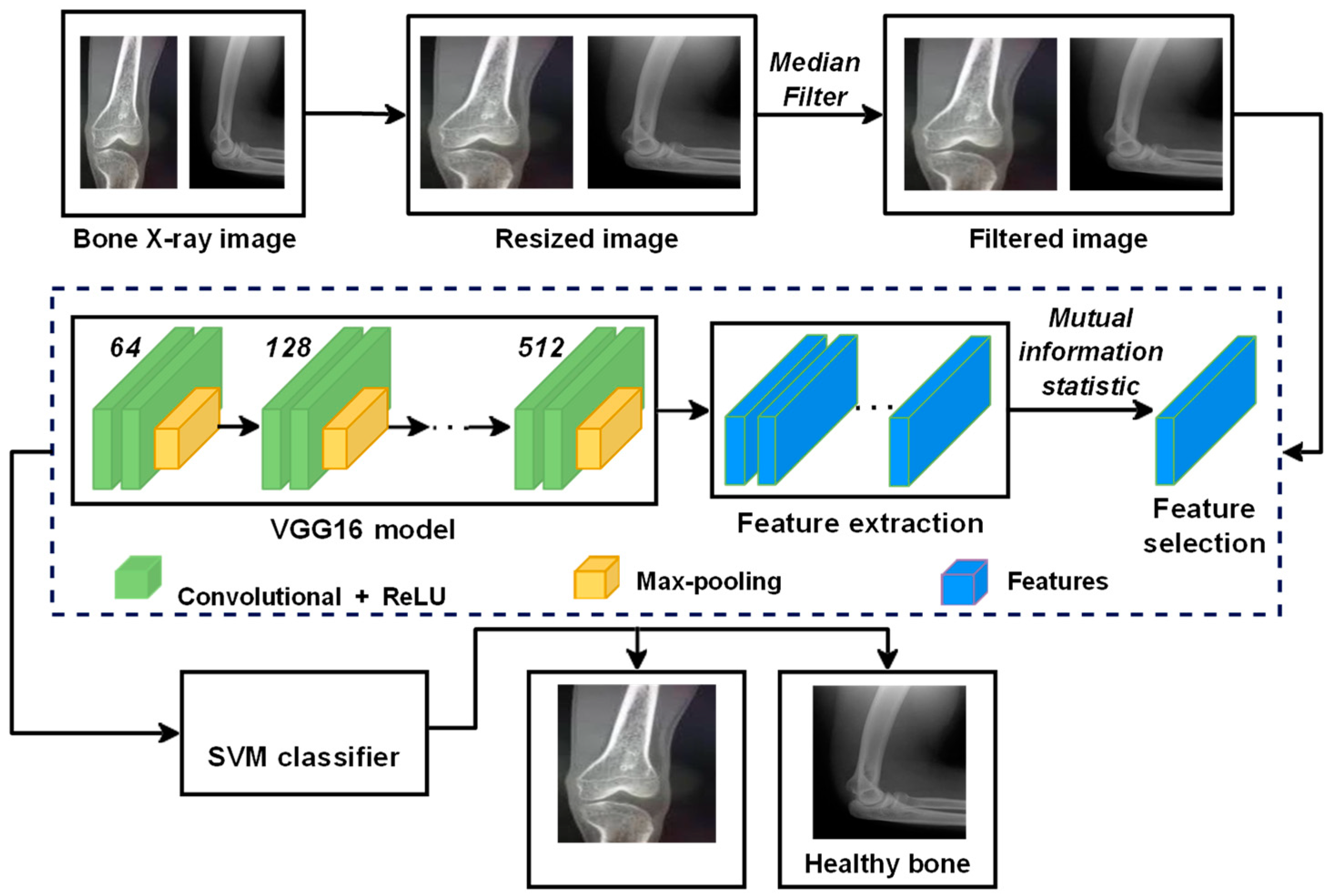
3. Proposed System
| Algorithm 1: Feature Extraction and Classification Using the DTBV System |
| Input: Bone X-ray image I Output: Classified image C 1. procedure FEATURE_EXTRACTION(dataset) 2. for I in dataset do 3. Read the image I←cv2.imread(image_path) 4. Resize the image 5. Apply median filter to remove noise from the image I←cv2.medianBlur(I, 3) 6. Extract the features from the filtered image using the VGG16 model feature_extractor←vgg16() f←feature_extractor(I) 7. end for 8. end procedure 9. procedure CLASSIFICATION(dataset, f) 10. Select the best features from the extracted features using mutual information statistic f s←SelectKBest(mutual_info_classif) 11. Split the dataset into training_dataset and testing_dataset 12. Train the SVM model with the features selected for the training_dataset classifier←SVC() C←classifier(fs) 13. Classify the testing_dataset using the selected features into healthy and malignant images 14. end procedure |
4. Results and Discussions
4.1. Experimental Setup
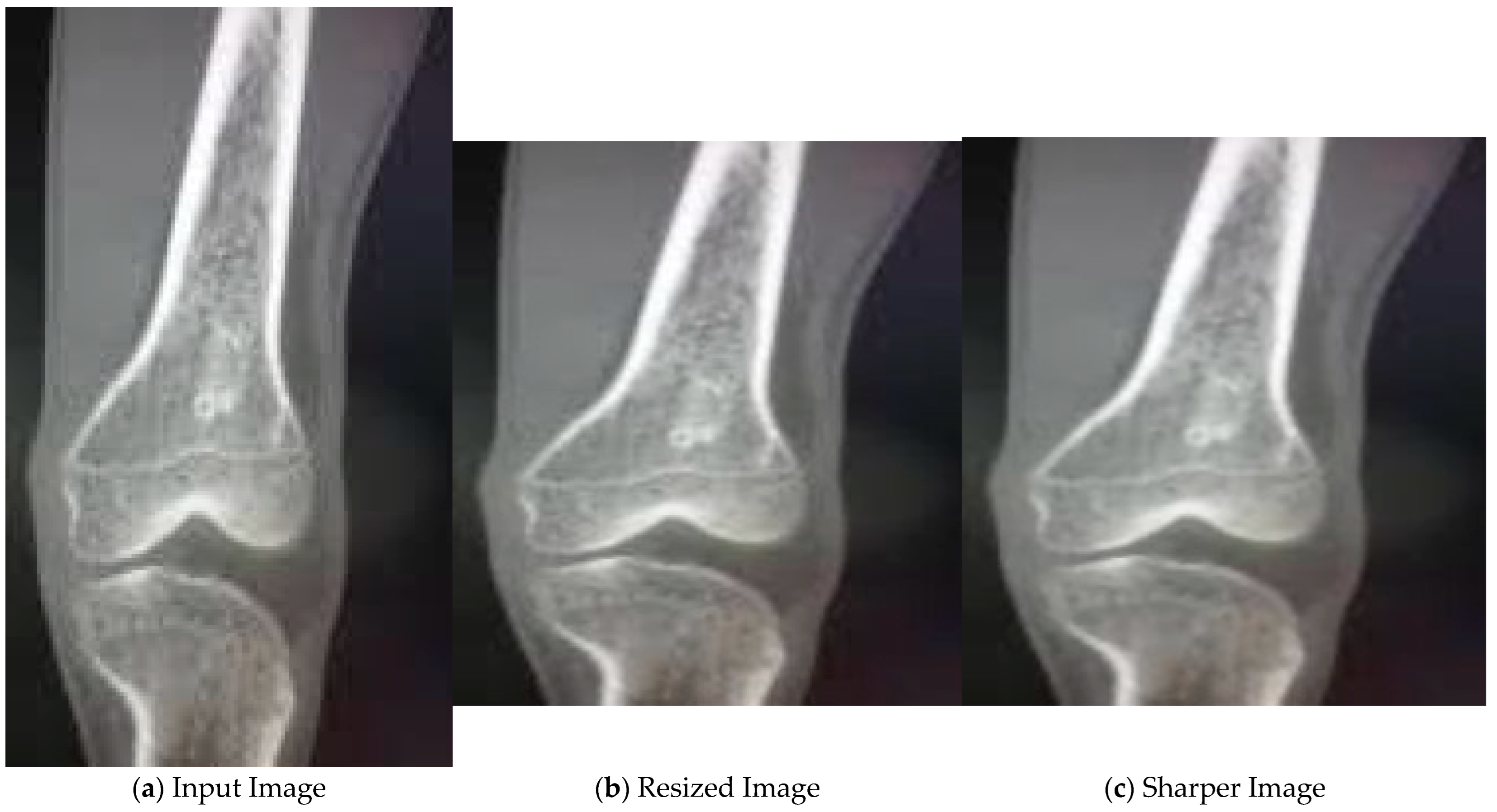
4.2. Results for Pre-Processing
4.3. Results for Feature Extraction and Classification
4.4. Comparison of Model Performance
5. Conclusions and Future Works
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muhammad, K.; Khan, S.; Ser, J.D.; Albuquerque, V.H.C.D. Deep Learning for Multigrade Brain Tumor Classification in Smart Healthcare Systems: A Prospective Survey. IEEE Trans. Neural Netw. Learn. Syst. 2021, 32, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Rashid, S.M.; Nayem Ferdous Khan, M.; Biswas, A.; Mahmud, A. Symptom Wise Age Prediction of Cancer Patients using Classifier Comparison and Feature Selection. In Proceedings of the 2019 22nd International Conference on Computer and Information Technology (ICCIT), Dhaka, Bangladesh, 18–20 December 2019; pp. 1–6. [Google Scholar]
- Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/bones.html (accessed on 1 January 2021).
- Gaume, M.; Chevret, S.; Campagna, R.; Larousserie, F.; Biau, D. The appropriate and sequential value of standard radiograph, computed tomography and magnetic resonance imaging to characterize a bone tumor. Sci. Rep. 2022, 12, 6196. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xing, F.; Braun, J.; Traub, F.; Rommens, P.M.; Xiang, Z.; Ritz, U. Progress of Phototherapy Applications in the Treatment of Bone Cancer. Int. J. Mol. Sci. 2021, 22, 11354. [Google Scholar] [CrossRef] [PubMed]
- Naveen, P.; Diwan, B. Pre-trained VGG-16 with CNN Architecture to classify X-Rays images into Normal or Pneumonia. In Proceedings of the 2021 International Conference on Emerging Smart Computing and Informatics (ESCI), Pune, India, 5–7 March 2021; pp. 102–105. [Google Scholar]
- Ranjitha, M.M.; Taranath, N.L.; Arpitha, C.N.; Subbaraya, C.K. Bone Cancer Detection Using K-Means Segmentation and Knn Classification. In Proceedings of the 2019 1st International Conference on Advances in Information Technology (ICAIT), Chikmagalur, India, 25–27 July 2019; pp. 76–80. [Google Scholar]
- Eweje, F.R.; Bao, B.; Wu, J.; Dalal, D.; Liao, W.; He, Y.; Luo, Y.; Lu, S.; Zhang, P.; Peng, X.; et al. Deep Learning for Classification of Bone Lesions on Routine MRI. EBioMedicine 2021, 68, 103402. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-W.; Oh, S.-J.; Kim, K.-S.; Jang, M.-C.; Kim, I.S.; Lee, Y.-K.; Chung, M.J.; Cho, B.H.; Seo, S.-W. Artificial intelligence-based classification of bone tumors in the proximal femur on plain radiographs: System development and validation. PLoS ONE 2022, 17, e0264140. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Qin, Z.; Ni, J.; Lin, X.; Shen, X. Practical and Secure SVM Classification for Cloud-Based Remote Clinical Decision Services. IEEE Trans. Comput. 2021, 70, 1612–1625. [Google Scholar] [CrossRef]
- Chaganti, S.Y.; Nanda, I.; Pandi, K.R.; Prudhvith, T.G.; Kumar, N. Image Classification using SVM and CNN. In Proceedings of the 2020 International Conference on Computer Science, Engineering and Applications (ICCSEA), Gunupur, India, 13–14 March 2020; pp. 1–5. [Google Scholar]
- Liu, H.; Gong, H.; Ding, X. Food Image Recognition Algorithm Base on Improved VGG16. In Proceedings of the 2021 IEEE 2nd International Conference on Information Technology, Big Data and Artificial Intelligence (ICIBA), Chongqing, China, 17–19 December 2021; pp. 899–903. [Google Scholar]
- Kushwaha, P.K.; Kumaresan, M. Machine learning algorithm in healthcare system: A Review. In Proceedings of the 2021 International Conference on Technological Advancements and Innovations (ICTAI), Tashkent, Uzbekistan, 10–12 November 2021; pp. 478–481. [Google Scholar]
- Chang, Y.; Yan, L.; Chen, M.; Fang, H.; Zhong, S. Two-Stage Convolutional Neural Network for Medical Noise Removal via Image Decomposition. IEEE Trans. Instrum. Meas. 2020, 69, 2707–2721. [Google Scholar] [CrossRef]
- Satti, P.; Sharma, N.; Garg, B. Min-Max Average Pooling Based Filter for Impulse Noise Removal. IEEE Signal Process. Lett. 2020, 27, 1475–1479. [Google Scholar] [CrossRef]
- Jabber, B.; Shankar, M.; Rao, P.V.; Krishna, A.; Basha, C.Z. SVM Model based Computerized Bone Cancer Detection. In Proceedings of the 2020 4th International Conference on Electronics, Communication and Aerospace Technology (ICECA), Coimbatore, India, 5–7 November 2020; pp. 407–411. [Google Scholar]
- Abegaz, B.W. A Parallelized Self-Driving Vehicle Controller Using Unsupervised Machine Learning. IEEE Trans. Ind. Appl. 2022, 58, 5148–5156. [Google Scholar] [CrossRef]
- Fu, K.; Cai, X.; Yuan, B.; Yang, Y.; Yao, X. An Efficient Surrogate Assisted Particle Swarm Optimization for Antenna Synthesis. IEEE Trans. Antennas Propag. 2022, 70, 4977–4984. [Google Scholar] [CrossRef]
- Salau, A.O.; Jain, S. Feature Extraction: A Survey of the Types, Techniques, Applications. In Proceedings of the 2019 International Conference on Signal Processing and Communication (ICSC), Noida, India, 7–9 March 2019; pp. 158–164. [Google Scholar]
- Sharma, A.; Yadav, D.P.; Garg, H.; Kumar, M.; Sharma, B.; Koundal, D. Bone Cancer Detection Using Feature Extraction Based Machine Learning Model. Comput. Math. Methods Med. 2021, 2021, 7433186. [Google Scholar] [CrossRef] [PubMed]
- Sarvamangala, D.R.; Kulkarni, R.V. Convolutional neural networks in medical image understanding: A survey. Evol. Intell. 2021, 15, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, A.; Asgharnezhad, H.; Jokandan, S.S.; Khosravi, A.; Kebria, P.M.; Nahavandi, D.; Nahavandi, S.; Srinivasan, D. An Uncertainty-Aware Transfer Learning-Based Framework for COVID-19 Diagnosis. IEEE Trans. Neural Netw. Learn. Syst. 2021, 32, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Hossain, E.; Rahaman, M.A. Bone Cancer Detection Classification Using Fuzzy Clustering Neuro Fuzzy Classifier. In Proceedings of the 2018 4th International Conference on Electrical Engineering and Information Communication Technology (ICEEICT), Dhaka, Bangladesh, 13–15 September 2018; pp. 541–546. [Google Scholar]
- Yadav, D.P.; Rathor, S. Bone Fracture Detection and Classification using Deep Learning Approach. In Proceedings of the 2020 International Conference on Power Electronics IoT Applications in Renewable Energy and its Control (PARC), Mathura, India, 28–29 February 2020; pp. 282–285. [Google Scholar]
- Papandrianos, N.; Papageorgiou, E.; Anagnostis, A.; Papageorgiou, K. Efficient Bone Metastasis Diagnosis in Bone Scintigraphy Using a Fast Convolutional Neural Network Architecture. Diagnostics 2020, 10, 532. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.; Alnajjar, F.; Jassmi, H.A.; Gocho, M.; Khan, W.; Serhani, M.A. Performance Evaluation of Deep CNN-Based Crack Detection and Localization Techniques for Concrete Structures. Sensors 2021, 21, 1688. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Z.; Zhou, Z.; Deng, H.; Zhao, W.; Wang, C.; Guo, Y. Anomaly detection of industrial control systems based on transfer learning. Tsinghua Sci. Technol. 2021, 26, 821–832. [Google Scholar] [CrossRef]
- Simonyan, K.; Zisserman, A. Very Deep Convolutional Networks for Large-Scale Image Recognition. arXiv 2015, arXiv:1409.1556. [Google Scholar]
- Sekhar, A.; Biswas, S.; Hazra, R.; Sunaniya, A.K.; Mukherjee, A.; Yang, L. Brain Tumor Classification Using Fine-Tuned GoogLeNet Features and Machine Learning Algorithms: IoMT Enabled CAD System. IEEE J. Biomed. Health Inform. 2022, 26, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Liu, Y.; Hou, C.; Fan, J.; Zheng, B.; Yin, J. Expediting the Accuracy-Improving Process of SVMs for Class Imbalance Learning. IEEE Trans. Knowl. Data Eng. 2021, 33, 3550–3567. [Google Scholar] [CrossRef]





| Model | Accuracy (%) |
|---|---|
| DTBV | 93.9 |
| InceptionV3 | 81.8 |
| VGG19 | 79.2 |
| ResNet50 | 76.2 |
| Performance Metric (%) | DTBV | Logistic Regression | Decision Tree | KNN | Random Forest |
|---|---|---|---|---|---|
| Accuracy | 93.9 | 87.9 | 84.8 | 81.8 | 75.8 |
| Recall | 93.3 | 93.3 | 100 | 86.7 | 86.7 |
| Specificity | 94.4 | 83.3 | 72.2 | 77.8 | 66.7 |
| Precision | 93.3 | 82.4 | 75 | 76.5 | 68.4 |
| FPR | 5.6 | 16.7 | 27.8 | 22.2 | 33.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suganeshwari, G.; Balakumar, R.; Karuppanan, K.; Prathiba, S.B.; Anbalagan, S.; Raja, G. DTBV: A Deep Transfer-Based Bone Cancer Diagnosis System Using VGG16 Feature Extraction. Diagnostics 2023, 13, 757. https://doi.org/10.3390/diagnostics13040757
Suganeshwari G, Balakumar R, Karuppanan K, Prathiba SB, Anbalagan S, Raja G. DTBV: A Deep Transfer-Based Bone Cancer Diagnosis System Using VGG16 Feature Extraction. Diagnostics. 2023; 13(4):757. https://doi.org/10.3390/diagnostics13040757
Chicago/Turabian StyleSuganeshwari, G., R. Balakumar, Kalimuthu Karuppanan, Sahaya Beni Prathiba, Sudha Anbalagan, and Gunasekaran Raja. 2023. "DTBV: A Deep Transfer-Based Bone Cancer Diagnosis System Using VGG16 Feature Extraction" Diagnostics 13, no. 4: 757. https://doi.org/10.3390/diagnostics13040757
APA StyleSuganeshwari, G., Balakumar, R., Karuppanan, K., Prathiba, S. B., Anbalagan, S., & Raja, G. (2023). DTBV: A Deep Transfer-Based Bone Cancer Diagnosis System Using VGG16 Feature Extraction. Diagnostics, 13(4), 757. https://doi.org/10.3390/diagnostics13040757





