α- and β-Genotyping of Thalassemia Patients Based on a Multimodal Liver MRI Radiomics Model: A Preliminary Study in Two Centers
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Data
2.2. MRI Scanning Method
2.3. Screening of Clinical Features
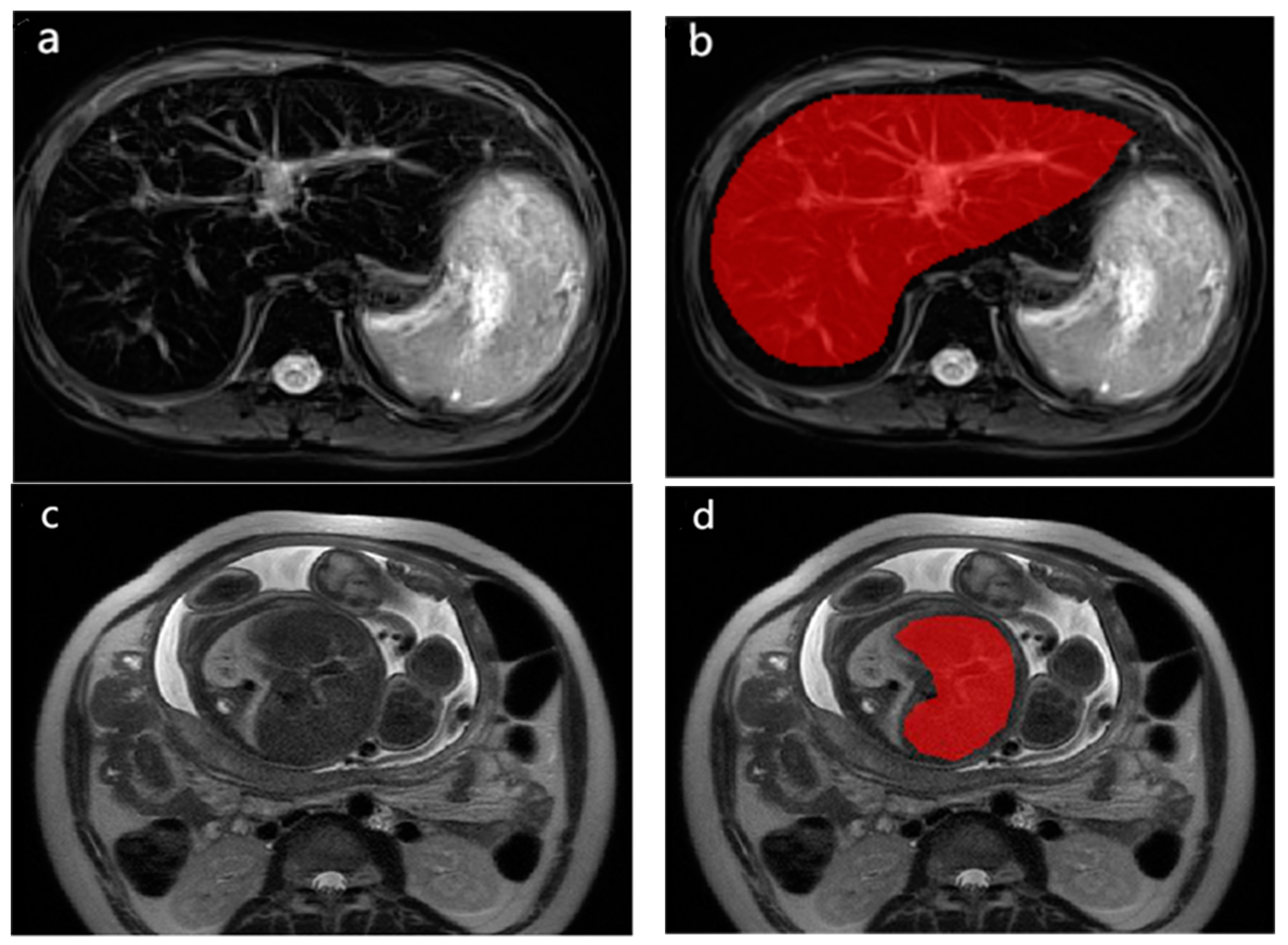
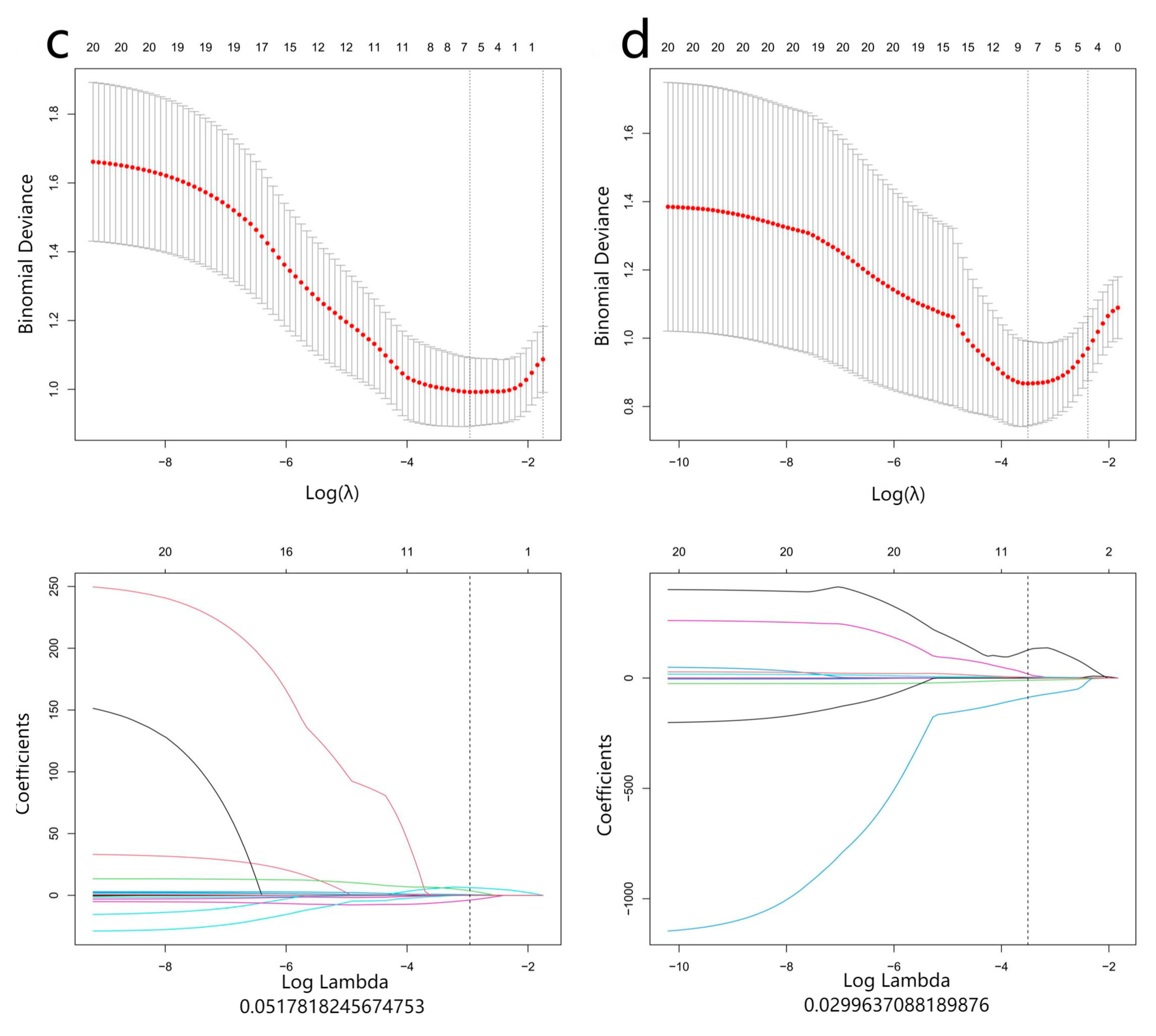
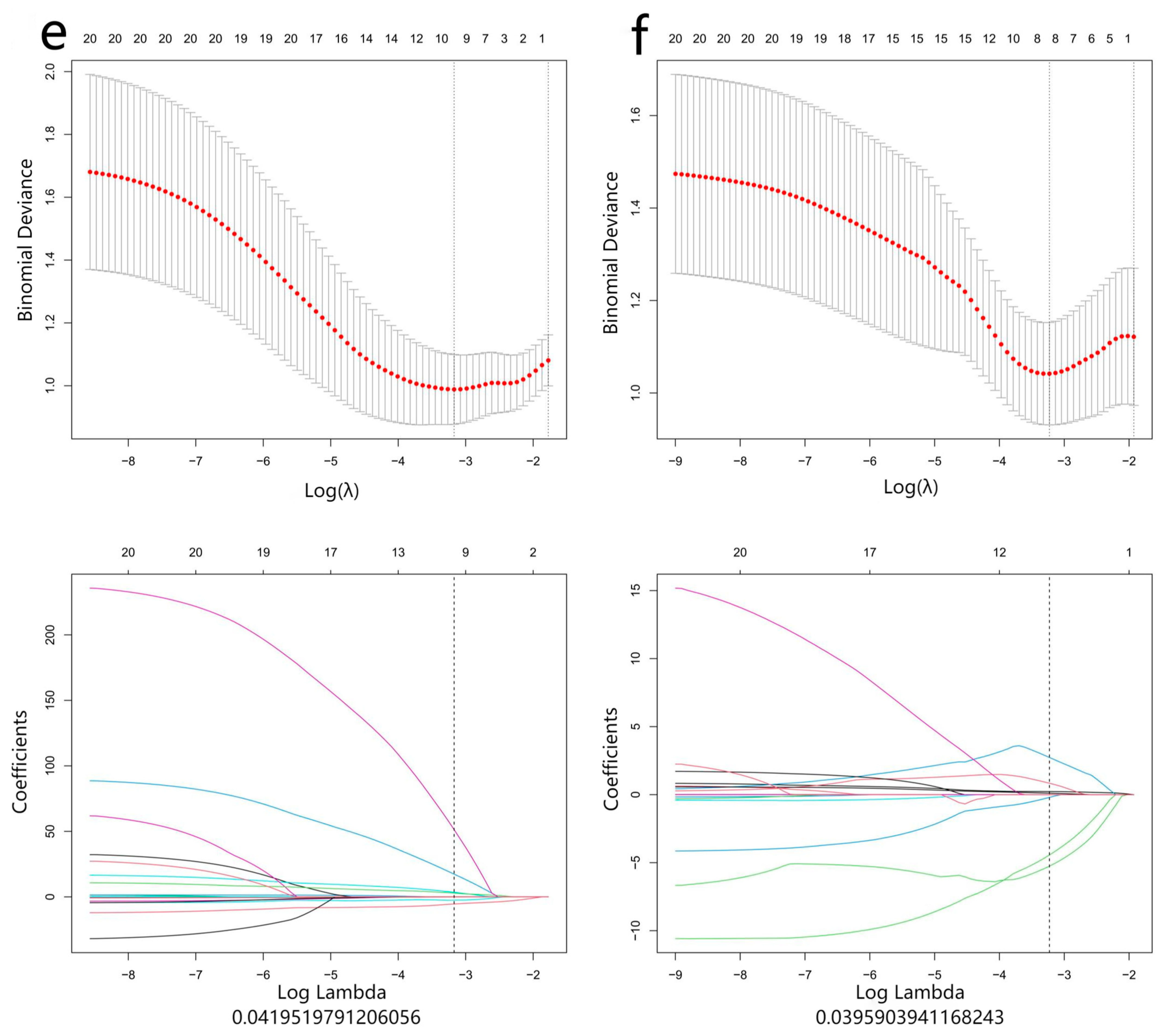
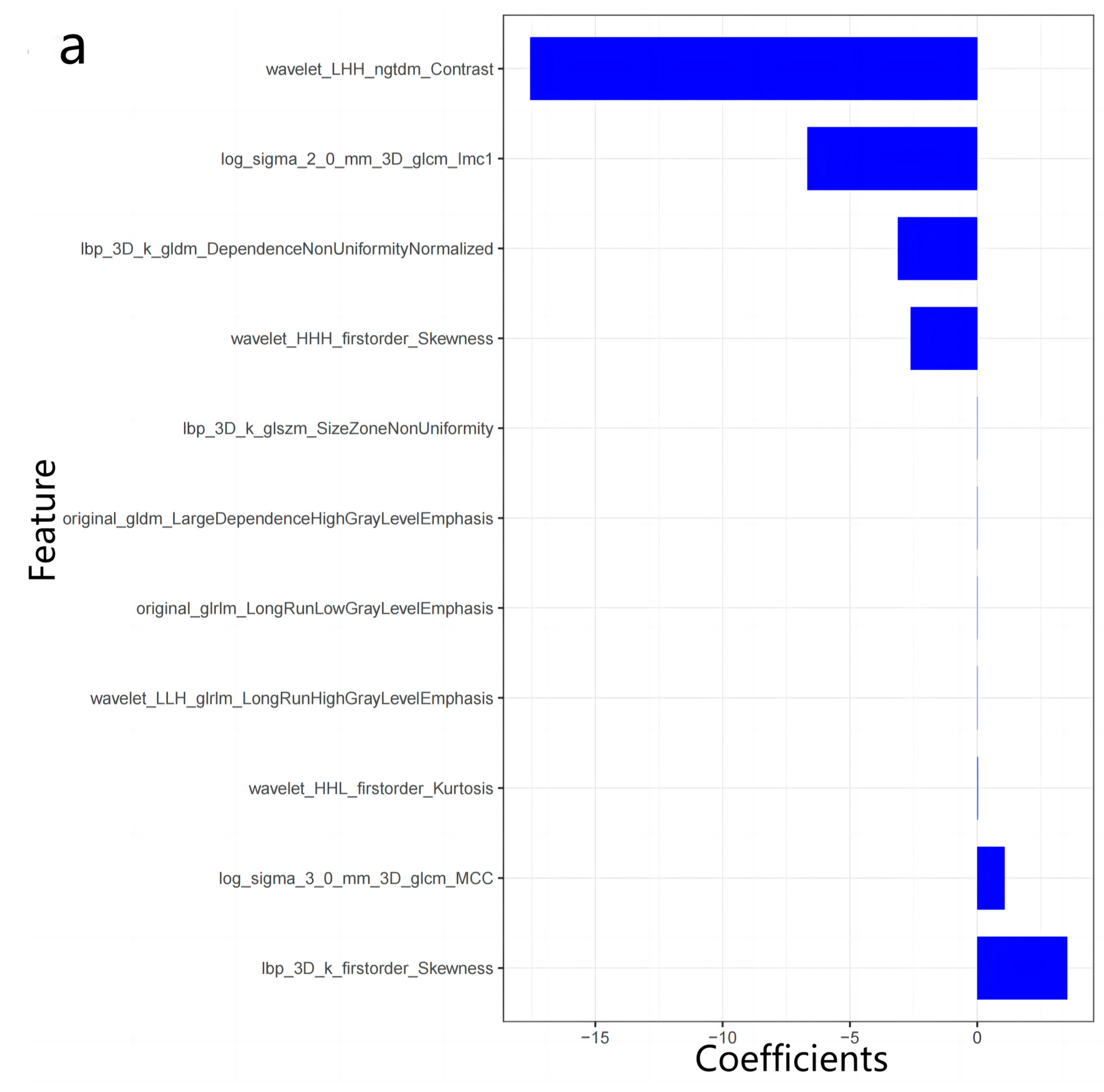
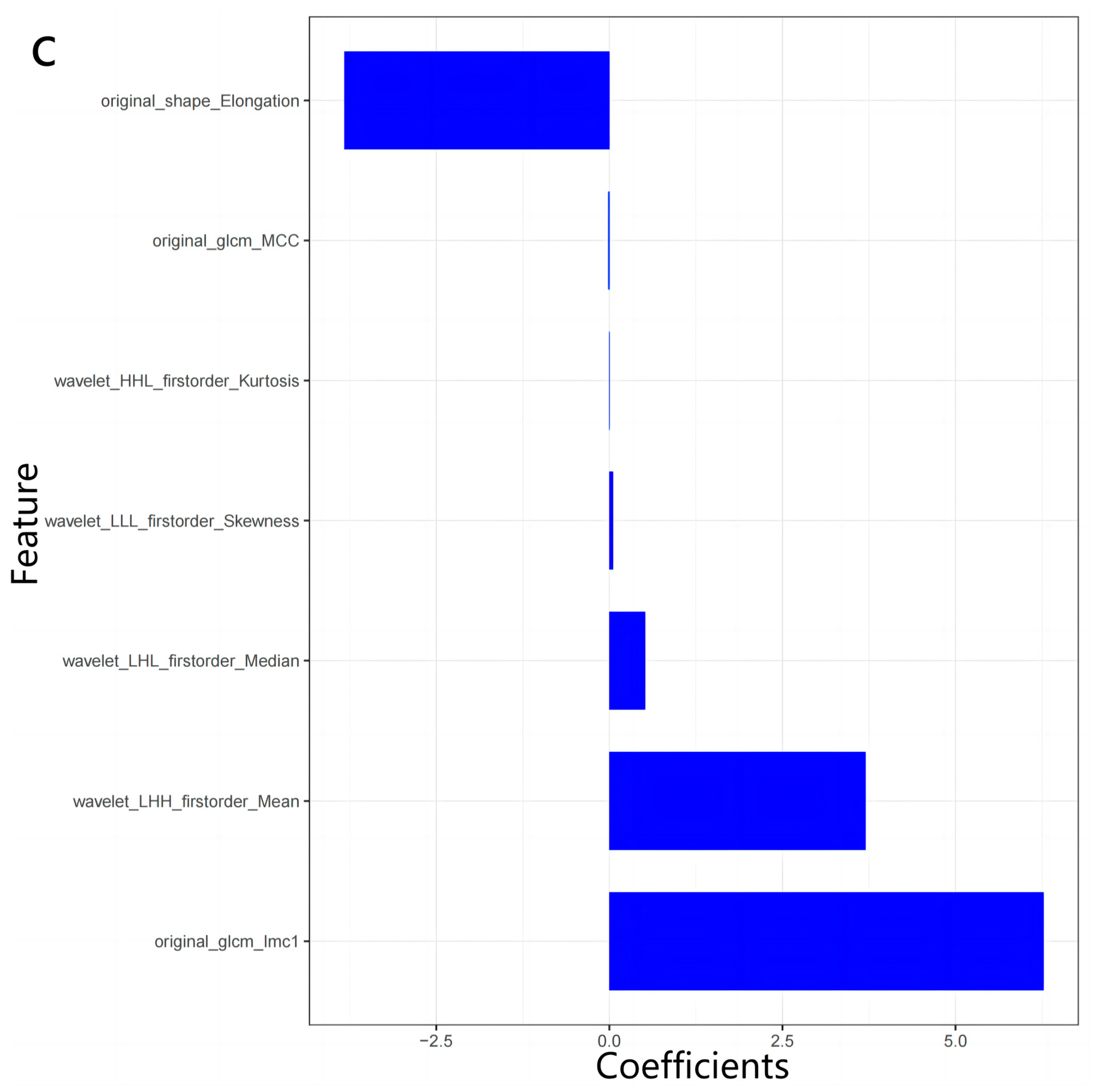
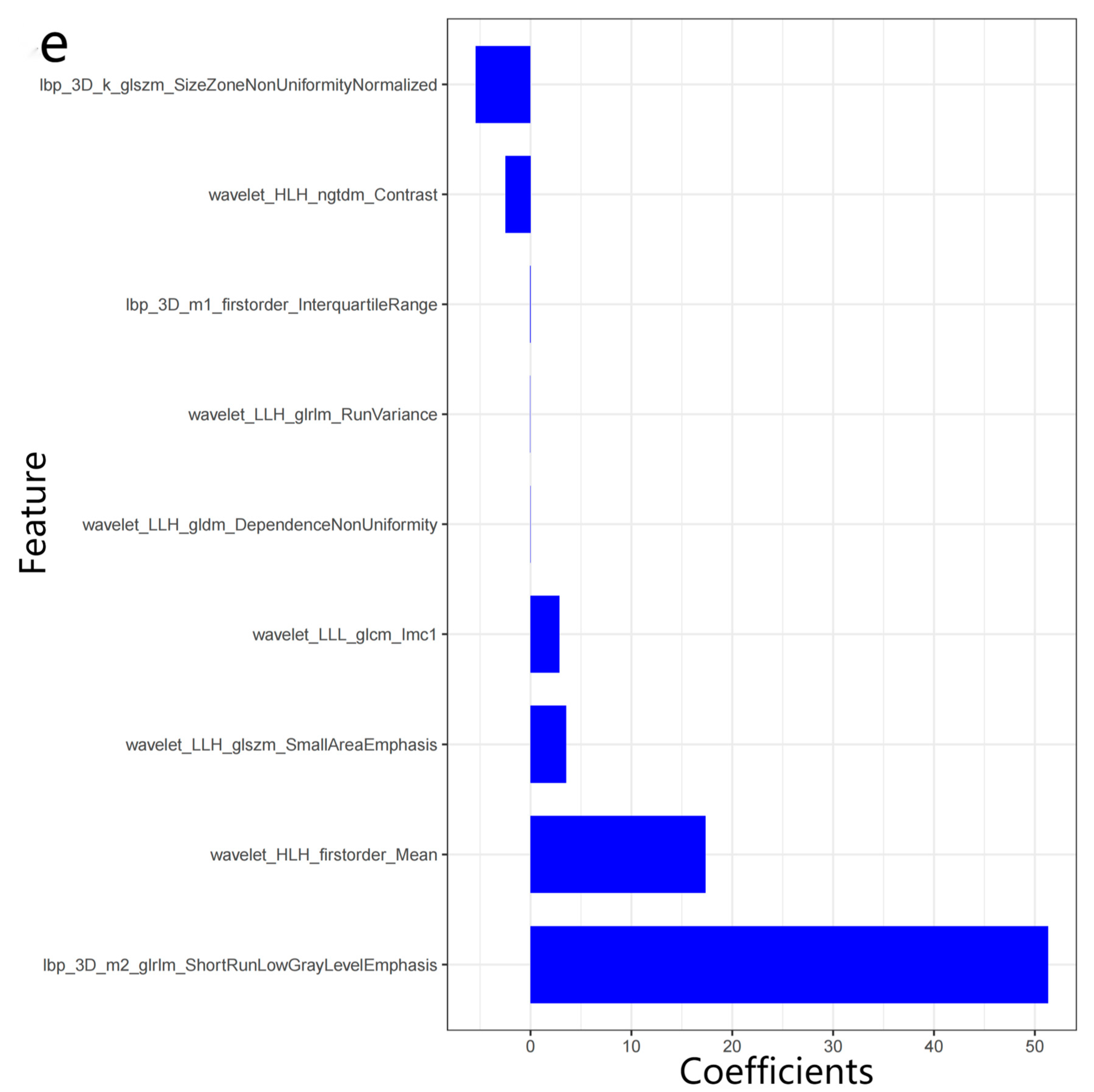
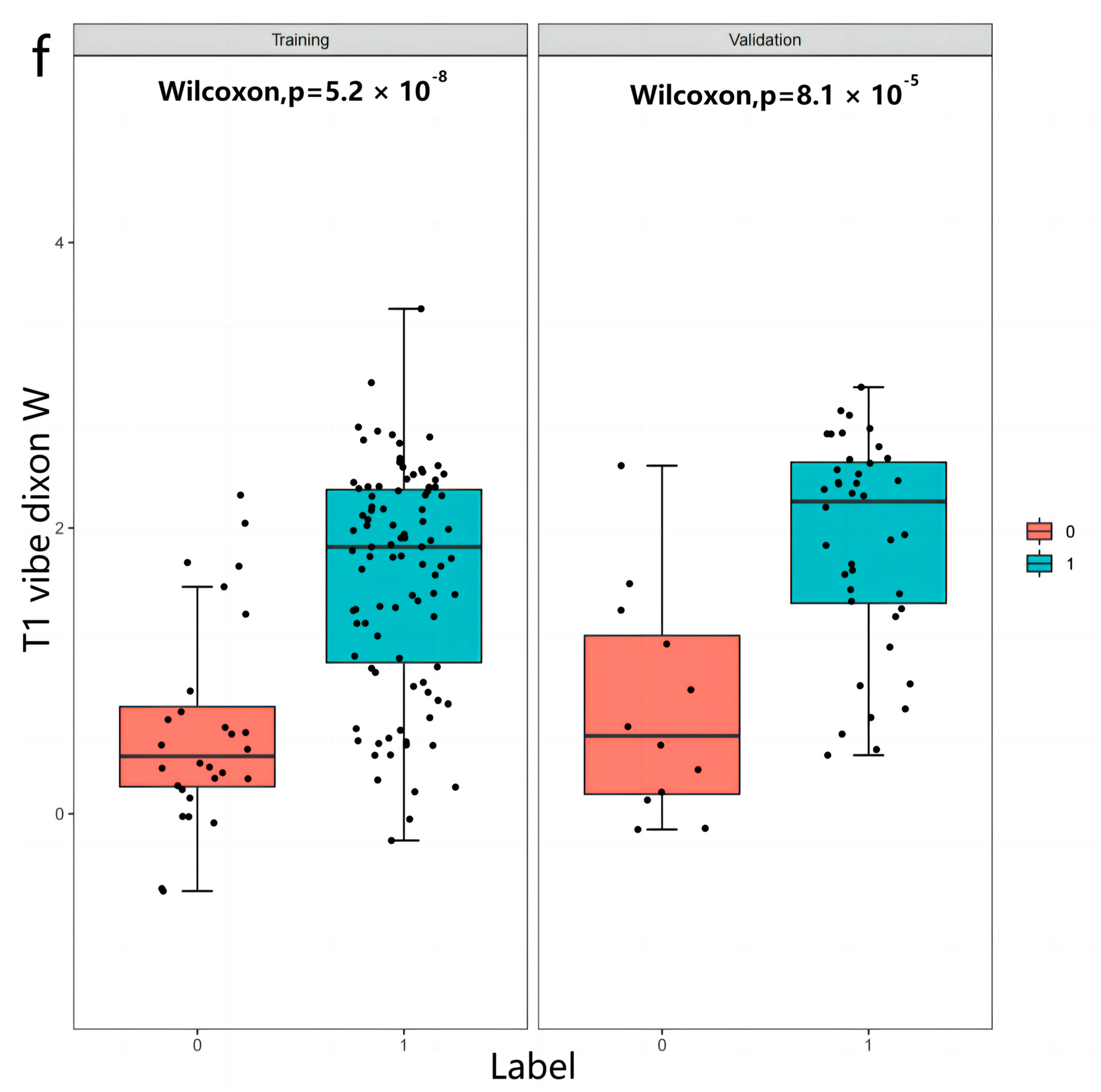
2.4. Radiomics Analysis
2.5. Statistical Analysis
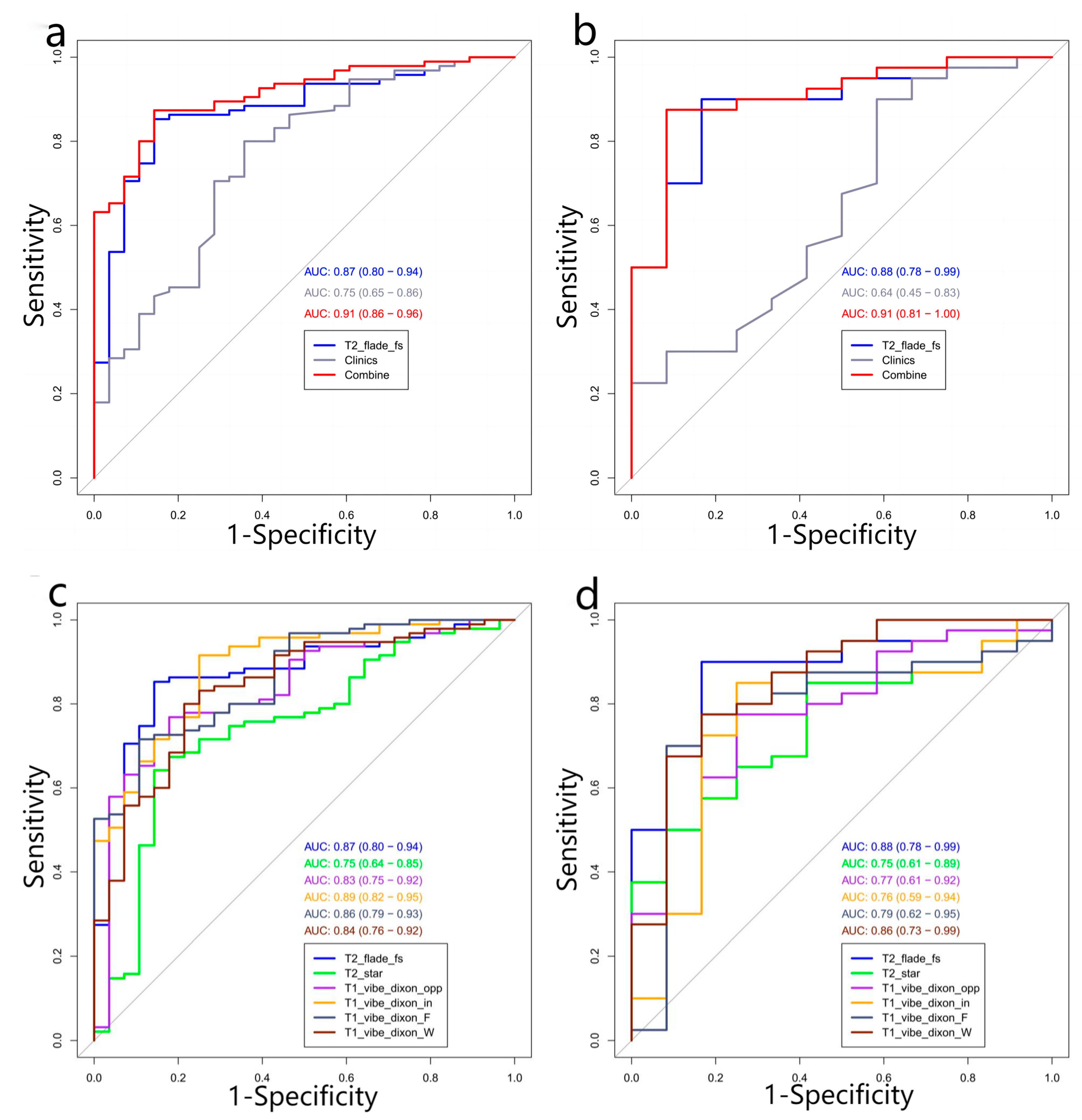
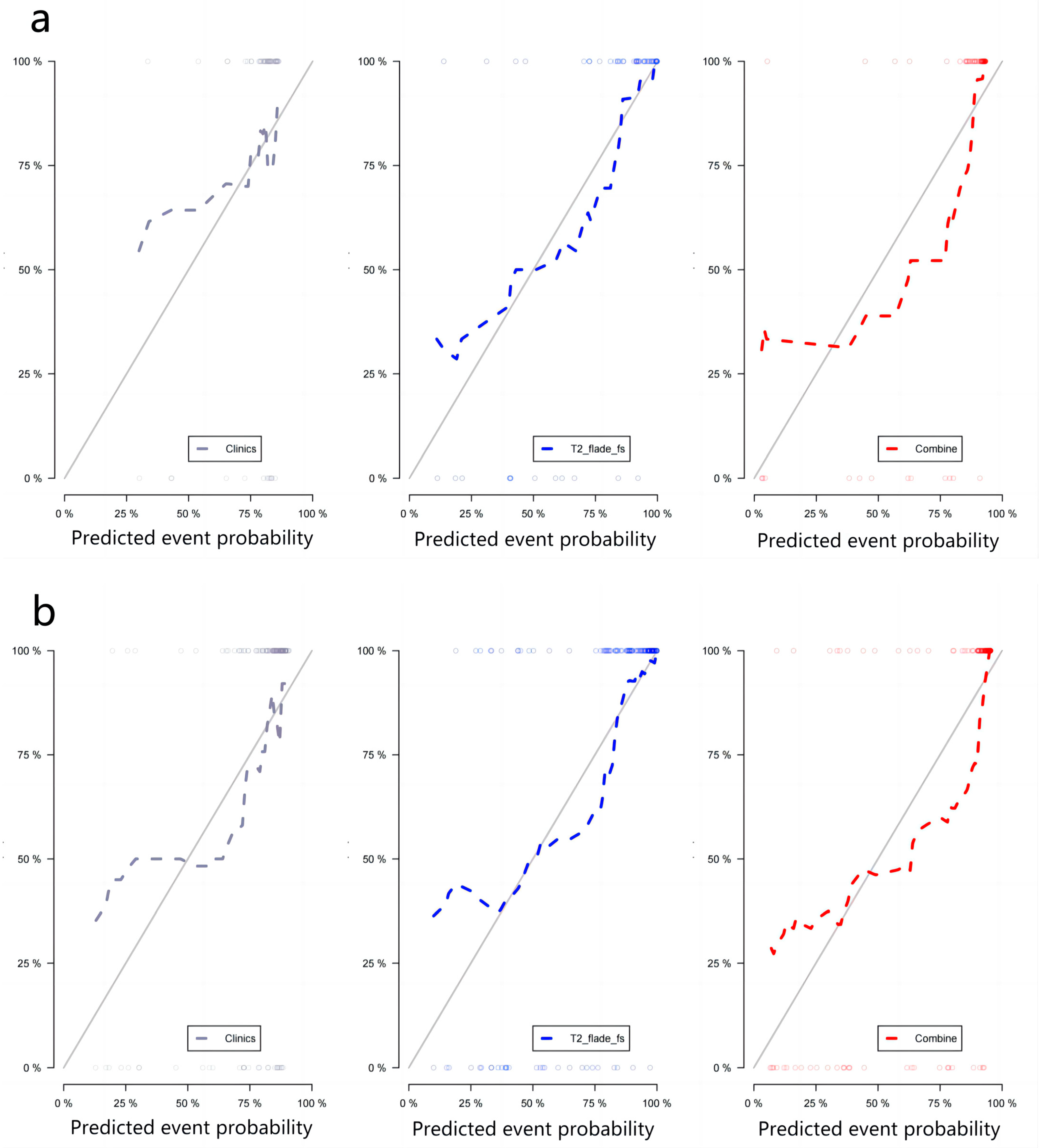
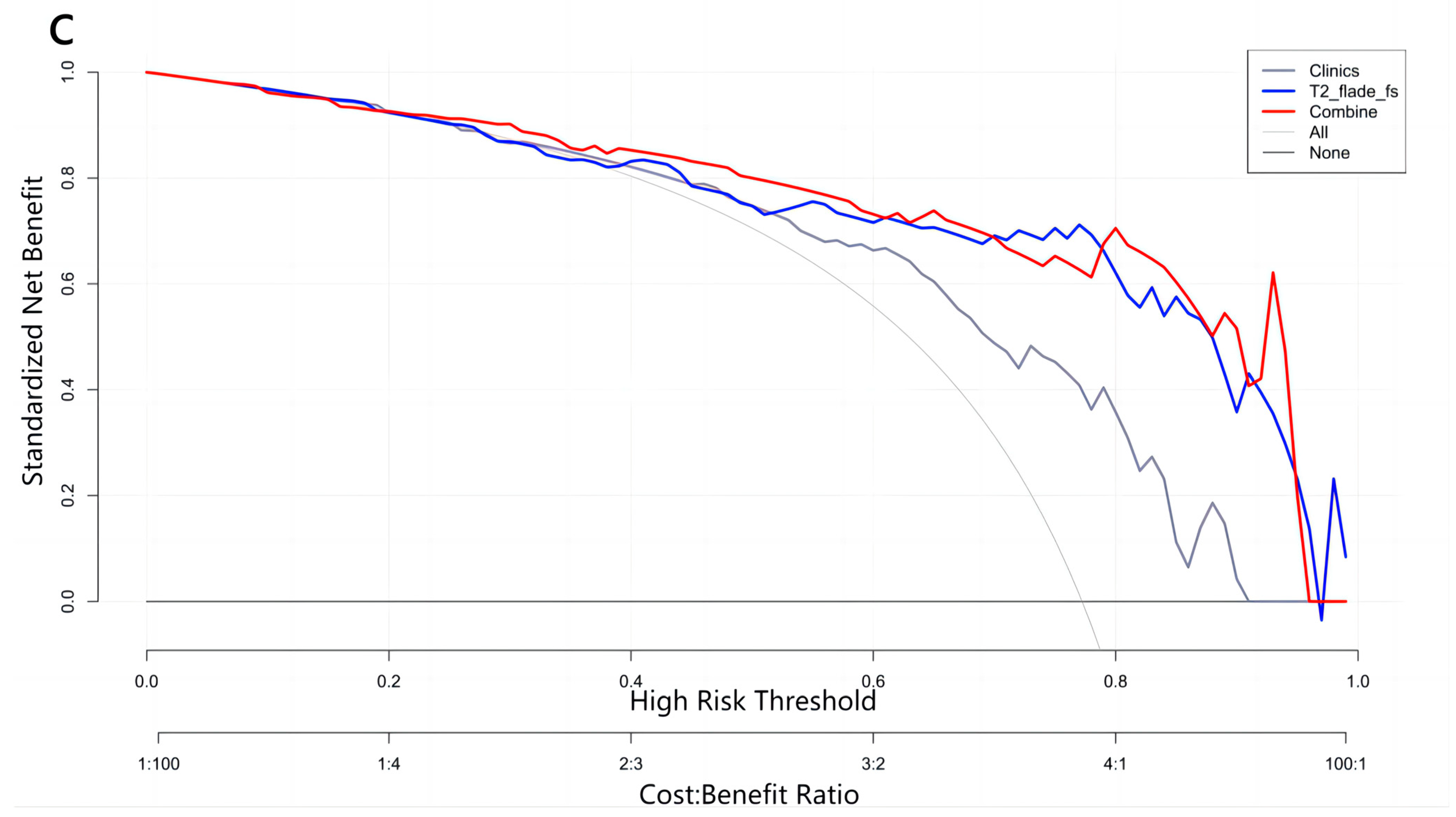
2.6. Performance of Radiomics Prediction Models
2.7. Intra-Observer and Inter-Observer Consistency
3. Results
3.1. Clinical Features
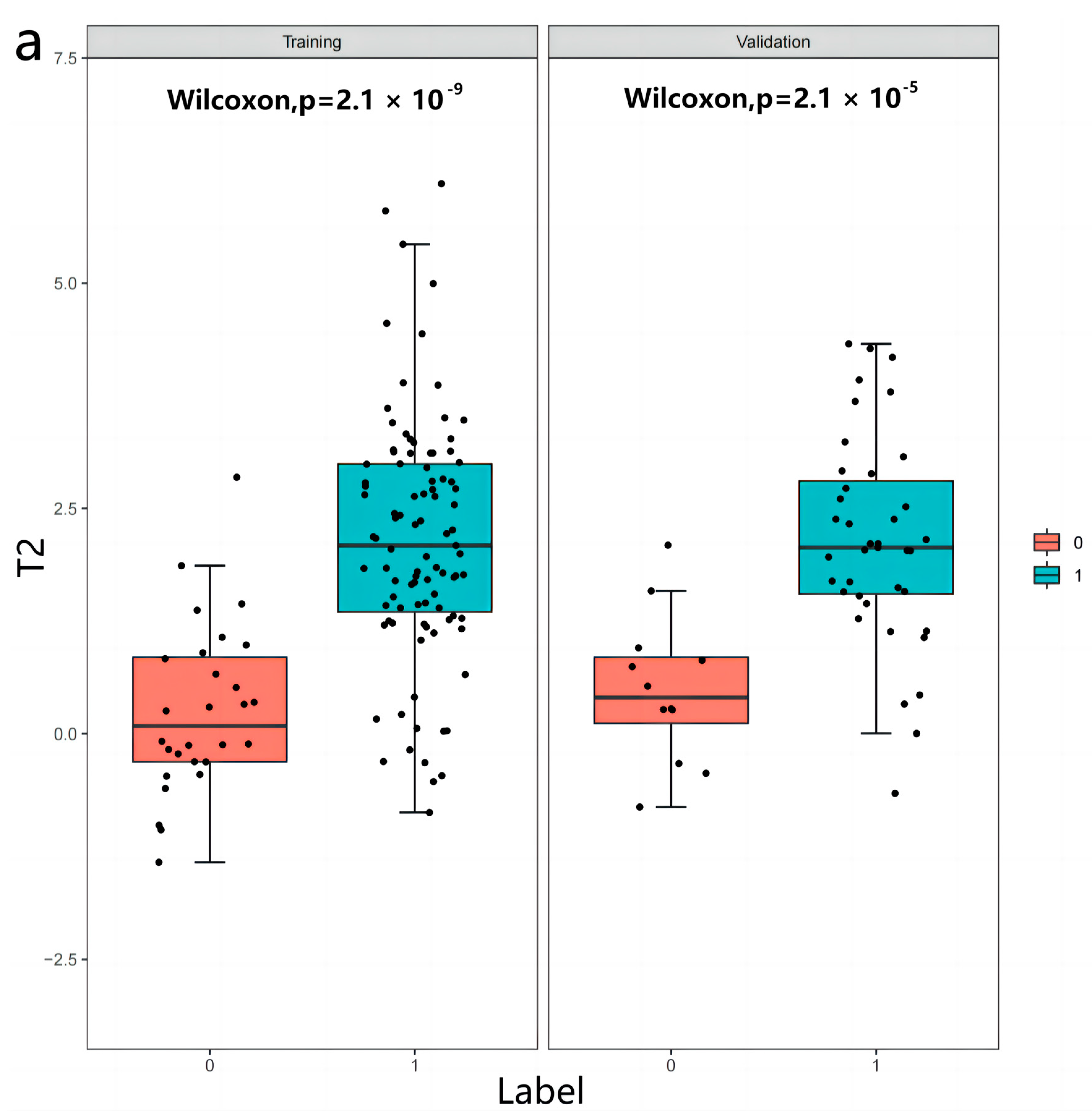
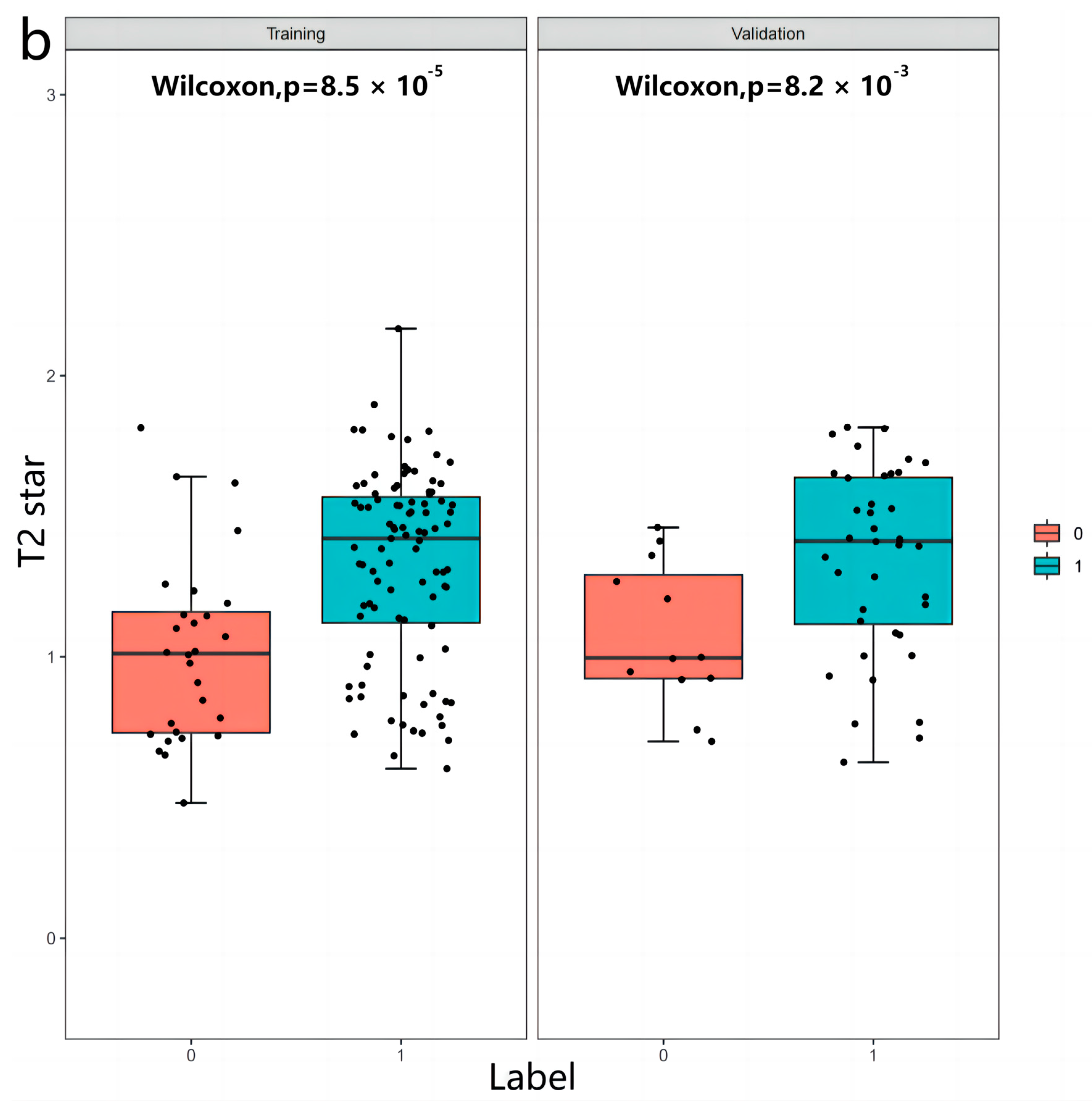
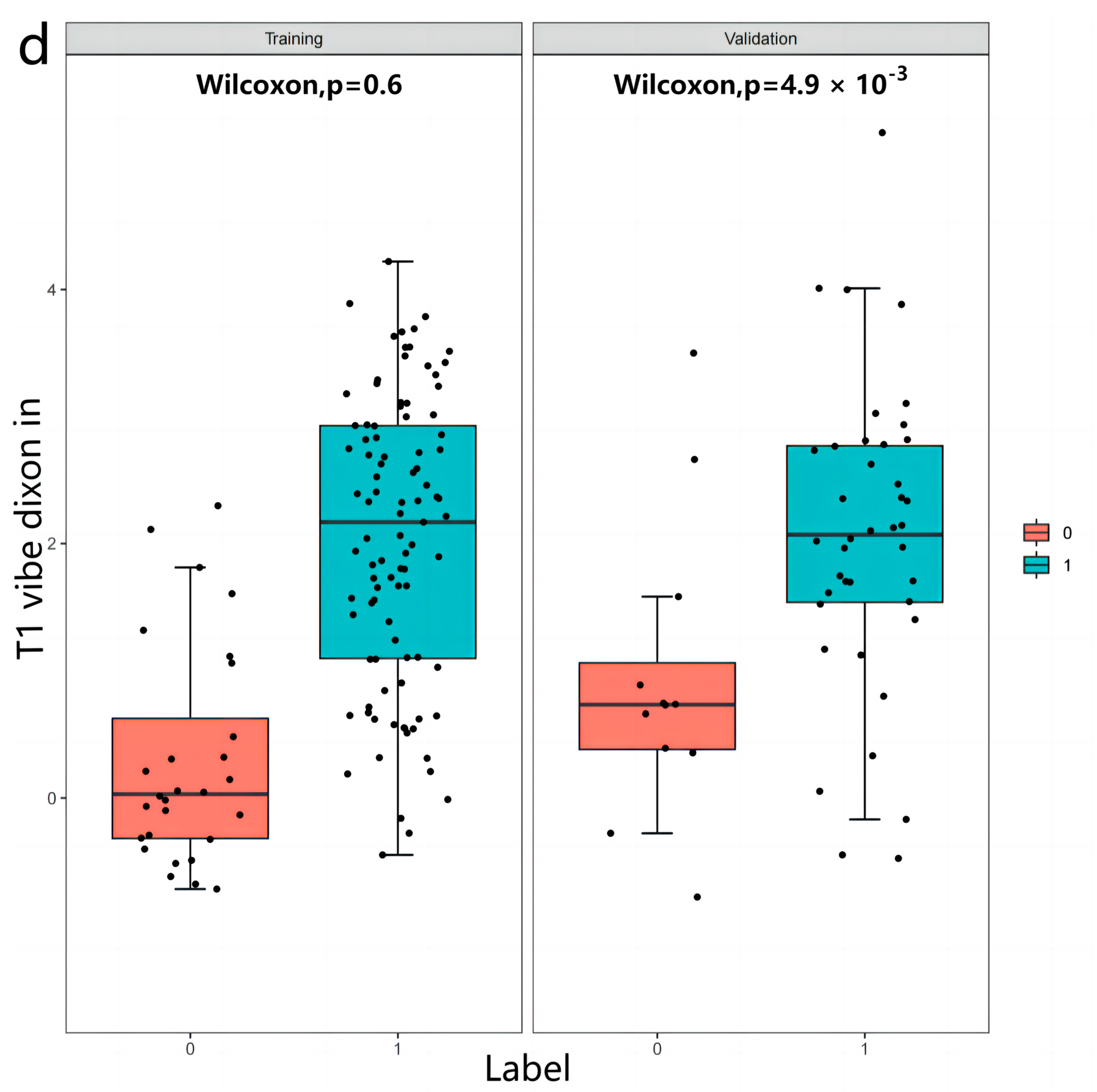
3.2. Radiomics Features
3.3. Inter-Observer and Intra-Observer Reproducibility of Radiomics Feature Extraction
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Radiomics Models | χ2 | p-Value |
|---|---|---|
| T2 train | 5.244 | 0.731 |
| T2 test | 3.695 | 0.884 |
| T2 star train | 5.354 | 0.719 |
| T2 star test | 7.998 | 0.434 |
| T1 vibe dixon opp train | 12.58 | 0.127 |
| T1 vibe dixon opp test | 13.666 | 0.091 |
| T1 vibe dixon in train | 5.244 | 0.731 |
| T1 vibe dixon in test | 3.695 | 0.884 |
| T1 vibe dixon F train | 5.354 | 0.719 |
| T1 vibe dixon F test | 7.998 | 0.434 |
| T1 vibe dixon W train | 12.58 | 0.127 |
| T1 vibe dixon W test | 13.666 | 0.091 |
References
- Li, D.-Z.; Yang, Y.-D. Invasive prenatal diagnosis of fetal thalassemia. Best Pr. Res. Clin. Obstet. Gynaecol. 2017, 39, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Sayani, F.A.; Kwiatkowski, J.L. Increasing prevalence of thalassemia in America: Implications for primary care. Ann. Med. 2015, 47, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Motta, I. New therapeutic targets in transfusion-dependent and -independent thalassemia. Hematol. Am. Soc. Hematol. Educ. Program 2017, 2017, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Traeger-Synodinos, J.; Harteveld, C.L.; Old, J.M.; Petrou, M.; Galanello, R.; Giordano, P.; Angastioniotis, M.; De la Salle, B.; Henderson, S.; May, A. EMQN Best Practice Guidelines for molecular and haematology methods for carrier identification and prenatal diagnosis of the haemoglobinopathies. Eur. J. Hum. Genet. EJHG 2015, 23, 560. [Google Scholar] [CrossRef]
- Giardine, B.; van Baal, S.; Kaimakis, P.; Riemer, C.; Miller, W.; Samara, M.; Kollia, P.; Anagnou, N.P.; Chui, D.H.; Wajcman, H.; et al. HbVar database of human hemoglobin variants and thalassemia mutations: 2007 update. Hum. Mutat. 2007, 28, 206. [Google Scholar] [CrossRef] [PubMed]
- Vichinsky, E. Complexity of alpha thalassemia: Growing health problem with new approaches to screening, diagnosis, and therapy. Ann. N. Y. Acad. Sci. 2010, 1202, 180–187. [Google Scholar] [CrossRef]
- Musallam, K.M.; Cappellini, M.D.; Daar, S.; El-Beshlawy, A.; Taher, A.T. Magnitude of cumulative iron overload correlates with the severity of anemia in untreated non-transfusion-dependent β-thalassemia. Ann. Hematol. 2023, 102, 467–469. [Google Scholar] [CrossRef]
- Munkongdee, T.; Chen, P.; Winichagoon, P.; Fucharoen, S.; Paiboonsukwong, K. Update in Laboratory Diagnosis of Thalassemia. Front. Mol. Biosci. 2020, 7, 74. [Google Scholar] [CrossRef]
- Afzal, M.; Naeem, M.A.; Ahmed, S.; Amin, N.; Rahim, A.; Munawar, M.; Ishaq, M.; Rathore, A.; Maria, K. Noninvasive prenatal testing of beta-thalassemia for common Pakistani mutations: A comparative study using cell-free fetal DNA from maternal plasma and chorionic villus sampling. Hematology 2022, 27, 353–359. [Google Scholar] [CrossRef]
- Swanson, A.; Sehnert, A.J.; Bhatt, S. Non-invasive Prenatal Testing: Technologies, Clinical Assays and Implementation Strategies for Women’s Healthcare Practitioners. Curr. Genet. Med. Rep. 2013, 1, 113–121. [Google Scholar] [CrossRef]
- Henninger, B.; Plaikner, M.; Zoller, H.; Viveiros, A.; Kannengiesser, S.; Jaschke, W.; Kremser, C. Performance of different Dixon-based methods for MR liver iron assessment in comparison to a biopsy-validated R2* relaxometry method. Eur. Radiol. 2021, 31, 2252–2262. [Google Scholar] [CrossRef] [PubMed]
- Henninger, B.; Alustiza, J.; Garbowski, M.; Gandon, Y. Practical guide to quantification of hepatic iron withMRI. Eur. Radiol. 2020, 30, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Akbari, H.; Rozycki, M.; Abdullah, K.G.; Nasrallah, M.P.; Binder, Z.A.; Davuluri, R.V.; Lustig, R.A.; Dahmane, N.; Bilello, M.; et al. Radiomic MRI signature reveals three distinct subtypes of glioblastoma with different clinical and molecular characteristics, offering prognostic value beyond IDH1. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, B.; Miles, K.A.; Babikir, S.; Shortman, R.; Afaq, A.; Ardeshna, K.M.; Groves, A.M.; Kayani, I. CT-based texture analysis potentially provides prognostic information complementary to interim fdg-pet for patients with hodgkin’s and aggressive non-hodgkin’s lymphomas. Eur. Radiol. 2017, 27, 1012–1020. [Google Scholar] [CrossRef]
- Groheux, D.; Martineau, A.; Teixeira, L.; Espié, M.; de Cremoux, P.; Bertheau, P.; Merlet, P.; Lemarignier, C. 18FDG-PET/CT for predicting the outcome in ER+/HER2- breast cancer patients: Comparison of clinicopathological parameters and PET image-derived indices including tumor texture analysis. Breast Cancer Res. BCR 2017, 19, 3. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, T.; Han, M. The application of radiomics in predicting gene mutations in cancer. Eur. Radiol. 2022, 32, 4014–4024. [Google Scholar] [CrossRef]
- Taher, A.T.; Saliba, A.N. Iron overload in thalassemia: Different organs at different rates. Hematol. Am. Soc. Hematol. Educ. Program 2017, 2017, 265–271. [Google Scholar] [CrossRef]
- Labranche, R.; Gilbert, G.; Cerny, M.; Vu, K.-N.; Soulières, D.; Olivié, D.; Billiard, J.-S.; Yokoo, T.; Tang, A. Liver Iron Quantification with MR Imaging: A Primer for Radiologists. Radiographics 2018, 38, 392–412. [Google Scholar] [CrossRef]
- Huang, Z.; Lyu, M.; Ai, Z.; Chen, Y.; Liang, Y.; Xiang, Z. Pre-operative Prediction of Ki-67 Expression in Various Histological Subtypes of Lung Adenocarcinoma Based on CT Radiomic Features. Front. Surg. 2021, 8, 736737. [Google Scholar] [CrossRef]
- Xi, L.J.; Guo, Z.Y.; Yang, X.K.; Ping, Z.G. Zhonghua yu fang yi xue za zhi. Chin. J. Prev. Med. 2023, 57, 107–111. [Google Scholar]
- Zhang, M.; Wong, S.W.; Wright, J.N.; Wagner, M.W.; Toescu, S.; Han, M.; Tam, L.T.; Zhou, Q.; Ahmadian, S.S.; Shpanskaya, K.; et al. MRI Radiogenomics of Pediatric Medulloblastoma: A Multicenter Study. Radiology 2022, 304, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Perez-Johnston, R.; Araujo-Filho, J.A.; Connolly, J.G.; Caso, R.; Whiting, K.; Tan, K.S.; Zhou, J.; Gibbs, P.; Rekhtman, N.; Ginsberg, M.S.; et al. CT-based Radiogenomic Analysis of Clinical Stage I Lung Adenocarcinoma with Histopathologic Features and Oncologic Outcomes. Radiology 2022, 303, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Charoenkwan, P.; Tantiworawit, A. Treatment strategies for haemoglobin E thalassaemia. Lancet Glob. Health 2022, 10, e18–e19. [Google Scholar] [CrossRef]
- Shu, Z.; Fang, S.; Ding, Z.; Mao, D.; Cai, R.; Chen, Y.; Pang, P.; Gong, X. MRI-based Radiomics nomogram to detect primary rectal cancer with synchronous liver metastases. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.S.; Kannengiesser, S.A.; Ward, R.; Kuo, K.; Sussman, M.S. Prospective Evaluation of an R2* Method for Assessing Liver Iron Concentration (LIC) Against FerriScan: Derivation of the Calibration Curve and Characterization of the Nature and Source of Uncertainty in the Relationship. J. Magn. Reson. Imaging 2019, 49, 1467–1474. [Google Scholar] [CrossRef]
- Ng, F.; Kozarski, R.; Ganeshan, B.; Goh, V. Assessment of tumor heterogeneity by CT texture analysis: Can the largest cross-sectional area be used as an alternative to whole tumor analysis? Eur. J. Radiol. 2013, 82, 342–348. [Google Scholar] [CrossRef]







| Variables | α-Genome (n = 40) | β-Genome (n = 135) | ||
|---|---|---|---|---|
| M | Interquartile Range | M | Interquartile Range | |
| Liver T2 star (ms) | 2.48 | 1.523–4.09 | 1.24 | 0.98–1.8 |
| Serum ferritin (mg/mL) | 474.86 | 311.478–895.198 | 3918.495 | 2343.943–6937.428 |
| WBC (109/L) | 6.59 | 5.36–8.82 | 7.13 | 5.388–10.79 |
| RBC (1012/L) | 4.46 | 3.690–4.880 | 3.635 | 3.11–4.128 |
| HGB (g/L) | 88 | 79.3–101.2 | 92.3 | 82.925–109.975 |
| PLT (109/L) | 300.1 | 222.3–348 | 263.2 | 181.525–426.925 |
| NEU percentage (%) | 0.574 | 0.459–0.63 | 0.473 | 0.344–0.579 |
| LYM percentage (%) | 0.35 | 0.295–0.418 | 0.381 | 0.314–0.504 |
| MONO percentage (%) | 0.061 | 0.057–0.079 | 0.077 | 0.061–0.101 |
| EO percentage (%) | 0.015 | 0.012–0.027 | 0.023 | 0.012–0.039 |
| BISO percentage (%) | 0.004 | 0.003–0.007 | 0.004 | 0.003–0.007 |
| NEU (109/L) | 3.99 | 2.62–5.27 | 3.61 | 2.148–5.59 |
| LYM (109/L) | 2.36 | 1.66–2.83 | 2.69 | 1.903–4.115 |
| MONO (109/L) | 0.45 | 0.31–0.59 | 0.51 | 0.343–1.01 |
| EOS (109/L) | 0.12 | 0.07–0.18 | 0.155 | 0.073–0.365 |
| BASO (109/L) | 0.03 | 0.01–0.05 | 0.03 | 0.02–0.06 |
| MCV (fl) | 73.39 | 67.6–75.5 | 81.22 | 74.925–85.225 |
| MCH (pg) | 20.37 | 19–21.55 | 26.58 | 24.543–28.365 |
| MCHC (g/L) | 278 | 269.9–293 | 330.25 | 322.775–339.5 |
| HCT (%) | 0.316 | 0.278–0.35 | 0.283 | 0.247–0.335 |
| RDWCV (%) | 0.25 | 0.23–0.3 | 0.19 | 0.14–0.25 |
| PDW (%) | 0.16 | 0.14–0.19 | 0.16 | 0.11–0.18 |
| PCT (%) | 0.31 | 0.195–0.44 | 0.231 | 0.16–0.392 |
| MPV (fl) | 9.5 | 8.09–12.1 | 8.845 | 7.773–10.05 |
| Variable | Odds Ratio | Lower | Upper | p-Value |
|---|---|---|---|---|
| Age | 0.904 | 0.857 | 0.954 | <0.001 |
| Gender | 1.42 | 0.607 | 3.325 | 0.419 |
| TBiL | 0.994 | 0.982 | 1.007 | 0.366 |
| DBiL | 1.003 | 0.975 | 1.031 | 0.849 |
| IBil | 0.985 | 0.967 | 1.004 | 0.131 |
| DB versus TB ratio | 25.277 | 0.237 | 2695.483 | 0.175 |
| ALB | 0.801 | 0.681 | 0.942 | 0.007 |
| GLO | 0.967 | 0.889 | 1.053 | 0.44 |
| A versus G ratio | 1.096 | 0.29 | 4.137 | 0.893 |
| GGT | 1.005 | 0.99 | 1.019 | 0.538 |
| TBA | 1.095 | 1 | 1.198 | 0.05 |
| AST | 1.029 | 0.996 | 1.062 | 0.088 |
| ALT | 1.021 | 0.997 | 1.044 | 0.081 |
| AST versus ALT ratio | 0.604 | 0.352 | 1.038 | 0.068 |
| PA | 0.993 | 0.984 | 1.003 | 0.167 |
| CHE | 1 | 1 | 1 | 0.397 |
| UREA | 1.023 | 0.714 | 1.465 | 0.901 |
| CREA | 0.901 | 0.834 | 0.973 | 0.008 |
| RBP | 0.971 | 0.921 | 1.023 | 0.27 |
| HCO3 | 0.953 | 0.801 | 1.135 | 0.591 |
| Ccr | 0.994 | 0.97 | 1.018 | 0.611 |
| CysC | 3.603 | 0.142 | 91.551 | 0.437 |
| Variable | Odds Ratio | CI.95% | p-Value |
|---|---|---|---|
| Age | 0.939 | [0.896;0.983] | 0.009 |
| ALB | 0.831 | [0.716;0.964] | 0.044 |
| TBA | 1.064 | [0.989;1.144] | 0.107 |
| CREA | 0.936 | [0.871;1.005] | 0.14 |
| Model | TP | TN | FP | FN | Accuracy | Sensitivity | Specificity | Pos. Pred. Value | Neg. Pred. Value |
|---|---|---|---|---|---|---|---|---|---|
| T2 test | 35 | 10 | 2 | 5 | 0.865 | 0.875 | 0.833 | 0.946 | 0.667 |
| T2 star test | 26 | 8 | 4 | 14 | 0.654 | 0.65 | 0.667 | 0.867 | 0.364 |
| T1 vibe dixon opp test | 32 | 6 | 6 | 8 | 0.731 | 0.8 | 0.5 | 0.842 | 0.429 |
| T1 vibe dixon in test | 35 | 4 | 8 | 5 | 0.75 | 0.875 | 0.333 | 0.814 | 0.444 |
| T1 vibe dixon F test | 32 | 8 | 4 | 8 | 0.769 | 0.8 | 0.667 | 0.889 | 0.5 |
| T1 vibe dixon W test | 35 | 8 | 4 | 5 | 0.827 | 0.875 | 0.667 | 0.897 | 0.615 |
| Clinics test | 32 | 5 | 7 | 8 | 0.712 | 0.8 | 0.417 | 0.821 | 0.385 |
| Combine test | 36 | 8 | 4 | 4 | 0.846 | 0.9 | 0.667 | 0.9 | 0.667 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Feng, Q.; Yi, J.; Tang, C.; Lin, H.; Liang, B.; Luo, C.; Guan, K.; Li, T.; Peng, P. α- and β-Genotyping of Thalassemia Patients Based on a Multimodal Liver MRI Radiomics Model: A Preliminary Study in Two Centers. Diagnostics 2023, 13, 958. https://doi.org/10.3390/diagnostics13050958
Xu F, Feng Q, Yi J, Tang C, Lin H, Liang B, Luo C, Guan K, Li T, Peng P. α- and β-Genotyping of Thalassemia Patients Based on a Multimodal Liver MRI Radiomics Model: A Preliminary Study in Two Centers. Diagnostics. 2023; 13(5):958. https://doi.org/10.3390/diagnostics13050958
Chicago/Turabian StyleXu, Fengming, Qing Feng, Jixing Yi, Cheng Tang, Huashan Lin, Bumin Liang, Chaotian Luo, Kaiming Guan, Tao Li, and Peng Peng. 2023. "α- and β-Genotyping of Thalassemia Patients Based on a Multimodal Liver MRI Radiomics Model: A Preliminary Study in Two Centers" Diagnostics 13, no. 5: 958. https://doi.org/10.3390/diagnostics13050958
APA StyleXu, F., Feng, Q., Yi, J., Tang, C., Lin, H., Liang, B., Luo, C., Guan, K., Li, T., & Peng, P. (2023). α- and β-Genotyping of Thalassemia Patients Based on a Multimodal Liver MRI Radiomics Model: A Preliminary Study in Two Centers. Diagnostics, 13(5), 958. https://doi.org/10.3390/diagnostics13050958






