Enhancing the Reliability of Intraoperative Ultrasound in Pediatric Space-Occupying Brain Lesions
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dixon, L.; Lim, A.; Grech-Sollars, M.; Nandi, D.; Camp, S. Intraoperative Ultrasound in Brain Tumor Surgery: A Review and Implementation Guide. Neurosurg. Rev. 2022, 45, 2503–2515. [Google Scholar] [CrossRef]
- de Laurentis, C.; Bteich, F.; Beuriat, P.A.; Mottolese, C.; Giussani, C.; Szathmari, A.; Vinchon, M.; Di Rocco, F. Sodium Fluorescein in Pediatric Neurosurgery: A Systematic Review with Technical Considerations and Future Perspectives. Childs Nerv. Syst. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- de Laurentis, C.; Höhne, J.; Cavallo, C.; Restelli, F.; Falco, J.; Broggi, M.; Bosio, L.; Vetrano, I.G.; Schiariti, M.; Zattra, C.M.; et al. The Impact of Fluorescein-Guided Technique in the Surgical Removal of CNS Tumors in a Pediatric Population: Results from a Multicentric Observational Study. J. Neurosurg. Sci. 2019, 63, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Falco, J.; Broggi, M.; Schiariti, M.; Vetrano, I.G.; Esposito, S.; Ferroli, P.; Acerbi, F. The Role of Sodium Fluorescein in Pediatric Supratentorial Intra-Axial Tumor Resection: New Insights from a Monocentric Series of 33 Consecutive Patients. Childs Nerv. Syst. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Tonn, J.-C.; Goetz, C.; Ullrich, W.; Stepp, H.; Bink, A.; Pietsch, T.; Pichlmeier, U. 5-Aminolevulinic Acid-Derived Tumor Fluorescence: The Diagnostic Accuracy of Visible Fluorescence Qualities as Corroborated by Spectrometry and Histology and Postoperative Imaging. Neurosurgery 2014, 74, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.P.; Deepa, S.F.A.J.; Moinca, I.; Suresh, P.; Naidu, K.V.J.R. Medulloblastoma: A Common Pediatric Tumor: Prognostic Factors and Predictors of Outcome. Asian J. Neurosurg. 2015, 10, 50. [Google Scholar] [CrossRef]
- McCrea, H.J.; Bander, E.D.; Venn, R.A.; Reiner, A.S.; Iorgulescu, J.B.; Puchi, L.A.; Schaefer, P.M.; Cederquist, G.; Greenfield, J.P. Sex, Age, Anatomic Location, and Extent of Resection Influence Outcomes in Children with High-Grade Glioma. Neurosurgery 2015, 77, 443–453. [Google Scholar] [CrossRef]
- Roth, J.; Biyani, N.; Beni-Adani, L.; Constantini, S. Real-Time Neuronavigation with High-Quality 3D Ultrasound SonoWand in Pediatric Neurosurgery. Pediatr. Neurosurg. 2007, 43, 185–191. [Google Scholar] [CrossRef]
- Souweidane, M.M. The Evolving Role of Surgery in the Management of Pediatric Brain Tumors. J. Child Neurol. 2009, 24, 1366–1374. [Google Scholar] [CrossRef]
- Sun, M.Z.; Ivan, M.E.; Clark, A.J.; Oh, M.C.; Delance, A.R.; Oh, T.; Safaee, M.; Kaur, G.; Bloch, O.; Molinaro, A.; et al. Gross Total Resection Improves Overall Survival in Children with Choroid Plexus Carcinoma. J. Neurooncol. 2014, 116, 179–185. [Google Scholar] [CrossRef]
- Ullrich, N.J.; Pomeroy, S.L.; Kapur, K.; Manley, P.E.; Goumnerova, L.C.; Loddenkemper, T. Incidence, Risk Factors, and Longitudinal Outcome of Seizures in Long-Term Survivors of Pediatric Brain Tumors. Epilepsia 2015, 56, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Avula, S.; Mallucci, C.L.; Pizer, B.; Garlick, D.; Crooks, D.; Abernethy, L.J. Intraoperative 3-Tesla MRI in the Management of Paediatric Cranial Tumours—Initial Experience. Pediatr. Radiol. 2012, 42, 158–167. [Google Scholar] [CrossRef] [PubMed]
- El Beltagy, M.A.; Aggag, M.; Kamal, M. Role of Intraoperative Ultrasound in Resection of Pediatric Brain Tumors. Childs Nerv. Syst. 2010, 26, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.H.; Hall, W.A.; Truwit, C.L.; Liu, H. Intra-Operative MRI-Guided Approaches to the Pediatric Posterior Fossa Tumors. Pediatr. Neurosurg. 2001, 34, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, T.M.; Titsworth, W.L. The Evolution of IMRI Utilization for Pediatric Neurosurgery: A Single Center Experience. Acta Neurochir. Suppl. 2011, 109, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Mutchnick, I.; Moriarty, T.M. Intraoperative MRI in Pediatric Neurosurgery—An Update. Transl. Pediatr. 2014, 3, 236–246. [Google Scholar] [CrossRef]
- Chacko, A.G.; Kumar, N.K.S.; Chacko, G.; Athyal, R.; Rajshekhar, V. Intraoperative Ultrasound in Determining the Extent of Resection of Parenchymal Brain Tumours—A Comparative Study with Computed Tomography and Histopathology. Acta Neurochir. 2003, 145, 743–748, discussion 748. [Google Scholar] [CrossRef]
- Unsgaard, G.; Ommedal, S.; Muller, T.; Gronningsaeter, A.; Nagelhus Hernes, T.A. Neuronavigation by Intraoperative Three-Dimensional Ultrasound: Initial Experience during Brain Tumor Resection. Neurosurgery 2002, 50, 804–812, discussion 812. [Google Scholar] [CrossRef]
- Ulrich, N.H.; Burkhardt, J.-K.; Serra, C.; Bernays, R.-L.; Bozinov, O. Resection of Pediatric Intracerebral Tumors with the Aid of Intraoperative Real-Time 3-D Ultrasound. Childs Nerv. Syst. 2012, 28, 101–109. [Google Scholar] [CrossRef]
- Moiyadi, A.; Shetty, P. Objective Assessment of Utility of Intraoperative Ultrasound in Resection of Central Nervous System Tumors: A Cost-Effective Tool for Intraoperative Navigation in Neurosurgery. J. Neurosci. Rural Pract. 2011, 2, 4–11. [Google Scholar] [CrossRef]
- Choudhri, A.F.; Klimo, P.; Auschwitz, T.S.; Whitehead, M.T.; Boop, F.A. 3T Intraoperative MRI for Management of Pediatric CNS Neoplasms. Am. J. Neuroradiol. 2014, 35, 2382–2387. [Google Scholar] [CrossRef]
- Roth, J.; Beni-Adani, L.; Biyani, N.; Constantini, S. Classical and Real-Time Neuronavigation in Pediatric Neurosurgery. Childs Nerv. Syst. 2006, 22, 1065–1071. [Google Scholar] [CrossRef]
- Giussani, C.; Trezza, A.; Ricciuti, V.; Di Cristofori, A.; Held, A.; Isella, V.; Massimino, M. Intraoperative MRI versus Intraoperative Ultrasound in Pediatric Brain Tumor Surgery: Is Expensive Better than Cheap? A Review of the Literature. Childs Nerv. Syst. 2022, 38, 1445–1454. [Google Scholar] [CrossRef]
- Mosteiro, A.; Di Somma, A.; Ramos, P.R.; Ferrés, A.; De Rosa, A.; González-Ortiz, S.; Enseñat, J.; González, J.J. Is Intraoperative Ultrasound More Efficient than Magnetic Resonance in Neurosurgical Oncology? An Exploratory Cost-Effectiveness Analysis. Front. Oncol. 2022, 12, 1016264. [Google Scholar] [CrossRef]
- Barzaghi, L.R.; Capitanio, J.F.; Giudice, L.; Panni, P.; Acerno, S.; Mortini, P. Usefulness of Ultrasound-Guided Microsurgery in Cavernous Angioma Removal. World Neurosurg. 2018, 116, e414–e420. [Google Scholar] [CrossRef]
- Prada, F.; Del Bene, M.; Moiraghi, A.; Casali, C.; Legnani, F.G.; Saladino, A.; Perin, A.; Vetrano, I.G.; Mattei, L.; Richetta, C.; et al. From Grey Scale B-Mode to Elastosonography: Multimodal Ultrasound Imaging in Meningioma Surgery-Pictorial Essay and Literature Review. Biomed Res. Int. 2015, 2015, 925729. [Google Scholar] [CrossRef]
- Prada, F.; Perin, A.; Martegani, A.; Aiani, L.; Solbiati, L.; Lamperti, M.; Casali, C.; Legnani, F.; Mattei, L.; Saladino, A.; et al. Intraoperative Contrast-Enhanced Ultrasound for Brain Tumor Surgery. Neurosurgery 2014, 74, 542–552, discussion 552. [Google Scholar] [CrossRef]
- Sweeney, J.F.; Smith, H.; Taplin, A.; Perloff, E.; Adamo, M.A. Efficacy of Intraoperative Ultrasonography in Neurosurgical Tumor Resection. J. Neurosurg. Pediatr. 2018, 21, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Carai, A.; De Benedictis, A.; Calloni, T.; Onorini, N.; Paternò, G.; Randi, F.; Colafati, G.S.; Mastronuzzi, A.; Marras, C.E. Intraoperative Ultrasound-Assisted Extent of Resection Assessment in Pediatric Neurosurgical Oncology. Front. Oncol. 2021, 11, 660805. [Google Scholar] [CrossRef] [PubMed]
- El Beltagy, M.A.; Atteya, M.M.E. The Benefits of Navigated Intraoperative Ultrasonography during Resection of Fourth Ventricular Tumors in Children. Childs Nerv. Syst. 2013, 29, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Gosal, J.S.; Tiwari, S.; Sharma, T.; Agrawal, M.; Garg, M.; Mahal, S.; Bhaskar, S.; Sharma, R.K.; Janu, V.; Jha, D.K. Simulation of Surgery for Supratentorial Gliomas in Virtual Reality Using a 3D Volume Rendering Technique: A Poor Man’s Neuronavigation. Neurosurg. Focus 2021, 51, E23. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, S.; Marziali, G.; Pezzullo, G.; Guadalupi, P.; Giordano, C.; Infante, A.; Benenati, M.; Ramaglia, A.; Massimi, L.; Gessi, M.; et al. Role of Susceptibility-Weighted Imaging and Intratumoral Susceptibility Signals in Grading and Differentiating Pediatric Brain Tumors at 1.5 T: A Preliminary Study. Neuroradiology 2020, 62, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, S.; Martucci, M.; Botto, A.; Ruberto, E.; Leone, E.; Infante, A.; Ramaglia, A.; Caldarelli, M.; Frassanito, P.; Triulzi, F.M.; et al. Brain DSC MR Perfusion in Children: A Clinical Feasibility Study Using Different Technical Standards of Contrast Administration. Am. J. Neuroradiol. 2019, 40, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Rosado, E.; Riccabona, M. Off-Label Use of Ultrasound Contrast Agents for Intravenous Applications in Children: Analysis of the Existing Literature. J. Ultrasound. Med. 2016, 35, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, P.S.; Cantisani, V.; Deganello, A.; Dietrich, C.F.; Duran, C.; Franke, D.; Harkanyi, Z.; Kosiak, W.; Miele, V.; Ntoulia, A.; et al. Role of Contrast-Enhanced Ultrasound (CEUS) in Paediatric Practice: An EFSUMB Position Statement. J. Ultrasound 2017, 38, 33–43. [Google Scholar] [CrossRef]
- Saß, B.; Zivkovic, D.; Pojskic, M.; Nimsky, C.; Bopp, M.H.A. Navigated Intraoperative 3D Ultrasound in Glioblastoma Surgery: Analysis of Imaging Features and Impact on Extent of Resection. Front. Neurosci. 2022, 16, 883584. [Google Scholar] [CrossRef]
- Keleş, A.; Türe, U. Cottonoid-Guided Intraoperative Ultrasonography in Neurosurgery: A Proof-of-Concept Single Surgeon Case Series. Neurosurg. Rev. 2022, 45, 2289–2303. [Google Scholar] [CrossRef]
- Selbekk, T.; Jakola, A.S.; Solheim, O.; Johansen, T.F.; Lindseth, F.; Reinertsen, I.; Unsgård, G. Ultrasound Imaging in Neurosurgery: Approaches to Minimize Surgically Induced Image Artefacts for Improved Resection Control. Acta Neurochir. 2013, 155, 973–980. [Google Scholar] [CrossRef]
- Šteňo, A.; Karlík, M.; Mendel, P.; Čík, M.; Šteňo, J. Navigated Three-Dimensional Intraoperative Ultrasound-Guided Awake Resection of Low-Grade Glioma Partially Infiltrating Optic Radiation. Acta Neurochir. 2012, 154, 1255–1262. [Google Scholar] [CrossRef]
- Unsgård, G.; Sagberg, L.M.; Müller, S.; Selbekk, T. A New Acoustic Coupling Fluid with Ability to Reduce Ultrasound Imaging Artefacts in Brain Tumour Surgery—A Phase I Study. Acta Neurochir. 2019, 161, 1475–1486. [Google Scholar] [CrossRef]
- Piscaglia, F.; Nolsøe, C.; Dietrich, C.F.; Cosgrove, D.O.; Gilja, O.H.; Bachmann Nielsen, M.; Albrecht, T.; Barozzi, L.; Bertolotto, M.; Catalano, O.; et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): Update 2011 on Non-Hepatic Applications. Ultraschall Med. 2012, 33, 33–59. [Google Scholar] [CrossRef] [PubMed]
- Prada, F.; Bene, M.D.; Fornaro, R.; Vetrano, I.G.; Martegani, A.; Aiani, L.; Sconfienza, L.M.; Mauri, G.; Solbiati, L.; Pollo, B.; et al. Identification of Residual Tumor with Intraoperative Contrast-Enhanced Ultrasound during Glioblastoma Resection. Neurosurg. Focus 2016, 40, E7. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, N.; Tucer, B.; Mavili, E.; Menkü, A.; Kurtsoy, A. Ultrasound Guidance in Intracranial Tumor Resection: Correlation with Postoperative Magnetic Resonance Findings. Acta Radiol. 2005, 46, 743–749. [Google Scholar] [CrossRef] [PubMed]
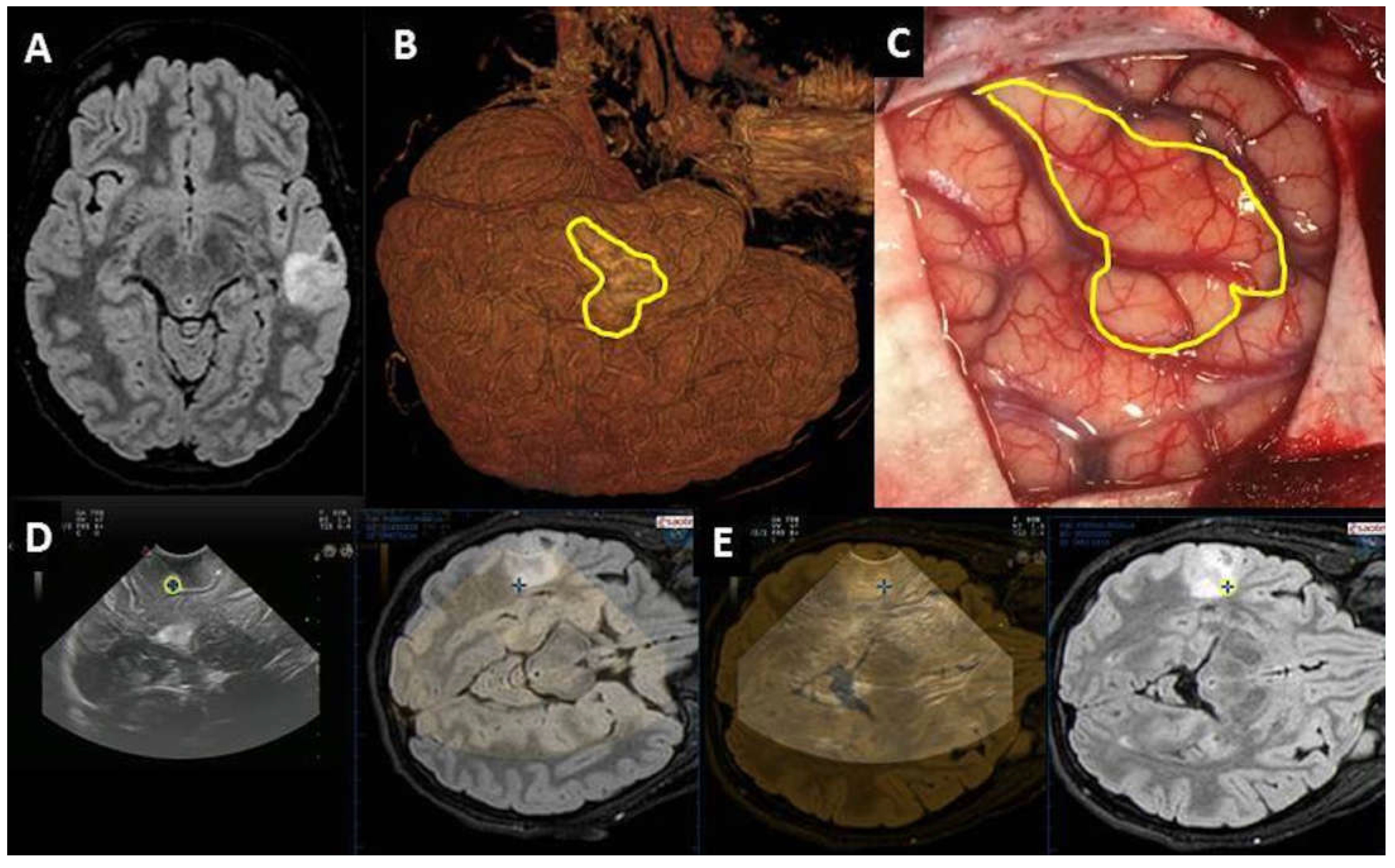




| Number of cases | 45 |
| M:F | 23:22 |
| Age | 3m–18 years (median 6.4 years) |
| Pathology | 16 LG gliomas 12 HG gliomas 8 gangliogliomas 7 DNTs 5 cavernomas 5 other (2 FCDs, 1 meningioma, 1 SEGA, 1 histiocytosis) |
| Size | 24 (<2 cm) 21 (>2 cm) |
| Site | 15 temporal > 8 frontal > 6 parietal > 5 intraventricular > 4 occipital–4 diencephalic > 2 thalamic > 1 insular |
| Pre-IOUS | Problems | Solutions |
|---|---|---|
| Localization | Deep-seated lesion |
|
| Localization | Superficial lesion not distinguishable from brain parenchyma |
|
| Definition | Vascular relationship |
|
| Definition | Vascular pattern |
|
| Surgical route | Deep-seated lesion |
|
| Post-IOUS | ||
| EOR | Collapsed surgical cavity |
|
| EOR | Open ventricle |
|
| EOR | Artifacts |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frassanito, P.; Stifano, V.; Bianchi, F.; Tamburrini, G.; Massimi, L. Enhancing the Reliability of Intraoperative Ultrasound in Pediatric Space-Occupying Brain Lesions. Diagnostics 2023, 13, 971. https://doi.org/10.3390/diagnostics13050971
Frassanito P, Stifano V, Bianchi F, Tamburrini G, Massimi L. Enhancing the Reliability of Intraoperative Ultrasound in Pediatric Space-Occupying Brain Lesions. Diagnostics. 2023; 13(5):971. https://doi.org/10.3390/diagnostics13050971
Chicago/Turabian StyleFrassanito, Paolo, Vito Stifano, Federico Bianchi, Gianpiero Tamburrini, and Luca Massimi. 2023. "Enhancing the Reliability of Intraoperative Ultrasound in Pediatric Space-Occupying Brain Lesions" Diagnostics 13, no. 5: 971. https://doi.org/10.3390/diagnostics13050971
APA StyleFrassanito, P., Stifano, V., Bianchi, F., Tamburrini, G., & Massimi, L. (2023). Enhancing the Reliability of Intraoperative Ultrasound in Pediatric Space-Occupying Brain Lesions. Diagnostics, 13(5), 971. https://doi.org/10.3390/diagnostics13050971







