Diagnostic Approaches to Vascular Injury in Polytrauma—A Literature Review
Abstract
:1. Introduction
2. Clinical Diagnosis of Vascular Injury
3. Diagnostic Tools
3.1. Ankle Brachial Index (ABI)
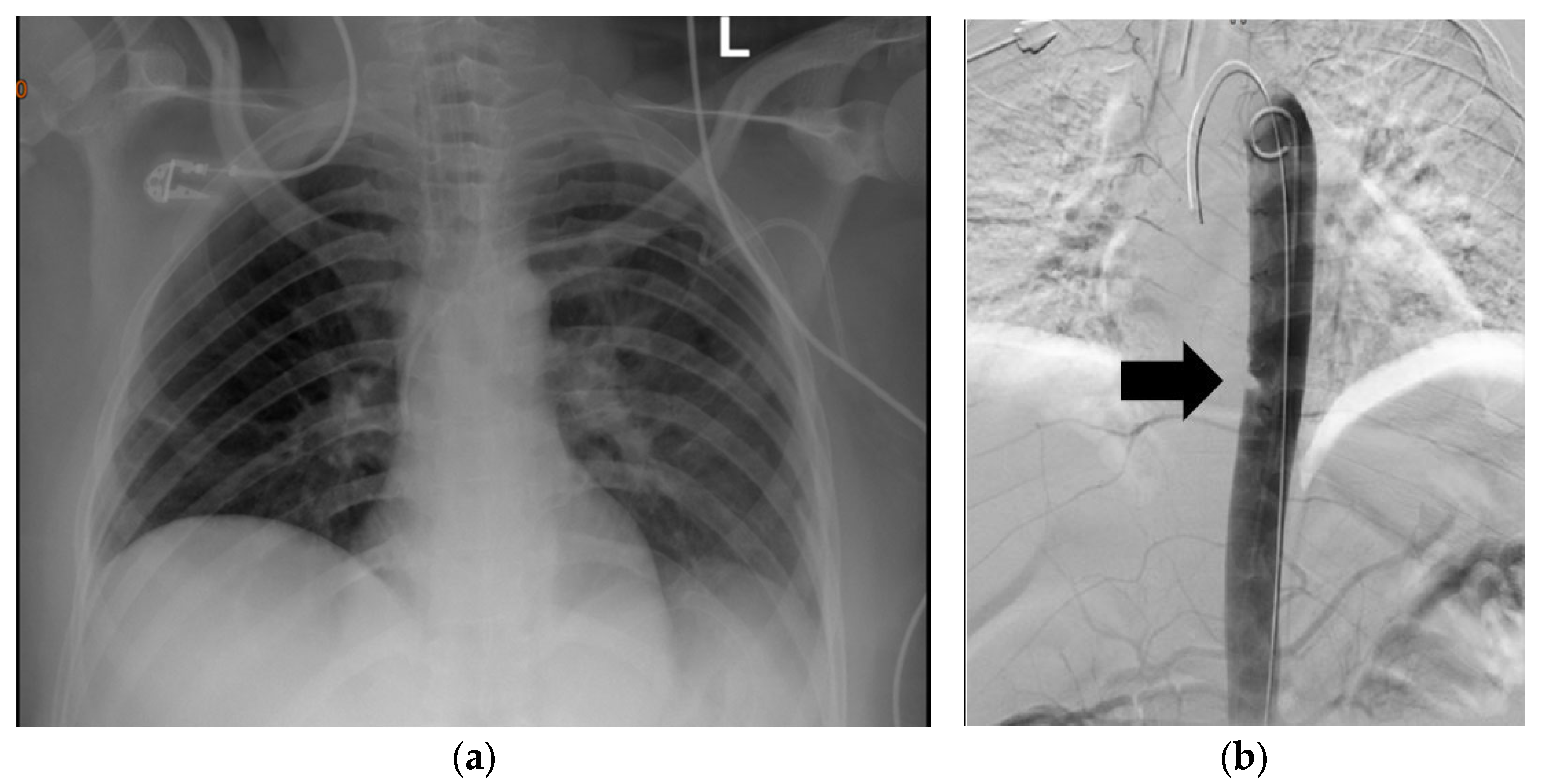
3.2. Plain Chest Radiography
3.3. Extended Focused Assessment with Sonar in Trauma (eFAST)
3.4. Duplex Sonography (DUS)
3.5. Echocardiography
3.6. Computed Tomography Angiography (CTA)
3.7. Magnetic Resonance Imaging/Angiography (MRA)
3.8. Digital Subtraction Angiography (DSA)
3.9. Intra-Vascular Ultrasound (IVUS)
4. Approach to and Use of on-Table Imaging
5. Differences in Approach to Blunt vs. Penetrating Injuries
5.1. Neck Trauma and Chest Trauma
5.2. Abdominal Trauma
Extremity Trauma
6. Haemodynamically Stable Patient vs. Untable Patient
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moodley, N.B.; Aldous, C.; Clarke, D. An audit of trauma-related mortality in a provincial capital in South Africa. South Afr. J. Surg. 2014, 52, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Starnes, B.W.; Arthurs, Z.M. Endovascular management of vascular trauma. Perspect. Vasc. Surg. Endovasc. Ther. 2006, 18, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Muckart, D.; Pillay, B.; Hardcastle, T.; Skinner, D. Vascular injuries following blunt polytrauma. Eur. J. Trauma Emerg. Surg. 2014, 40, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, W.; Wang, A.; Meng, H.; Wang, Y.; Liu, S.; Li, R.; Lu, S.; Peng, J. Diagnosis and treatment of traumatic vascular injury of limbs in military and emergency medicine: A systematic review. Medicine 2019, 98, e15406. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, R.S.; Van Haren, R.M.; Yokobori, S.; Cohen, D.; Beckerman, S.R.; Ahmad, F.; Bullock, M.R. Management of simultaneous traumatic brain injury and aortic injury. J. Neurosurg. 2013, 119, 324–331. [Google Scholar] [CrossRef]
- Liu, J.-L.; Li, J.-Y.; Jiang, P.; Jia, W.; Tian, X.; Cheng, Z.-Y.; Zhang, Y.-X. Literature review of peripheral vascular trauma: Is the era of intervention coming? Chin. J. Traumatol. 2020, 23, 5–9. [Google Scholar] [CrossRef]
- Gopireddy, D.R.; Kee-Sampson, J.W.; Vulasala, S.S.R.; Stein, R.; Kumar, S.; Virarkar, M. Imaging of penetrating vascular trauma of the body and extremities secondary to ballistic and stab wounds. J. Clin. Imaging Sci. 2023, 13, 1. [Google Scholar] [CrossRef]
- Wani, M.L.; Sheikh, M.T.; Irshad, I.; Ahangar, A.G.; Ganie, F.A.; Sheikh, M.T.; Wani, S.N. Evaluating peripheral vascular injuries: Is color Doppler enough for diagnosis? Int. Cardiovasc. Res. J. 2014, 8, 15. [Google Scholar]
- Held, M.; Laubscher, M.; Von Bormann, R.; Walters, J.; Roche, S.; Banderker, A.; Navsaria, P.; Nicol, A.; Maqungo, S. High rate of popliteal artery injuries and limb loss in 96 knee dislocations. SA Orthop. J. 2016, 15, 72–76. [Google Scholar] [CrossRef]
- Hemingway, J.; Adjei, E.; Desikan, S.; Gross, J.; Tran, N.; Singh, N.; Starnes, B.; Quiroga, E. Re-evaluating the safety and effectiveness of the 0.9 ankle-brachial index threshold in penetrating lower extremity trauma. J. Vasc. Surg. 2020, 72, 1305–1311.e1. [Google Scholar] [CrossRef]
- Andring, N.; Olszewski, C.; Strickland, L.; Beck, E.C.; Bang, K.; Pilson, H.; Carroll, E.; Halvorson, J. No false elevation in ankle brachial index in patients with tibial plateau fractures and vascular risk factors. J. Orthop. 2022, 30, 115–119. [Google Scholar] [CrossRef]
- Mouawad, N.J.; Paulisin, J.; Hofmeister, S.; Thomas, M.B. Blunt thoracic aortic injury–concepts and management. J. Cardiothorac. Surg. 2020, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Spering, C.; Brauns, S.D.; Lefering, R.; Bouillon, B.; Dobroniak, C.C.; Füzesi, L.; Seitz, M.T.; Jaeckle, K.; Dresing, K.; Lehmann, W.; et al. Diagnostic value of chest radiography in the early management of severely injured patients with mediastinal vascular injury. Eur. J. Trauma Emerg. Surg. 2022, 48, 4223–4231. [Google Scholar] [CrossRef]
- Dyer, D.S.; Moore, E.E.; Ilke, D.N.; McIntyre, R.C.; Bernstein, S.M.; Durham, J.D.; Mestek, M.F.; Heinig, M.J.; Russ, P.D.; Symonds, D.L.; et al. Thoracic aortic injury: How predictive is mechanism and is chest computed tomography a reliable screening tool? A prospective study of 1,561 patients. J. Trauma Acute Care Surg. 2000, 48, 673–683. [Google Scholar] [CrossRef]
- Topcu, A.C.; Ozeren-Topcu, K.; Bolukcu, A.; Sahin, S.; Seyhan, A.U.; Kayacioglu, I. Blunt traumatic aortic injury: 10-year single-center experience. AORTA 2020, 8, 163–168. [Google Scholar] [CrossRef]
- Naidoo, S.; Hardcastle, T.C. Traumatic injury to the great vessels of the chest. Mediastinum 2021, 5, 26. [Google Scholar] [CrossRef]
- Rippey, J.C.; Royse, A.G. Ultrasound in trauma. Best Pract. Res. Clin. Anaesthesiol. 2009, 23, 343–362. [Google Scholar] [CrossRef]
- Bloom, B.A.; Gibbons, R.C. Focused Assessment with Sonography for Trauma. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Mowery, N.T.; Gunter, O.L.; Collier, B.R.; Diaz, J., Jr.; Haut, E.; Hildreth, A.; Holevar, M.; Mayberry, J.; Streib, E. Practice management guidelines for management of hemothorax and occult pneumothorax. J. Trauma Acute Care Surg. 2011, 70, 510–518. [Google Scholar] [CrossRef] [Green Version]
- Dawson, D.L. Imaging for the evaluation and treatment of vascular trauma. In Rich’s Vascular Trauma; Elsevier: Amsterdam, The Netherlands, 2016; pp. 44–55. [Google Scholar]
- Azizzadeh, A.; Keyhani, K.; Miller, C.C., III; Coogan, S.M.; Safi, H.J.; Estrera, A.L. Blunt traumatic aortic injury: Initial experience with endovascular repair. J. Vasc. Surg. 2009, 49, 1403–1408. [Google Scholar] [CrossRef] [Green Version]
- Vignon, P.; Boncoeur, M.-P.; François, B.; Rambaud, G.; Maubon, A.; Gastinne, H. Comparison of multiplane transesophageal echocardiography and contrast-enhanced helical CT in the diagnosis of blunt traumatic cardiovascular injuries. J. Am. Soc. Anesthesiol. 2001, 94, 615–622. [Google Scholar] [CrossRef]
- Girón-Arango, L.; d’Empaire, P.P. Is There a Role for Transesophageal Echocardiography in the Perioperative Trauma Patient? Curr. Anesthesiol. Rep. 2022, 12, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, L.; Coimbra, R.; Goes, A.M., Jr.; Reva, V.; Santorelli, J.; Moore, E.E.; Galante, J.; Abu-Zidan, F.; Peitzman, A.B.; Ordonez, C.; et al. American Association for the Surgery of Trauma–World Society of Emergency Surgery guidelines on diagnosis and management of peripheral vascular injuries. J. Trauma Acute Care Surg. 2020, 89, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Van Waes, O.J.; Navsaria, P.H.; Verschuren, R.C.; Vroon, L.C.; Van Lieshout, E.M.; Halm, J.A.; Nicol, A.J.; Vermeulen, J. Management of penetrating injuries of the upper extremities. Ulus. Travma Ve Acil Cerrahi Derg. 2013, 19, 405–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chillo, P.; Kisenge, P. Magnitude and associated factors of contrast induced nephropathy among patients undergoing coronary angiography and interventions at a cardiac referral hospital in Tanzania-a cross-sectional study. Pan Afr. Med. J. 2021, 38, 311. [Google Scholar] [CrossRef] [PubMed]
- Giles, T.; Weaver, N.; Varghese, A.; Way, T.L.; Abel, C.; Choi, P.; Briggs, G.D.; Balogh, Z.J. Acute kidney injury development in polytrauma and the safety of early repeated contrast studies: A retrospective cohort study. J. Trauma Acute Care Surg. 2022, 93, 872–881. [Google Scholar] [CrossRef]
- Hinson, J.S.; Ehmann, M.R.; Fine, D.M.; Fishman, E.K.; Toerper, M.F.; Rothman, R.E.; Klein, E.Y. Risk of acute kidney injury after intravenous contrast media administration. Ann. Emerg. Med. 2017, 69, 577–586.e4. [Google Scholar] [CrossRef]
- Aycock, R.D.; Westafer, L.M.; Boxen, J.L.; Majlesi, N.; Schoenfeld, E.M.; Bannuru, R.R. Acute kidney injury after computed tomography: A meta-analysis. Ann. Emerg. Med. 2018, 71, 44–53.e4. [Google Scholar] [CrossRef]
- Patterson, B.; Holt, P.; Cleanthis, M.; Tai, N.; Carrell, T.; Loosemore, T. Imaging vascular trauma. J. Br. Surg. 2012, 99, 494–505. [Google Scholar] [CrossRef]
- Abu Mughli, R.; Wu, T.; Li, J.; Moghimi, S.; Alem, Z.; Nasir, M.U.; Abdellatif, W.; Nicolaou, S. An update in imaging of blunt vascular neck injury. Can. Assoc. Radiol. J. 2020, 71, 281–292. [Google Scholar] [CrossRef] [Green Version]
- Kassem, A.M.M.; Fathy, A.M.; Alnekidy, A.A.M.; Morsy, M.A.; El Shafei, M.M. The role of combined computed tomographic angiography and digital subtraction angiography in the management of cervico-facial vascular lesions. Egypt. J. Radiol. Nucl. Med. 2021, 52, 88. [Google Scholar] [CrossRef]
- Scholtz, P.; Beningfield, S.; Candy, S. A retrospective study of computed tomography angiography versus digital subtraction angiography in penetrating neck trauma at Groote Schuur Hospital, South Africa. SA J. Radiol. 2014, 18, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ares, W.J.; Jankowitz, B.T.; Tonetti, D.A.; Gross, B.A.; Grandhi, R. A comparison of digital subtraction angiography and computed tomography angiography for the diagnosis of penetrating cerebrovascular injury. Neurosurg. Focus 2019, 47, E16. [Google Scholar] [CrossRef] [Green Version]
- Etheridge, J.C.; Ahanchi, S.S.; Dexter, D.J.; Cain, B.C.; Collins, J.N.; Panneton, J.M. The impact of intravascular ultrasound on outcomes of endovascular repair of blunt traumatic aortic injury. Trauma 2019, 21, 208–214. [Google Scholar] [CrossRef]
- Gross, T.; Messmer, P.; Amsler, F.; Füglistaler-Montali, I.; Zürcher, M.; Hügli, R.W.; Regazzoni, P.; Jacob, A.L. Impact of a multifunctional image-guided therapy suite on emergency multiple trauma care. J. Br. Surg. 2010, 97, 118–127. [Google Scholar] [CrossRef]
- Thippeswamy, P.B.; Rajasekaran, R.B. Imaging in polytrauma–Principles and current concepts. J. Clin. Orthop. Trauma 2021, 16, 106–113. [Google Scholar] [CrossRef]
- Miracle, A.C.; Uzelac, A. Imaging blunt and penetrating trauma to the neck: Clinical relevance and management. Appl. Radiol. 2016, 45, 14–19. [Google Scholar] [CrossRef]
- Mama, N.; Jemni, H.; Achour, N.A.; Sidiya, O.C.; Kadri, K.; Gaha, M.; Hasni, I.; Tlilli, K. Abdominal trauma imaging. In Abdominal Surgery; IntechOpen: London, UK, 2012. [Google Scholar]
- Radwan, M.M.; Abu-Zidan, F.M. Focussed Assessment Sonograph Trauma (FAST) and CT scan in blunt abdominal trauma: Surgeon’s perspective. Afr. Health Sci. 2006, 6, 187–190. [Google Scholar]
- Wiewióra, M.; Sosada, K.; Piecuch, J.; Żurawiński, W. The role of laparoscopy in abdominal trauma–review of the literature. Videosurgery Other Miniinvasive Tech. 2011, 6, 121–126. [Google Scholar] [CrossRef]
- Koto, M.Z.; Matsevych, O.Y.; Aldous, C. Diagnostic laparoscopy for trauma: How not to miss injuries. J. Laparoendosc. Adv. Surg. Tech. 2018, 28, 506–513. [Google Scholar] [CrossRef]
- Huber, G.H.; Manna, B. Vascular extremity trauma. In StatPearls [Internet] 2021; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Montorfano, M.A.; Pla, F.; Vera, L.; Cardillo, O.; Nigra, S.G.; Montorfano, L.M. Point-of-care ultrasound and Doppler ultrasound evaluation of vascular injuries in penetrating and blunt trauma. Crit. Ultrasound J. 2017, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- Walkoff, L.; Nagpal, P.; Khandelwal, A. Imaging primer for CT angiography in peripheral vascular trauma. Emerg. Radiol. 2021, 28, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.E.; Souza, J.M.; Eidt, J.F. Severe Lower Extremity Injury in The Adult Patient. Up-to-Date 2021. Available online: https://www.medilib.ir/uptodate/show/15150 (accessed on 1 March 2023).
- Velmahos, G.C.; Toutouzas, K.G.; Vassiliu, P.; Sarkisyan, G.; Chan, L.S.; Hanks, S.H.; Berne, T.V.; Demetriades, D. A prospective study on the safety and efficacy of angiographic embolization for pelvic and visceral injuries. J. Trauma Acute Care Surg. 2002, 53, 303–308. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntola, V.C.; Hardcastle, T.C. Diagnostic Approaches to Vascular Injury in Polytrauma—A Literature Review. Diagnostics 2023, 13, 1019. https://doi.org/10.3390/diagnostics13061019
Ntola VC, Hardcastle TC. Diagnostic Approaches to Vascular Injury in Polytrauma—A Literature Review. Diagnostics. 2023; 13(6):1019. https://doi.org/10.3390/diagnostics13061019
Chicago/Turabian StyleNtola, Vuyolwethu C., and Timothy C. Hardcastle. 2023. "Diagnostic Approaches to Vascular Injury in Polytrauma—A Literature Review" Diagnostics 13, no. 6: 1019. https://doi.org/10.3390/diagnostics13061019





