PET Imaging of Neuro-Inflammation with Tracers Targeting the Translocator Protein (TSPO), a Systematic Review: From Bench to Bedside
Abstract
:1. Introduction
2. Materials and Methods
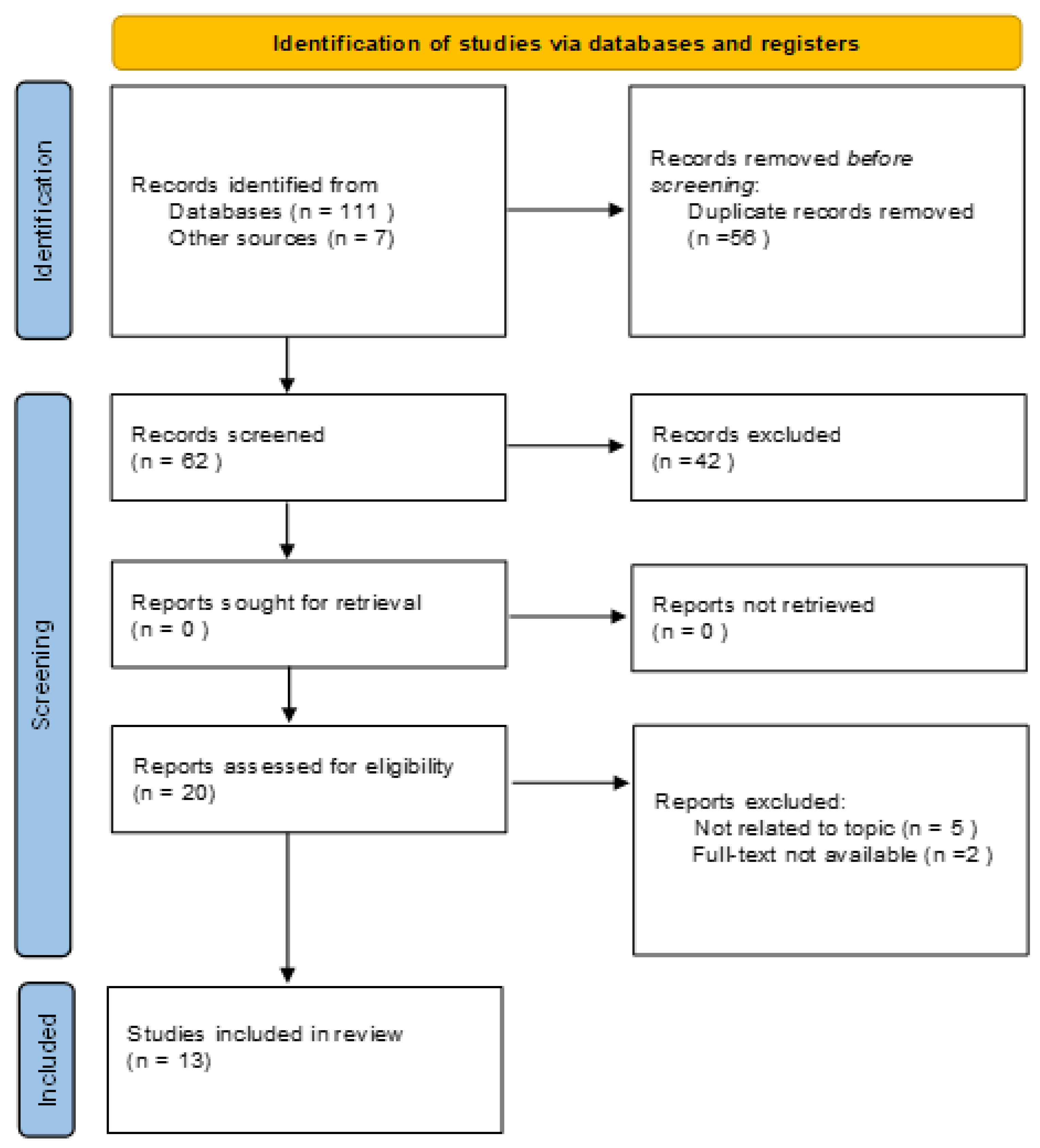
2.1. Search Strategy and Study Selection
2.2. Data Extraction and Methodological Quality Assessment
3. Results
3.1. Analysis of the Evidence
3.1.1. (R)-11C-PK11195
3.1.2. [18F]-FEPPA
3.1.3. 11C-PBR28
3.1.4. [11C]-DPA-713
3.1.5. [18F]-DPA-714
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Hu, K.; Shao, T.; Hou, L.; Zhang, S.; Ye, W.; Josephson, L.; Meyer, J.H.; Zhang, M.R.; Vasdev, N.; et al. Recent developments on PET radiotracers for TSPO and their applications in neuroimaging. Acta Pharm. Sin. B 2021, 11, 373–393. [Google Scholar] [CrossRef]
- Lee, Y.; Park, Y.; Nam, H.; Lee, J.W.; Yu, S.W. Translocator protein (TSPO): The new story of the old protein in neuroinflammation. BMB Rep. 2020, 53, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Werry, E.L.; Bright, F.M.; Piguet, O.; Ittner, L.M.; Halliday, G.M.; Hodges, J.R.; Kiernan, M.C.; Loy, C.T.; Kril, J.J.; Kassiou, M. Recent Developments in TSPO PET Imaging as A Biomarker of Neuroinflammation in Neurodegenerative Disorders. Int. J. Mol. Sci. 2019, 20, 3161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrazzano, G.; Frantellizzi, V.; De Bartolo, M.I.; De Feo, M.S.; Conte, A.; Fabbrini, G.; De Vincentis, G.; Berardelli, A. Isolated head tremor: A DAT-SPECT and somatosensory temporal discrimination study. Park. Relat. Disord. 2020, 81, 56–59. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Lavelli, V.; Ferrari, C.; Sardaro, A.; Farcomeni, A.; Pacilio, M.; Borrazzo, C.; Pani, R.; Rubini, G.; Vincentis, G. Diagnostic Value of the Early Heart-to-Mediastinum Count Ratio in Cardiac 123I-mIBG Imaging for Parkinson’s Disease. Curr. Radiopharm. 2021, 14, 64–69. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef] [Green Version]
- Frantellizzi, V.; Pani, A.; Ricci, M.; Locuratolo, N.; Fattapposta, F.; De Vincentis, G. Neuroimaging in Vascular Cognitive Impairment and Dementia: A Systematic Review. J. Alzheimers Dis. 2020, 73, 1279–1294. [Google Scholar] [CrossRef] [PubMed]
- Frantellizzi, V.; Conte, M.; De Vincentis, G. Hybrid Imaging of Vascular Cognitive Impairment. Semin. Nucl. Med. 2021, 51, 286–295. [Google Scholar] [CrossRef]
- Filippi, L.; Schillaci, O.; Palumbo, B. Neuroimaging with PET/CT in chronic traumatic encephalopathy: What nuclear medicine can do to move the field forward. Expert Rev. Mol. Diagn. 2022, 22, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.R.; Guo, Q.; Kalk, N.J.; Colasanti, A.; Kalogiannopoulou, D.; Dimber, R.; Lewis, Y.L.; Libri, V.; Barletta, J.; Ramada-Magalhaes, J.; et al. Determination of [(11)C]PBR28 binding potential in vivo: A first human TSPO blocking study. J. Cereb. Blood Flow Metab. 2014, 34, 989–994. [Google Scholar] [CrossRef] [Green Version]
- Ouchi, Y.; Yoshikawa, E.; Sekine, Y.; Futatsubashi, M.; Kanno, T.; Ogusu, T.; Torizuka, T. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann. Neurol. 2005, 57, 168–175. [Google Scholar] [CrossRef]
- Kobylecki, C.; Counsell, S.J.; Cabanel, N.; Wächter, T.; Turkheimer, F.E.; Eggert, K.; Oertel, W.; Brooks, D.J.; Gerhard, A. Diffusion-weighted imaging and its relationship to microglial activation in parkinsonian syndromes. Park. Relat. Disord. 2013, 19, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Edison, P.; Ahmed, I.; Fan, Z.; Hinz, R.; Gelosa, G.; Ray Chaudhuri, K.; Walker, Z.; Turkheimer, F.E.; Brooks, D.J. Microglia, amyloid, and glucose metabolism in Parkinson’s disease with and without dementia. Neuropsychopharmacology 2013, 38, 938–949. [Google Scholar] [CrossRef]
- Iannaccone, S.; Cerami, C.; Alessio, M.; Garibotto, V.; Panzacchi, A.; Olivieri, S.; Gelsomino, G.; Moresco, R.M.; Perani, D. In vivo microglia activation in very early dementia with Lewy bodies, comparison with Parkinson’s disease. Park. Relat. Disord. 2013, 19, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Gerhard, A.; Pavese, N.; Hotton, G.; Turkheimer, F.; Es, M.; Hammers, A.; Eggert, K.; Oertel, W.; Banati, R.B.; Brooks, D.J. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis. 2006, 21, 404–412. [Google Scholar] [CrossRef]
- Koshimori, Y.; Ko, J.H.; Mizrahi, R.; Rusjan, P.; Mabrouk, R.; Jacobs, M.F.; Christopher, L.; Hamani, C.; Lang, A.E.; Wilson, A.A.; et al. Imaging Striatal Microglial Activation in Patients with Parkinson’s Disease. PLoS ONE 2015, 10, e0138721. [Google Scholar] [CrossRef]
- Ghadery, C.; Koshimori, Y.; Coakeley, S.; Harris, M.; Rusjan, P.; Kim, J.; Houle, S.; Strafella, A.P. Microglial activation in Parkinson’s disease using [(18)F]-FEPPA. J. Neuroinflamm. 2017, 14, 8. [Google Scholar] [CrossRef] [Green Version]
- Ghadery, C.; Koshimori, Y.; Christopher, L.; Kim, J.; Rusjan, P.; Lang, A.E.; Houle, S.; Strafella, A.P. The Interaction Between Neuroinflammation and β-Amyloid in Cognitive Decline in Parkinson’s Disease. Mol. Neurobiol. 2020, 57, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Jucaite, A.; Svenningsson, P.; Rinne, J.O.; Cselényi, Z.; Varnäs, K.; Johnström, P.; Amini, N.; Kirjavainen, A.; Helin, S.; Minkwitz, M.; et al. Effect of the myeloperoxidase inhibitor AZD3241 on microglia: A PET study in Parkinson’s disease. Brain 2015, 138, 2687–2700. [Google Scholar] [CrossRef] [Green Version]
- Varnäs, K.; Cselényi, Z.; Jucaite, A.; Halldin, C.; Svenningsson, P.; Farde, L.; Varrone, A. PET imaging of [(11)C]PBR28 in Parkinson’s disease patients does not indicate increased binding to TSPO despite reduced dopamine transporter binding. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 367–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jucaite, A.; Cselényi, Z.; Kreisl, W.C.; Rabiner, E.A.; Varrone, A.; Carson, R.E.; Rinne, J.O.; Savage, A.; Schou, M.; Johnström, P.; et al. Glia Imaging Differentiates Multiple System Atrophy from Parkinson’s Disease: A Positron Emission Tomography Study with [(11) C]PBR28 and Machine Learning Analysis. Mov. Disord. 2022, 37, 119–129. [Google Scholar] [CrossRef]
- Terada, T.; Yokokura, M.; Yoshikawa, E.; Futatsubashi, M.; Kono, S.; Konishi, T.; Miyajima, H.; Hashizume, T.; Ouchi, Y. Extrastriatal spreading of microglial activation in Parkinson’s disease: A positron emission tomography study. Ann. Nucl. Med. 2016, 30, 579–587. [Google Scholar] [CrossRef]
- Fang, Y.D.; McConathy, J.E.; Yacoubian, T.A.; Zhang, Y.; Kennedy, R.E.; Standaert, D.G. Image Quantification for TSPO PET with a Novel Image-Derived Input Function Method. Diagnostics 2022, 12, 1161. [Google Scholar] [CrossRef]
- Rinne, J.O.; Hublin, C.; Någren, K.; Helenius, H.; Partinen, M. Unchanged striatal dopamine transporter availability in narcolepsy: A PET study with [11C]-CFT. Acta Neurol. Scand. 2004, 109, 52–55. [Google Scholar] [CrossRef]
- Rabinovici, G.D.; Furst, A.J.; O’Neil, J.P.; Racine, C.A.; Mormino, E.C.; Baker, S.L.; Chetty, S.; Patel, P.; Pagliaro, T.A.; Klunk, W.E.; et al. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology 2007, 68, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Zanotti-Fregonara, P.; Zhang, Y.; Jenko, K.J.; Gladding, R.L.; Zoghbi, S.S.; Fujita, M.; Sbardella, G.; Castellano, S.; Taliani, S.; Martini, C.; et al. Synthesis and evaluation of translocator 18 kDa protein (TSPO) positron emission tomography (PET) radioligands with low binding sensitivity to human single nucleotide polymorphism rs6971. ACS Chem. Neurosci. 2014, 5, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Unterrainer, M.; Mahler, C.; Vomacka, L.; Lindner, S.; Havla, J.; Brendel, M.; Böning, G.; Ertl-Wagner, B.; Kümpfel, T.; Milenkovic, V.M.; et al. TSPO PET with [(18)F]GE-180 sensitively detects focal neuroinflammation in patients with relapsing-remitting multiple sclerosis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
| Author | Year of Publication | Country | Tracer | Population | Characteristics |
|---|---|---|---|---|---|
| Ouchi et al. [11] | 2005 | Japan | 11 C-(R)PK11195 | 20 patients | 10 Parkinson’s Disease 10 Healthy subjects |
| Kobylecky et al. [12] | 2013 | UK | 11 C-(R)PK11195 | 20 patients | 11 Atypical Parkinsonian syndromes 9 Parkinson’s Disease |
| Edison et al. [13] | 2012 | UK | 11 C-(R)PK11195; 11 C-PIB; 18 F-FDG | 19 patients | 19 Parkinson’s Disease |
| Iannaccone et al. [14] | 2012 | Italy | 11 C-(R)PK11195 | 12 patients | 6 Parkinson’s Disease 6 Dementia with Levy Bodies |
| Gerhard et al. [15] | 2006 | UK | 11 C-(R)PK11195 | 29 patients | 18 Parkinson’s Disease 11 Healthy subjects |
| Koshimori et al. [16] | 2015 | Canada | 18 F-FEPPA | 36 patients | 19 Parkinson’s Disease 17 Healthy Subjects |
| Ghadery et al. [17] | 2017 | Canada | 18 F-FEPPA | 52 patients | 30 Parkinson’s Disease 22 Healthy subjects |
| Ghadery et al. [18] | 2020 | Canada | 18 F-FEPPA; 11 C-PIB | 41 patients | 29 Parkinson’s Disease 12 Healthy subjects |
| Jucaite et al. [19] | 2015 | Sweden; Finland | 11 C-PBR28; 18 F-FE-PE21 | 29 patients | 29 Parkinson’s Disease |
| Varnas et al. [20] | 2019 | Sweden | 11 C-PBR28 | 32 patients | 16 Parkinson’s Disease 16 Healthy subjects |
| Jucaite et al. [21] | 2022 | Sweden | 11 C-PBR28 | 90 patients | 66 Multiple System Atrophy 24 Parkinson’s Disease |
| Tarada et al. [22] | 2016 | Japan | 11 C-DPA713 | 11 patients | 11 Parkinson’s Disease |
| Fang et al. [23] | 2022 | USA | 18 F-DPA714 | 5 patients | 3 Parkinson’s Disease 2 Healthy subjects |
| 1. Was There a Clear Question for the Study to Address? | 2. Was There a Comparison with an Appropriate Reference Standard? | 3. Did All Patients Get the Diagnostic Test and Reference Standard? | 4. Could the Results of the Test Have Been Influenced by the Results of the Reference Standard? | 5. Is the Disease Status of the Tested Population Clearly Described? | 6. Were the Methods for Performing the Test Described in Sufficient Detail? | 7. What Are the Results? | 8. How Sure Are We about the Results? Consequences and Cost of Alternatives Performed? | 9. Can the Results Be Applied to Your Patients/the Population of Interest? | 10. Can the Test Be Applied to Your Patient or Population of Interest? | 11. Were All Outcomes Important to the Individual or Population Considered? | 12. What Would Be the Impact of Using This Test on Your Patients/Population? | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ouchi et al. 2005 [11] | ☺ | ☺ | ☺ | ? | ☺ | ☺ | ☺ | ? | ☺ | ☺ | ☺ | It may show patterns of neuroinflammation in patients with PD. |
| Kobilecky et al. 2013 [12] | ☺ | ☺ | ☺ | ? | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | It may correlate diffusion-weighted images and PET images in patients with PD. |
| Edison et al. 2012 [13] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | Comparison of different radiotracers in PD. |
| Iannaccone et al. 2012 [14] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ? | ☺ | ☺ | ☺ | Comparison of neuroinflammation patterns in PD and DLB. |
| Gerhard et al. 2006 [15] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | It may explain the self-maintaining process of neuroinflammation over time. |
| Koshimori et al. 2015 [16] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ? | ? | ? | ☹ | Differentiates binding potential between HABs and MABs. |
| Ghadery et al. 2017 [17] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ? | ? | ? | ☺ | Differentiates binding potential between HABs and MABs and correlate the binding potential with the severity of the disease. |
| Ghadery et al. 2020 [18] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ? | ? | ☺ | Studies the relationship between brain β-amyloid and neuroinflammation in patients affected by PD according to their disease severity. |
| Jucaite et al. 2015 [19] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | Investigates the effects of MPO inhibition on neuroinflammation |
| Varnas et al. 2019 [20] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☹ | ☺ | ☺ | ☹ | Correlates DAT and TSPO expression in patients with PD. |
| Jucaite et al. 2022 [21] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | May differentiate between MSA and PD. |
| Tarada et al. 2016 [22] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | It may explain patterns of neuroinflammation in early-stage disease. |
| Fang et al. 2022 [23] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ? | ? | ? | ? | May provide an image-derived input function method for 18F-DPA-714. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corica, F.; De Feo, M.S.; Gorica, J.; Sidrak, M.M.A.; Conte, M.; Filippi, L.; Schillaci, O.; De Vincentis, G.; Frantellizzi, V. PET Imaging of Neuro-Inflammation with Tracers Targeting the Translocator Protein (TSPO), a Systematic Review: From Bench to Bedside. Diagnostics 2023, 13, 1029. https://doi.org/10.3390/diagnostics13061029
Corica F, De Feo MS, Gorica J, Sidrak MMA, Conte M, Filippi L, Schillaci O, De Vincentis G, Frantellizzi V. PET Imaging of Neuro-Inflammation with Tracers Targeting the Translocator Protein (TSPO), a Systematic Review: From Bench to Bedside. Diagnostics. 2023; 13(6):1029. https://doi.org/10.3390/diagnostics13061029
Chicago/Turabian StyleCorica, Ferdinando, Maria Silvia De Feo, Joana Gorica, Marko Magdi Abdou Sidrak, Miriam Conte, Luca Filippi, Orazio Schillaci, Giuseppe De Vincentis, and Viviana Frantellizzi. 2023. "PET Imaging of Neuro-Inflammation with Tracers Targeting the Translocator Protein (TSPO), a Systematic Review: From Bench to Bedside" Diagnostics 13, no. 6: 1029. https://doi.org/10.3390/diagnostics13061029
APA StyleCorica, F., De Feo, M. S., Gorica, J., Sidrak, M. M. A., Conte, M., Filippi, L., Schillaci, O., De Vincentis, G., & Frantellizzi, V. (2023). PET Imaging of Neuro-Inflammation with Tracers Targeting the Translocator Protein (TSPO), a Systematic Review: From Bench to Bedside. Diagnostics, 13(6), 1029. https://doi.org/10.3390/diagnostics13061029







