Computed Tomography Bronchus Sign Subclassification during Radial Endobronchial Ultrasound-Guided Transbronchial Biopsy: A Retrospective Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
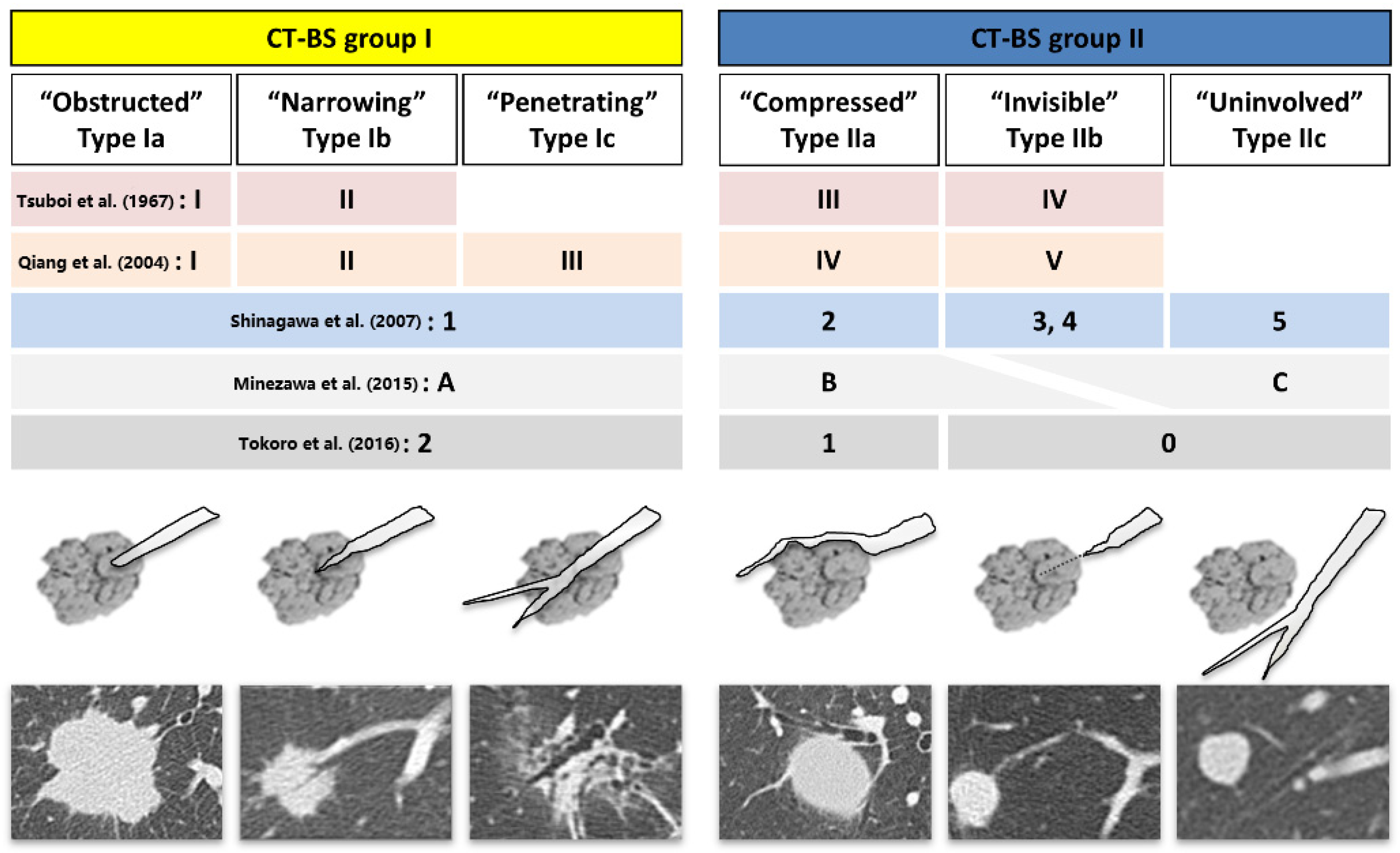
2.2. Bronchus Type Classification
2.3. Procedures
2.4. Diagnosis
2.5. Data Collection and Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [Green Version]
- Rivera, M.P.; Mehta, A.C.; Wahidi, M.M. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, E142S–E165S. [Google Scholar] [CrossRef]
- Kurimoto, N.; Miyazawa, T.; Okimasa, S.; Maeda, A.; Oiwa, H.; Miyazu, Y.; Murayama, M. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004, 126, 959–965. [Google Scholar] [CrossRef] [Green Version]
- Ishida, T.; Asano, F.; Yamazaki, K.; Shinagawa, N.; Oizumi, S.; Moriya, H.; Munakata, M.; Nishimura, M. Virtual Navigation in Japan Trial Group. Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions: A randomised trial. Thorax 2011, 66, 1072–1077. [Google Scholar] [CrossRef] [Green Version]
- Eberhardt, R.; Anantham, D.; Ernst, A.; Feller-Kopman, D.; Herth, F. Multimodality bronchoscopic diagnosis of peripheral lung lesions: A randomized controlled trial. Am. J. Respir. Crit. Care Med. 2007, 176, 36–41. [Google Scholar] [CrossRef]
- Oki, M.; Saka, H.; Ando, M.; Asano, F.; Kurimoto, N.; Morita, K.; Kitagawa, C.; Kogure, Y.; Miyazawa, T. Ultrathin bronchoscopy with multimodal devices for peripheral pulmonary lesions. A randomized trial. Am. J. Respir. Crit. Care Med. 2015, 192, 468–476. [Google Scholar] [CrossRef] [Green Version]
- Ost, D.E.; Ernst, A.; Lei, X.; Kovitz, K.L.; Benzaquen, S.; Diaz-Mendoza, J.; Greenhill, S.; Toth, J.; Feller-Kopman, D.; Puchalski, J.; et al. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. Results of the AQuIRE Registry. Am. J. Respir. Crit. Care Med. 2016, 193, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Oki, M.; Saka, H.; Imabayashi, T.; Himeji, D.; Nishii, Y.; Nakashima, H.; Minami, D.; Okachi, S.; Mizumori, Y.; Ando, M. Guide sheath versus non-guide sheath method for endobronchial ultrasound-guided biopsy of peripheral pulmonary lesions: A multicentre randomised trial. Eur. Respir. J. 2022, 59, 2101678. [Google Scholar] [CrossRef]
- Tay, J.H.; Irving, L.; Antippa, P.; Steinfort, D.P. Radial probe endobronchial ultrasound: Factors influencing visualization yield of peripheral pulmonary lesions. Respirology 2013, 18, 185–190. [Google Scholar] [CrossRef]
- Shinagawa, N.; Yamazaki, K.; Onodera, Y.; Asahina, H.; Kikuchi, E.; Asano, F.; Miyasaka, K.; Nishimura, M. Factors related to diagnostic sensitivity using an ultrathin bronchoscope under CT guidance. Chest 2007, 131, 549–553. [Google Scholar] [CrossRef]
- Nakai, T.; Matsumoto, Y.; Suzuk, F.; Tsuchida, T.; Izumo, T. Predictive factors for a successful diagnostic bronchoscopy of ground-glass nodules. Ann. Thorac. Med. 2017, 12, 171–176. [Google Scholar] [CrossRef]
- Seijo, L.M.; de Torres, J.P.; Lozano, M.D.; Bastarrika, G.; Alcaide, A.B.; Lacunza, M.M.; Zulueta, J.J. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a bronchus sign on CT imaging: Results from a prospective study. Chest 2010, 138, 1316–1321. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, Y.; Nakai, T.; Tanaka, M.; Imabayashi, T.; Tsuchida, T.; Ohe, Y. Diagnostic outcomes and safety of cryobiopsy added to conventional sampling methods: An observational study. Chest 2021, 160, 1890–1901. [Google Scholar] [CrossRef]
- Guvenc, C.; Yserbyt, J.; Testelmans, D.; Zanca, F.; Carbonez, A.; Ninane, V.; De Wever, W.; Dooms, C. Computed tomography characteristics predictive for radial EBUS-miniprobe-guided diagnosis of pulmonary lesions. J. Thorac. Oncol. 2015, 10, 472–478. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.S.; Sethi, J.; Taneja, A.; Musani, A.; Maldonado, F. Computed tomography bronchus sign and the diagnostic yield of guided bronchoscopy for peripheral pulmonary lesions. A systematic review and meta-analysis. Ann. Am. Thorac. Soc. 2018, 15, 978–987. [Google Scholar] [CrossRef]
- Bellinger, C.; Poon, R.; Dotson, T.; Sharma, D. Lesion characteristics affecting yield of electromagnetic navigational bronchoscopy. Respir. Med. 2021, 180, 106357. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, H.J.; Song, M.J.; Kwon, B.S.; Lim, S.Y.; Lee, Y.J.; Park, J.S.; Cho, Y.J.; Yoon, H.I.; Lee, J.H.; et al. Utility and safety of sole electromagnetic navigation bronchoscopy under moderate sedation for lung cancer diagnosis. Transl. Lung Cancer Res. 2022, 11, 462–471. [Google Scholar] [CrossRef]
- Tsuboi, E.; Ikeda, S.; Tajima, M.; Shimosato, Y.; Ishikawa, S. Transbronchial biopsy smear for diagnosis of peripheral pulmonary carcinomas. Cancer 1967, 20, 687–698. [Google Scholar] [CrossRef]
- Gaeta, M.; Pandolfo, I.; Volta, S.; Russi, E.G.; Bartiromo, G.; Girone, G.; La Spada, F.; Barone, M.; Casablanca, G.; Minutoli, A. Bronchus sign on CT in peripheral carcinoma of the lung: Value in predicting results of transbronchial biopsy. AJR Am. J. Roentgenol. 1991, 157, 1181–1185. [Google Scholar] [CrossRef]
- Minezawa, T.; Okamura, T.; Yatsuya, H.; Yamamoto, N.; Morikawa, S.; Yamaguchi, T.; Morishita, M.; Niwa, Y.; Takeyama, T.; Mieno, Y.; et al. Bronchus sign on thin-section computed tomography is a powerful predictive factor for successful transbronchial biopsy using endobronchial ultrasound with a guide sheath for small peripheral lung lesions: A retrospective observational study. BMC Med. Imaging 2015, 15, 21. [Google Scholar] [CrossRef] [Green Version]
- Tokoro, Y.; Yasuo, M.; Kobayashi, T.; Hama, M.; Ichiyama, T.; Horiuchi, T.; Ushiki, A.; Kawakami, S.; Honda, T.; Hanaoka, M. Computed tomography-guided bronchoscopy in the diagnosis of small peripheral pulmonary lesions: A retrospective study of 240 examinations in a single academic center. Respir. Investig. 2016, 54, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.S.; Trick, W.; Mba, B.I.; Mohananey, D.; Sethi, J.; Musani, A.I. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: A systematic review and meta-analysis. Respirology 2017, 22, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Naidich, D.P.; Sussman, R.; Kutcher, W.L.; Aranda, C.P.; Garay, S.M.; Ettenger, N.A. Solitary pulmonary nodules. CT-bronchoscopic correlation. Chest 1988, 93, 595–598. [Google Scholar] [CrossRef] [Green Version]
- Bilaçeroğlu, S.; Kumcuoğlu, Z.; Alper, H.; Osma, E.; Cağirici, U.; Günel, O.; Bayol, U.; Celikten, E.; Perim, K.; Köse, T. CT bronchus sign-guided bronchoscopic multiple diagnostic procedures in carcinomatous solitary pulmonary nodules and masses. Respiration 1998, 65, 49–55. [Google Scholar] [CrossRef]
- Qiang, J.W.; Zhou, K.R.; Lu, G.; Wang, Q.; Ye, X.G.; Xu, S.T.; Tan, L.J. The relationship between solitary pulmonary nodules and bronchi: Multi-slice CT-pathological correlation. Clin. Radiol. 2004, 59, 1121–1127. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Matsumoto, Y.; Tanaka, M.; Imabayashi, T.; Uchimura, K.; Tsuchida, T. Diagnostic value of bronchoscopy for peripheral metastatic lung tumors. Cancers 2022, 14, 375. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Izumo, T.; Sasada, S.; Tsuchida, T.; Ohe, Y. Diagnostic utility of endobronchial ultrasound with a guide sheath under the computed tomography workstation (ziostation) for small peripheral pulmonary lesions. Clin. Respir. J. 2017, 11, 185–192. [Google Scholar] [CrossRef]
- Hsu, L.H.; Liu, C.C.; Ko, J.S.; Chen, C.C.; Feng, A.C. Safety of interventional bronchoscopy through complication review at a cancer center. Clin. Respir. J. 2016, 10, 359–367. [Google Scholar] [CrossRef]
- Hayama, M.; Izumo, T.; Matsumoto, Y.; Chavez, C.; Tsuchida, T.; Sasada, S. Complications with endobronchial ultrasound with a guide sheath for the diagnosis of peripheral pulmonary lesions. Respiration 2015, 90, 129–135. [Google Scholar] [CrossRef]
- Gaeta, M.; Caruso, R.; Blandino, A.; Bartiromo, G.; Scribano, E.; Pandolfo, I. Radiolucencies and cavitation in bronchioloalveolar carcinoma: CT-pathologic correlation. Eur. Radiol. 1999, 9, 55–59. [Google Scholar] [CrossRef]
- Chavez, C.; Sasada, S.; Izumo, T.; Nakamura, Y.; Tsuta, K.; Tsuchida, T. Image-guided bronchoscopy for histopathologic diagnosis of pure ground glass opacity: A case report. J. Thorac. Dis. 2014, 6, E81–E84. [Google Scholar] [CrossRef]
- Nasu, S.; Okamoto, N.; Suzuki, H.; Shiroyama, T.; Tanaka, A.; Samejima, Y.; Kanai, T.; Noda, Y.; Morita, S.; Morishita, N.; et al. Comparison of the utilities of cryobiopsy and forceps biopsy for peripheral lung cancer. Anticancer Res. 2019, 39, 5683–5688. [Google Scholar] [CrossRef]
- Kim, H.; Park, C.M.; Yoon, S.H.; Hwang, E.J.; Lee, J.H.; Ahn, S.Y.; Goo, J.M. Open bronchus sign on CT: A risk factor for hemoptysis after percutaneous transthoracic biopsy. Korean J. Radiol. 2018, 19, 880–887. [Google Scholar] [CrossRef]
- Hong, K.S.; Lee, K.H.; Chung, J.H.; Shin, K.C.; Jin, H.J.; Jang, J.G.; Ahn, J.H. Utility of radial probe endobronchial ultrasound guided transbronchial lung biopsy in bronchus sign negative peripheral pulmonary lesions. J. Korean Med. Sci. 2021, 36, e176. [Google Scholar] [CrossRef]
- Sun, J.; Criner, G.J.; Dibardino, D.; Li, S.; Nader, D.; Lam, B.; Kopas, L.; Wahidi, M.M.; Majid, A.; Marron, R.; et al. Efficacy and safety of virtual bronchoscopic navigation with fused fluoroscopy and vessel mapping for access of pulmonary lesions. Respirology 2022, 27, 357–365. [Google Scholar] [CrossRef]
- Ikezawa, Y.; Shinagawa, N.; Sukoh, N.; Morimoto, M.; Kikuchi, H.; Watanabe, M.; Nakano, K.; Oizumi, S.; Nishimura, M. Usefulness of endobronchial ultrasonography with a guide sheath and virtual bronchoscopic navigation for ground-glass opacity lesions. Ann. Thorac Surg. 2017, 103, 470–475. [Google Scholar] [CrossRef] [Green Version]
- Panchabhai, T.S.; Abdelrazek, H.; Bremner, R.M. Follow the vessel approach for navigational bronchoscopy: The “unseen” bronchus sign? J. Bronchol. Interv. Pulmonol. 2019, 26, e8–e11. [Google Scholar] [CrossRef]
- Ho, E.; Cho, R.J.; Keenan, J.C.; Murgu, S. The feasibility of using the “artery sign” for pre-procedural planning in navigational bronchoscopy for parenchymal pulmonary lesion sampling. Diagnostics 2022, 12, 3059. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Nishii, Y.; Iketani, A.; Esumi, S.; Esumi, M.; Furuhashi, K.; Nakamura, Y.; Suzuki, Y.; Ito, K.; Fujiwara, K.; et al. Comparison of the analytical performance of the Oncomine dx target test focusing on bronchoscopic biopsy forceps size in non-small cell lung cancer. Thorac. Cancer 2022, 13, 1449–1456. [Google Scholar] [CrossRef]
- Kalchiem-Dekel, O.; Connolly, J.G.; Lin, I.H.; Husta, B.C.; Adusumilli, P.S.; Beattie, J.A.; Buonocore, D.J.; Dycoco, J.; Fuentes, P.; Jones, D.R.; et al. Shape-sensing robotic-assisted bronchoscopy in the diagnosis of pulmonary parenchymal lesions. Chest. 2022, 161, 572–582. [Google Scholar] [CrossRef]
- Agrawal, A.; Ho, E.; Chaddha, U.; Demirkol, B.; Bhavani, S.V.; Hogarth, D.K.; Murgu, S. Factors Associated with Diagnostic Accuracy of Robotic Bronchoscopy with 12-Month Follow-Up. Ann. Thorax. Surg. 2022. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, M.A.; Schampaert, S.; de Groot, J.A.H.; Schirmer, C.C.; van der Bom, I. Cone-beam CT with augmented fluoroscopy combined with electromagnetic navigation bronchoscopy for biopsy of pulmonary nodules. J. Bronchol. Interv. Pulmonol. 2018, 25, 274–282. [Google Scholar] [CrossRef] [PubMed]



| CT-BS Group I | |||||
|---|---|---|---|---|---|
| All Types | Type Ia | Type Ib | Type Ic | p-Value | |
| Characteristics | N = 792 | N = 400 | N = 287 | N = 105 | |
| Sex | <0.001 | ||||
| Male | 462 (58.3) | 262 (65.5) | 152 (53.0) | 48 (45.7) | |
| Female | 330 (41.7) | 138 (34.5) | 135 (47.0) | 57 (54.3) | |
| Age, median, years (range) | 70 (17–90) | 70 (17–90) | 70 (35–89) | 69 (38–85) | 0.853 |
| Lesion size, mm | <0.001 | ||||
| Median (range) | 25.7 (7.0–100.2) | 25.8 (7.0–90.3) | 27.3 (9.5–100.2) | 20.0 (8.9–81.1) | |
| ≤20 | 244 (30.8) | 115 (28.7) | 73 (25.4) | 56 (53.3) | |
| >20 to ≤30 | 273 (34.5) | 143 (35.8) | 93 (32.4) | 37 (35.3) | |
| >30 | 275 (34.7) | 142 (35.5) | 121 (42.2) | 12 (11.4) | |
| Lobar location | 0.642 | ||||
| RUL/LUS | 393 (49.6) | 204 (51.0) | 135 (47.0) | 54 (51.4) | |
| RML/lingula | 94 (11.9) | 51 (12.8) | 32 (11.1) | 11 (10.5) | |
| RLL/LLL | 305 (38.5) | 145 (36.2) | 120 (41.8) | 40 (38.1) | |
| Lesion location from the hilum | 0.662 | ||||
| Inner | 202 (25.5) | 107 (26.8) | 68 (23.7) | 27 (25.7) | |
| Outer | 590 (74.5) | 293 (73.2) | 219 (76.3) | 78 (74.3) | |
| Locational relationship with pleura | <0.001 | ||||
| Apart from the pleura | 443 (55.9) | 202 (50.5) | 151 (52.6) | 90 (85.7) | |
| Abutting on the pleura | 349 (44.1) | 198 (49.5) | 136 (47.4) | 15 (14.3) | |
| Lesion appearance on CT | <0.001 | ||||
| Solid | 576 (72.7) | 383 (95.8) | 166 (57.8) | 27 (25.7) | |
| Ground-glass | 216 (27.3) | 17 (4.2) | 121 (42.2) | 78 (74.3) | |
| Visibility on fluoroscopy | <0.001 | ||||
| Visible | 609 (76.9) | 335 (83.8) | 212 (73.9) | 62 (59.0) | |
| Invisible | 183 (23.1) | 65 (16.2) | 75 (26.1) | 43 (41.0) | |
| Final diagnosis | 0.433 | ||||
| Malignant | 660 (83.3) | 332 (83.0) | 236 (82.2) | 92 (87.6) | |
| Benign | 132 (16.7) | 68 (17.0) | 51 (17.8) | 13 (12.4) | |
| CT-BS Group II | |||||
|---|---|---|---|---|---|
| All Types | Type IIa | Type IIb | Type IIc | p-Value | |
| Characteristics | N = 229 | N = 106 | N = 95 | N = 28 | |
| Sex | 0.340 | ||||
| Male | 147 (64.2) | 67 (63.2) | 65 (68.4) | 13 (46.4) | |
| Female | 82 (35.8) | 39 (36.8) | 30 (31.6) | 15 (53.6) | |
| Age, median, years (range) | 70 (21–91) | 70.5 (21–85) | 69 (29–91) | 72 (27–81) | 0.930 |
| Lesion size, mm | <0.001 | ||||
| Median (range) | 15.6 (6.2–98.1) | 18.8 (6.9–98.1) | 13.9 (6.2–42.0) | 13.0 (6.5–28.9) | |
| ≤20 | 160 (69.9) | 58 (54.7) | 78 (82.1) | 24 (85.7) | |
| >20 to ≤30 | 35 (15.3) | 20 (18.9) | 11 (11.6) | 4 (14.3) | |
| >30 | 34 (14.8) | 28 (26.4) | 6 (6.3) | 0 (0) | |
| Lobar location | 0.149 | ||||
| RUL/LUS | 110 (48.0) | 52 (49.1) | 48 (50.5) | 10 (35.7) | |
| RML/lingula | 33 (14.4) | 18 (17.0) | 8 (8.4) | 7 (25.0) | |
| RLL/LLL | 86 (37.6) | 36 (34.0) | 39 (41.1) | 11 (39.3) | |
| Lesion location from the hilum | <0.001 | ||||
| Inner | 88 (38.4) | 63 (59.4) | 14 (14.7) | 11 (39.3) | |
| Outer | 141 (61.6) | 43 (40.6) | 81 (85.3) | 17 (60.7) | |
| Locational relationship with pleura | <0.001 | ||||
| Apart from the pleura | 172 (70.2) | 83 (78.3) | 49 (51.6) | 24 (85.7) | |
| Abutting on the pleura | 73 (29.8) | 23 (21.7) | 46 (48.4) | 4 (14.3) | |
| Lesion appearance on CT | 0.007 | ||||
| Solid | 186 (81.2) | 95 (89.6) | 72 (75.8) | 19 (67.9) | |
| Ground-glass | 43 (18.8) | 11 (10.4) | 23 (24.2) | 9 (32.1) | |
| Visibility on fluoroscopy | 0.021 | ||||
| Visible | 133 (54.3) | 68 (64.2) | 51 (53.7) | 10 (35.7) | |
| Invisible | 112 (45.8) | 38 (35.8) | 44 (46.3) | 18 (64.2) | |
| Final diagnosis | 0.846 | ||||
| Malignant | 180 (78.6) | 85 (80.2) | 73 (76.8) | 22 (78.6) | |
| Benign | 49 (21.4) | 21 (19.8) | 22 (23.2) | 6 (21.4) | |
| CT-BS Group I | CT-BS Group II | |||||||
|---|---|---|---|---|---|---|---|---|
| Type Ia | Type Ib | Type Ic | p-Value | Type IIa | Type IIb | Type IIc | p-Value | |
| Characteristics | N = 400 | N = 287 | N = 105 | N = 106 | N = 95 | N = 28 | ||
| rEBUS finding | 0.082 | 0.003 | ||||||
| Within | 241 (60.2) | 177 (61.7) | 49 (46.7) | 25 (23.6) | 34 (35.8) | 7 (25.0) | ||
| Adjacent to | 135 (33.8) | 91 (31.7) | 43 (41.0) | 61 (57.5) | 32 (33.7) | 9 (32.1) | ||
| Invisible | 24 (6.0) | 19 (6.6) | 13 (12.4) | 20 (18.9) | 29 (30.5) | 12 (42.9) | ||
| GS | <0.001 | <0.001 | ||||||
| Thin GS | 179 (44.8) | 71 (24.7) | 14 (13.3) | 25 (23.6) | 44 (46.3) | 5 (17.9) | ||
| Thick GS | 130 (32.4) | 124 (43.2) | 35 (33.4) | 22 (20.7) | 6 (6.3) | 0 (0) | ||
| Non-GS | 91 (22.8) | 92 (32.1) | 56 (53.3) | 59 (55.7) | 45 (47.4) | 23 (82.1) | ||
| Forceps size | (N = 379) | (N = 285) | (N = 105) | <0.001 | (N = 93) | (N = 90) | (N = 24) | 0.004 |
| 1.9 mm | 220 (55.0) | 215 (74.9) | 91 (86.7) | 68 (64.2) | 47 (49.5) | 19 (67.9) | ||
| 1.5 mm | 179 (44.8) | 70 (24.4) | 14 (13.3) | 25 (23.6) | 43 (45.3) | 5 (17.9) | ||
| TBNA | 42 (10.5) | 34 (11.8) | 15 (14.3) | 0.541 | 53 (50.0) | 20 (21.1) | 13 (46.4) | <0.001 |
| Brushing | 326 (81.5) | 203 (70.7) | 48 (45.7) | <0.001 | 47 (44.3) | 49 (51.6) | 8 (28.6) | <0.001 |
| Bronchial lavage | 7 (1.8) | 8 (2.8) | 0 (0) | 0.192 | 2 (1.9) | 1 (1.1) | 0 (0) | 0.707 |
| Variables | Total N = 792 | Diagnostic Cases (%) n = 601 (75.9) | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |||
| Sex | ||||||
| Male | 462 | 359 (77.7) | 1.27 (0.91–1.76) | 0.157 | ||
| Female | 330 | 242 (73.3) | 1.00 | |||
| Age, years | ||||||
| >70 | 369 | 281 (76.2) | 1.03 (0.74–1.42) | 0.869 | ||
| ≤70 | 423 | 320 (75.7) | 1.00 | |||
| Lesion size, mm | ||||||
| >20 | 548 | 453 (82.7) | 3.09 (2.20–4.34) | <0.001 | 1.86 (1.27–2.72) | 0.001 |
| ≤20 | 244 | 148 (60.7) | 1.00 | 1.00 | ||
| Lobar location | ||||||
| RUL/LUS | 393 | 300 (76.3) | 1.05 (0.76–1.45) | 0.768 | ||
| Others | 399 | 301 (75.4) | 1.00 | |||
| Lesion location from the hilum | ||||||
| Inner | 202 | 164 (81.2) | 1.51 (1.91–2.25) | 0.042 | 1.44 (0.92–2.24) | 0.108 |
| Outer | 590 | 437 (74.1) | 1.00 | 1.00 | ||
| Locational relationship with pleura | ||||||
| Apart from the pleura | 443 | 338 (76.3) | 1.05 (0.76–1.46) | 0.759 | ||
| Abutting on the pleura | 349 | 301 (86.2) | 1.00 | |||
| Lesion appearance on CT | ||||||
| Solid | 576 | 449 (78.0) | 1.49 (1.05–2.12) | 0.027 | 0.79 (0.51–1.25) | 0.317 |
| Ground-glass | 216 | 152 (70.4) | 1.00 | 1.00 | ||
| Visibility on fluoroscopy | ||||||
| Visible | 609 | 507(83.3) | 4.71 (3.28–6.74) | <0.001 | 2.96 (1.98–4.42) | <0.001 |
| Invisible | 183 | 94 (51.4) | 1.00 | 1.00 | ||
| Bronchus type | ||||||
| Type Ia | 400 | 320 (80.0) | 2.53 (1.64–3.88) 1 | <0.001 1 | 1.78 (1.04–3.05) 1 | 0.035 1 |
| Type Ib | 287 | 219 (76.3) | ||||
| Type Ic | 105 | 62 (59.0) | 1.00 | 1.00 | ||
| rEBUS finding | ||||||
| Within | 467 | 405 (86.7) | 8.04 (4.47–14.50) 2 | <0.001 2 | 4.86 (2.58–9.14) 2 | <0.001 2 |
| Adjacent to | 269 | 178 (66.2) | ||||
| Invisible | 56 | 18 (32.1) | 1.00 | 1.00 | ||
| Guide sheath | ||||||
| With | 553 | 440 (79.6) | 1.89 (1.34–2.65) | <0.001 | 1.49 (1.01–2.21) | 0.044 |
| Without | 239 | 161 (67.4) | 1.00 | 1.00 | ||
| TBNA | ||||||
| Performed | 91 | 73 (80.2) | 1.33 (0.77–2.29) | 0.305 | ||
| Not performed | 701 | 528 (75.3) | 1.00 | |||
| Variables | Total N = 229 | Diagnostic Cases (%) n = 110 (48.0) | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | Adjusted OR (95%CI) | p-Value | |||
| Sex | ||||||
| Male | 147 | 74 (50.3) | 1.30 (0.75–2.23) | 0.350 | ||
| Female | 82 | 36 (43.9) | 1.00 | |||
| Age, years | ||||||
| >70 | 113 | 59 (52.2) | 1.39 (0.83–2.34) | 0.212 | ||
| ≤70 | 116 | 51 (44.0) | 1.00 | |||
| Lesion size, mm | ||||||
| >20 | 69 | 39 (56.5) | 1.63 (0.92–2.88) | 0.093 | 0.81 (0.40–1.65) | 0.562 |
| ≤20 | 160 | 71 (44.4) | 1.00 | 1.00 | ||
| Lobar location | ||||||
| RUL/LUS | 110 | 57 (51.8) | 1.34 (0.80–2.25) | 0.271 | ||
| Others | 119 | 53 (44.5) | 1.00 | |||
| Lesion location from the hilum | ||||||
| Inner | 88 | 47 (53.4) | 1.06 (0.62–1.80) | 0.843 | ||
| Outer | 141 | 67 (47.5) | 1.00 | |||
| Locational relationship with pleura | ||||||
| Apart from the pleura | 156 | 77 (49.4) | 1.18 (0.68–2.06) | 0.558 | ||
| Abutting on the pleura | 73 | 33 (45.2) | 1.00 | |||
| Lesion appearance on CT | ||||||
| Solid | 186 | 100 (53.8) | 3.84 (1.79–8.24) | <0.001 | 2.64 (1.10–6.29) | 0.029 |
| Ground-glass | 43 | 10 (23.3) | 1.00 | 1.00 | ||
| Visibility on fluoroscopy | ||||||
| Visible | 129 | 79 (61.2) | 3.52 (2.02–6.11) | <0.001 | 2.27 (1.19–4.36) | 0.014 |
| Invisible | 100 | 31 (31.0) | 1.00 | 1.00 | ||
| Bronchus type | ||||||
| Type IIa | 106 | 56 (52.8) | 1.85 (1.09–3.13) 1 | 0.022 1 | 1.17 (0.62–2.23) 1 | 0.623 1 |
| Type IIb | 95 | 44 (46.3) | ||||
| Type IIc | 28 | 10 (35.7) | 1.00 | 1.00 | ||
| rEBUS finding | ||||||
| Within | 66 | 50 (75.8) | 10.20 (4.58–22.90) 2 | <0.001 2 | 8.43 (3.65–19.40) 2 | <0.001 2 |
| Adjacent to | 102 | 52 (51.0) | ||||
| Invisible | 61 | 8 (13.1) | 1.00 | 1.00 | ||
| Guide sheath | ||||||
| With | 102 | 55 (53.9) | 1.53 (0.91–2.59) | 0.111 | ||
| Without | 127 | 55 (43.3) | 1.00 | |||
| TBNA | ||||||
| Performed | 86 | 45 (52.3) | 1.32 (0.77–2.25) | 0.314 | ||
| Not performed | 143 | 65 (45.5) | 1.00 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imabayashi, T.; Matsumoto, Y.; Uchimura, K.; Furuse, H.; Tsuchida, T. Computed Tomography Bronchus Sign Subclassification during Radial Endobronchial Ultrasound-Guided Transbronchial Biopsy: A Retrospective Analysis. Diagnostics 2023, 13, 1064. https://doi.org/10.3390/diagnostics13061064
Imabayashi T, Matsumoto Y, Uchimura K, Furuse H, Tsuchida T. Computed Tomography Bronchus Sign Subclassification during Radial Endobronchial Ultrasound-Guided Transbronchial Biopsy: A Retrospective Analysis. Diagnostics. 2023; 13(6):1064. https://doi.org/10.3390/diagnostics13061064
Chicago/Turabian StyleImabayashi, Tatsuya, Yuji Matsumoto, Keigo Uchimura, Hideaki Furuse, and Takaaki Tsuchida. 2023. "Computed Tomography Bronchus Sign Subclassification during Radial Endobronchial Ultrasound-Guided Transbronchial Biopsy: A Retrospective Analysis" Diagnostics 13, no. 6: 1064. https://doi.org/10.3390/diagnostics13061064





