Diagnostic Performance of Prototype Handheld Ultrasound According to the Fifth Edition of BI-RADS for Breast Ultrasound Compared with Automated Breast Ultrasound among Females with Positive Lumps
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. HHUS
2.3. ABUS
2.4. Image Review
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Diagnostic Performance
3.2.1. ABUS versus Prototype HHUS
3.2.2. Interobserver Agreement of ABUS and HHUS in the Fifth Edition of BI-RADS Descriptors and Final Assessment
- Shape of the Lesion
- 2.
- Orientation of the lesion
- 3.
- Margin of the lesion
- 4.
- Echo pattern of the lesion
- 5.
- Posterior Acoustic Features of the lesion
- 6.
- Calcification of the lesion
- 7.
- BI-RADS Assessment of The Lesion
- 8.
- Location of the Lesion
- 9.
- Location of the Lesion
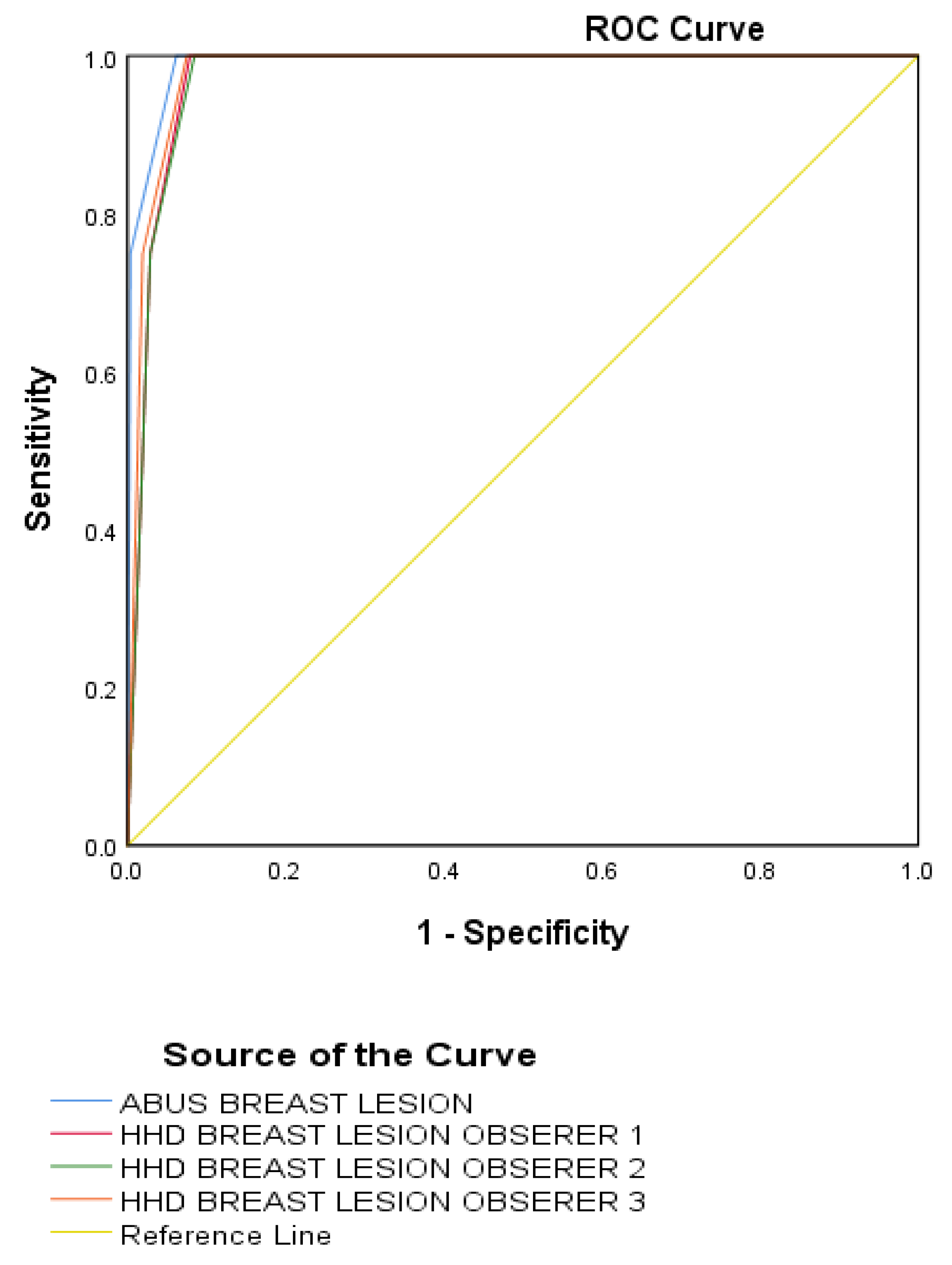
3.3. Sensitivity, Specificity, and Receiver Operating Characteristic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azizah, A.M.; Hashimah, B.; Nirmal, K.; Siti Zubaidah, A.R.; Puteri, N.A.; Nabihah, A.; Sukumaran, R.; Balqis, B.; Nadia, S.M.R.; Sharifah, S.S.S.; et al. Malaysian National Cancer Registry Report 2012–2016. Malaysia Cancer Statistics, Data and Figure; National Cancer Institute, Ministry of Health: Putrajaya, Malaysia, 2019. [Google Scholar]
- Nakamura, K.; Okada, E.; Ukawa, S.; Hirata, M.; Nagai, A.; Yamagata, Z.; Kiyohara, Y.; Muto, K.; Kamatani, Y.; Ninomiya, T.; et al. Characteristics and prognosis of Japanese female breast cancer patients: The BioBank Japan project. J. Epidemiol. 2017, 27, S58–S64. [Google Scholar] [CrossRef]
- Lee, S.B.; Sohn, G.; Kim, J.; Chung, I.Y.; Kim, H.J.; Ko, B.S.; Lee, J.W.; Son, B.H.; Kim, S.-B.; Ahn, S.-H. Chronological Improvement in Survival of Patients with Breast Cancer: A Large-Scale, Single-Center Study. J. Breast Cancer 2018, 21, 70–79. [Google Scholar] [CrossRef]
- National Registry of Diseases Office. Singapore Cancer Registry Annual Registry Report 2015; National Registry of Diseases Office: Sinapore, 2017. [Google Scholar]
- Pan, H.-B. The Role of Breast Ultrasound in Early Cancer Detection. J. Med. Ultrasound 2016, 24, 138–141. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, B.J.; Gil Choi, B.; Choi, J.J.; Lee, J.H.; Song, B.J.; Choe, B.J.; Park, S.; Kim, H. Radiologists’ Performance for Detecting Lesions and the Interobserver Variability of Automated Whole Breast Ultrasound. Korean J. Radiol. 2013, 14, 154–163. [Google Scholar] [CrossRef]
- Zanotel, M.; Bednarova, I.; Londero, V.; Linda, A.; Lorenzon, M.; Girometti, R.; Zuiani, C. Automated breast ultrasound: Basic principles and emerging clinical applications. La Radiol. Med. 2017, 123, 1–12. [Google Scholar] [CrossRef]
- Brem, R.F.; Tabár, L.; Duffy, S.W.; Inciardi, M.F.; Guingrich, J.A.; Hashimoto, B.E.; Lander, M.R.; Lapidus, R.L.; Peterson, M.K.; Rapelyea, J.A.; et al. Assessing Improvement in Detection of Breast Cancer with Three-dimensional Automated Breast US in Women with Dense Breast Tissue: The SomoInsight Study. Radiology 2015, 274, 663–673. [Google Scholar] [CrossRef]
- Vourtsis, A.; Kachulis, A. The performance of 3D ABUS versus HHUS in the visualisation and BI-RADS characterisation of breast lesions in a large cohort of 1886 women. Eur. Radiol. 2017, 28, 592–601. [Google Scholar] [CrossRef]
- Ibraheem, S.A.; Mahmud, R.; Saini, S.M.; Abu Hassan, H.; Keiteb, A.S.; Dirie, A.M. Evaluation of Diagnostic Performance of Automatic Breast Volume Scanner Compared to Handheld Ultrasound on Different Breast Lesions: A Systematic Review. Diagnostics 2022, 12, 541. [Google Scholar] [CrossRef]
- Choi, E.J.; Choi, H.; Park, E.H.; Song, J.S.; Youk, J.H. Evaluation of an automated breast volume scanner according to the fifth edition of BI-RADS for breast ultrasound compared with hand-held ultrasound. Eur. J. Radiol. 2018, 99, 138–145. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Leisenring, W.; Alono, T.; Pepe, M.S. Comparisons of Predictive Values of Binary Medical Diagnostic Tests for Paired Designs. Biometrics 2000, 56, 345–351. [Google Scholar] [CrossRef]
- Chen, H.; Han, M.; Jing, H.; Liu, Z.; Shang, H.; Wang, Q.; Cheng, W. Dependability of Automated Breast Ultrasound (ABUS) in Assessing Breast Imaging Reporting and Data System (BI-RADS) Category and Size of Malignant Breast Lesions Compared with Handheld Ultrasound (HHUS) and Mammography (MG). Int. J. Gen. Med. 2021, 14, 9193–9202. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, H.H.; Cha, J.H.; Park, J.H.; Lee, K.E.; Kim, J.H. Automated Ultrasound of the Breast for Diagnosis: Interobserver Agreement on Lesion Detection and Characterization. Am. J. Roentgenol. 2011, 197, 747–754. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Y.; Diao, X.-H.; Fang, L.; Pang, Y.; Cheng, A.-Q.; Li, W.-P.; Wang, Y. Comparative Study of Automated Breast 3-D Ultrasound and Handheld B-Mode Ultrasound for Differentiation of Benign and Malignant Breast Masses. Ultrasound Med. Biol. 2013, 39, 1735–1742. [Google Scholar] [CrossRef]
- Cheng, H.D.; Shan, J.; Ju, W.; Guo, Y.; Zhang, L. Automated breast cancer detection and classification using ultrasound images: A survey. Pattern Recognit. 2010, 43, 299–317. [Google Scholar] [CrossRef]
- Maier, A.; Heil, J.; Lauer, A.; Harcos, A.; Schaefgen, B.; von Au, A.; Spratte, J.; Riedel, F.; Rauch, G.; Hennigs, A.; et al. Inter-rater reliability and double reading analysis of an automated three-dimensional breast ultrasound system: Comparison of two independent examiners. Arch. Gynecol. Obstet. 2017, 296, 571–582. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Jiang, Y.-X.; Zhu, Q.-L.; Zhang, J.; Xiao, M.-S.; Liu, H.; Dai, Q.; Li, J.-C.; Sun, Q. Automated Breast Volume Scanning: Identifying 3-D Coronal Plane Imaging Features May Help Categorize Complex Cysts. Ultrasound Med. Biol. 2015, 42, 689–698. [Google Scholar] [CrossRef]
- Wang, L.; Qi, Z.-H. Automatic Breast Volume Scanner versus Handheld Ultrasound in Differentiation of Benign and Malignant Breast Lesions: A Systematic Review and Meta-analysis. Ultrasound Med. Biol. 2019, 45, 1874–1881. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Zhan, J.; Diao, X.; Pang, Y.; Chen, Y.; Wang, L. Inter-observer agreement on the evaluation of automated breast volume scanner for breast masses. Tradit. Med. Mod. Med. 2022, 1–11. [Google Scholar] [CrossRef]
- Zheng, F.-Y.; Yan, L.-X.; Huang, B.-J.; Xia, H.-S.; Wang, X.; Lu, Q.; Li, C.-X.; Wang, W.-P. Comparison of retraction phenomenon and BI-RADS-US descriptors in differentiating benign and malignant breast masses using an automated breast volume scanner. Eur. J. Radiol. 2015, 84, 2123–2129. [Google Scholar] [CrossRef]
- Zheng, F.-Y.; Lu, Q.; Huang, B.-J.; Xia, H.-S.; Yan, L.-X.; Wang, X.; Yuan, W.; Wang, W.-P. Imaging features of automated breast volume scanner: Correlation with molecular subtypes of breast cancer. Eur. J. Radiol. 2017, 86, 267–275. [Google Scholar] [CrossRef]
- Abdullah, N.; Mesurolle, B.; El-Khoury, M.; Kao, E. Breast Imaging Reporting and Data System Lexicon for US: Interobserver Agreement for Assessment of Breast Masses. Radiology 2009, 252, 665–672. [Google Scholar] [CrossRef]
- Jia, M.; Lin, X.; Zhou, X.; Yan, H.; Chen, Y.; Liu, P.; Bao, L.; Li, A.; Basu, P.; Qiao, Y.; et al. Diagnostic performance of automated breast ultrasound and handheld ultrasound in women with dense breasts. Breast Cancer Res. Treat. 2020, 181, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Jiang, Y.-X.; Zhu, Q.-L.; Zhang, J.; Dai, Q.; Liu, H.; Lai, X.-J.; Sun, Q. Differentiation of benign and malignant breast lesions: A comparison between automatically generated breast volume scans and handheld ultrasound examinations. Eur. J. Radiol. 2012, 81, 3190–3200. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Bao, L.; Zhu, L.; Tan, Y.; Xu, X.; Shan, Y.; Liu, J.; Zhu, Q.; Jiang, C.; Shen, Y. Diagnostic Performance of Automated Breast Ultrasound in Differentiating Benign and Malignant Breast Masses in Asymptomatic Women: A Comparison Study With Handheld Ultrasound. J. Ultrasound Med. 2019, 38, 2871–2880. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G.; DeVita, R.; Destounis, S.; Manzoni, F.; De Silvestri, A.; Tinelli, C. Agreement Between an Automated Volume Breast Scanner and Handheld Ultrasound for Diagnostic Breast Examinations. J. Ultrasound Med. 2017, 36, 2087–2092. [Google Scholar] [CrossRef]
- Zhan, J.; Diao, X.H.; Pang, Y.; Wang, Y.; Chen, L.; Chen, Y. Is there an extraclinical value of automated breast volume scanner compared with hand-held ultrasound?: A pilot study. Medicine 2017, 96, e7765. [Google Scholar] [CrossRef]
- Chang, J.M.; Moon, W.K.; Cho, N.; Park, J.S.; Kim, S.J. Radiologists’ performance in the detection of benign and malignant masses with 3D automated breast ultrasound (ABUS). Eur. J. Radiol. 2011, 78, 99–103. [Google Scholar] [CrossRef]
- Zhang, L.; Bao, L.Y.; Tan, Y.J.; Zhu, L.Q.; Xu, X.J.; Zhu, Q.Q.; Shan, Y.N.; Zhao, J.; Xie, L.S.; Liu, J. Diagnostic performance using automated breast ultrasound system for breast cancer in Chines Both e women aged 40 years or older: A comparative study. Ultrasound Med. Biol. 2019, 45, 3137–3144. [Google Scholar] [CrossRef]
- Meng, Z.; Chen, C.; Zhu, Y.; Zhang, S.; Wei, C.; Hu, B.; Yu, L.; Shen, E.; Hu, B. Diagnostic performance of the automated breast volume scanner: A systematic review of inter-rater reliability/agreement and meta-analysis of diagnostic accuracy for differentiating benign and malignant breast lesions. Eur. Radiol. 2015, 25, 3638–3647. [Google Scholar] [CrossRef]
- Wojcinski, S.; Gyapong, S.; Farrokh, A.; Soergel, P.; Hillemanns, P.; Degenhardt, F. Diagnostic performance and inter-observer concordance in lesion detection with the automated breast volume scanner (ABVS). BMC Med. Imaging 2013, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Skaane, P.; Gullien, R.; Eben, E.B.; Sandhaug, M.; Schulz-Wendtland, R.; Stoeblen, F. Interpretation of automated breast ultrasound (ABUS) with and without knowledge of mammography: A reader performance study. Acta Radiol. 2015, 56, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Schmachtenberg, C.; Fischer, T.; Hamm, B.; Bick, U. Diagnostic Performance of Automated Breast Volume Scanning (ABVS) Compared to Handheld Ultrasonography With Breast MRI as the Gold Standard. Acad. Radiol. 2017, 24, 954–961. [Google Scholar] [CrossRef]
- Chae, E.Y.; Shin, H.J.; Kim, H.J.; Yoo, H.; Baek, S.; Cha, J.H.; Kim, H.H. Diagnostic Performance of Automated Breast Ultrasound as a Replacement for a Hand-Held Second-Look Ultrasound for Breast Lesions Detected Initially on Magnetic Resonance Imaging. Ultrasound Med. Biol. 2013, 39, 2246–2254. [Google Scholar] [CrossRef]
- Chang, J.M.; Cha, J.H.; Park, J.S.; Kim, S.J.; Moon, W.K. Automated breast ultrasound system (ABUS): Reproducibility of mass localization, size measurement, and characterization on serial examinations. Acta Radiol. 2015, 56, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, B.; Li, W. Detection of Breast Lesions using an Automated Breast Volume Scanner System. J. Int. Med. Res. 2012, 40, 300–306. [Google Scholar] [CrossRef]
- Vourtsis, A. Three-dimensional automated breast ultrasound: Technical aspects and first results. Diagn. Interv. Imaging 2019, 100, 579–592. [Google Scholar] [CrossRef] [PubMed]


| Variables | Mean ± SD | Frequency N | Percentage % |
|---|---|---|---|
| Age (years) (min–max) | 31.80 ± 9.44 (19–59) | ||
| Race | |||
| Malay | 151 | 93.2 | |
| Chinese | 3 | 1.9 | |
| Indian | 4 | 2.5 | |
| Others | 4 | 2.5 | |
| Marital status | |||
| Single | 101 | 62.3 | |
| Married | 52 | 32.1 | |
| Divorced/separated | 6 | 3.7 | |
| Widowed | 3 | 1.9 | |
| Education level | |||
| Primary/secondary | 9 | 5.6 | |
| Diploma | 26 | 16.0 | |
| Degree | 76 | 46.9 | |
| Postgraduate | 51 | 31.5 | |
| Occupation | |||
| Student | 23 | 14.2 | |
| Working | 120 | 74.1 | |
| Housewife | 17 | 10.5 | |
| Retired | 2 | 1.2 | |
| Income (RM) | |||
| Less than RM 2500 | 45 | 27.8 | |
| RM 2501 to RM 5000 | 61 | 37.7 | |
| RM 5001 to RM 7500 | 23 | 14.2 | |
| RM 7501 to RM 10,000 | 16 | 9.9 | |
| RM 10,001 to RM 12,500 | 13 | 8.0 | |
| RM 12,501 and above | 4 | 2.5 | |
| Family history of BC | |||
| Yes | 39 | 24.1 | |
| No | 123 | 75.9 | |
| Family members | |||
| Mother | 17 | 43.6 | |
| Sister | 3 | 7.7 | |
| Others | 19 | 48.7 | |
| Menarche age (years) | 12.35 ± 1.24 | ||
| (min–max) | (8–16) |
| HHUS Observers | ABUS (Gold Standard) | p Value | |||
|---|---|---|---|---|---|
| Malignant | Non-Malignant | Total | |||
| Observer 1 | Malignant | 10 | 0 | 10 | |
| Non-Malignant | 4 | 960 | 964 | 0.072 | |
| Total | 14 | 960 | 974 | ||
| Observer 2 | Malignant | 9 | 0 | 9 | 0.053 |
| Non-Malignant | 3 | 961 | 964 | ||
| Total | 12 | 961 | 973 | ||
| Observer 3 | Malignant | 7 | 0 | 7 | 0.059 |
| Non-Malignant | 1 | 972 | 973 | ||
| Total | 8 | 972 | 980 | ||
| HHUS Observers | ABUS (Gold Standard) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right Breast | Rounded | Elliptical | Irregular | Total | p Value | Left Breast | Rounded | Elliptical | Irregular | Total | p Value | ||
| Observer 1 | Rounded | 329 | 11 | 0 | 340 | 0.12 | Observer 1 | Rounded | 286 | 5 | 0 | 291 | 1.01 |
| Elliptical | 14 | 180 | 0 | 194 | Elliptical | 4 | 142 | 0 | 146 | ||||
| Irregular | 0 | 0 | 2 | 2 | Irregular | 0 | 0 | 1 | 1 | ||||
| Total | 343 | 191 | 2 | 536 | Total | 290 | 147 | 1 | 438 | ||||
| Observer 2 | Rounded | 339 | 7 | 0 | 346 | 0.1 | Observer 2 | Rounded | 286 | 3 | 0 | 289 | 0.14 |
| Elliptical | 7 | 183 | 0 | 190 | Elliptical | 1 | 144 | 0 | 145 | ||||
| Irregular | 0 | 0 | 2 | 2 | Irregular | 0 | 0 | 1 | 1 | ||||
| Total | 346 | 190 | 2 | 538 | Total | 287 | 147 | 1 | 435 | ||||
| Observer 3 | Rounded | 338 | 6 | 0 | 344 | 0.05 | Observer 3 | Rounded | 289 | 5 | 0 | 294 | 0.12 |
| Elliptical | 9 | 185 | 0 | 194 | Elliptical | 1 | 144 | 0 | 145 | ||||
| Irregular | 0 | 0 | 2 | 2 | Irregular | 0 | 0 | 1 | 1 | ||||
| Total | 347 | 191 | 2 | 540 | Total | 290 | 149 | 1 | 440 | ||||
| HHUS Observers | ABUS (Gold Standard) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Right Breast | Parallel | Not Parallel | Total | p Value | Left Breast | Parallel | Not Parallel | Total | p Value | ||
| Observer 1 | Parallel | 533 | 1 | 534 | 0.000 * | Observer 1 | Parallel | 436 | 1 | 437 | 0.002 * |
| Not Parallel | 0 | 2 | 2 | Not Parallel | 0 | 1 | 1 | ||||
| Total | 533 | 3 | 536 | Total | 436 | 2 | 438 | ||||
| Observer 2 | Parallel | 536 | 1 | 537 | 0.000 * | Observer 2 | Parallel | 433 | 1 | 434 | 0.002 * |
| Not Parallel | 0 | 2 | 2 | Not Parallel | 0 | 1 | 1 | ||||
| Total | 536 | 3 | 539 | Total | 433 | 2 | 435 | ||||
| Observer 3 | Parallel | 538 | 1 | 539 | 0.000 * | Observer 3 | Parallel | 438 | 1 | 439 | 0.002 * |
| Not Parallel | 0 | 2 | 2 | Not Parallel | 0 | 1 | 1 | ||||
| Total | 538 | 3 | 541 | Total | 438 | 2 | 440 | ||||
| HHUS Observers | ABUS (Gold Standard) | |||||||
|---|---|---|---|---|---|---|---|---|
| Circumscribed | Indistinct | Angular | Microlobulated | Spiculated | Total | p Value | ||
| Right Breast | ||||||||
| Observer 1 | Circumscribed | 520 | 0 | - | 0 | 0 | 520 | 0.07 |
| Indistinct | 0 | 1 | - | 0 | 0 | 1 | ||
| Angular | - | 0 | - | - | - | - | ||
| Microlobulated | 3 | 0 | - | 11 | 0 | 14 | ||
| Spiculated | 0 | 0 | - | 0 | 1 | 1 | ||
| Total | 523 | 1 | - | 11 | 1 | 536 | ||
| Observer 2 | Circumscribed | 521 | 0 | - | 0 | 0 | 521 | 0.09 |
| Indistinct | 0 | 1 | - | 0 | 0 | 1 | ||
| Angular | - | - | - | - | - | - | ||
| Microlobulated | 5 | 0 | - | 11 | 0 | 16 | ||
| Spiculated | 0 | 0 | - | 0 | 1 | 1 | ||
| Total | 526 | 1 | - | 11 | 1 | 539 | ||
| Observer 3 | Circumscribed | 526 | 0 | - | 0 | 0 | 526 | 0.09 |
| Indistinct | 0 | 1 | - | 0 | 0 | 1 | ||
| Angular | - | - | - | - | - | - | ||
| Microlobulated | 2 | 0 | - | 11 | 0 | 13 | ||
| Spiculated | 0 | 0 | - | 0 | 1 | 1 | ||
| Total | 528 | 1 | - | 11 | 1 | 541 | ||
| Left Breast | ||||||||
| Observer 1 | Circumscribed | 425 | - | 1 | 0 | 0 | 426 | 1 |
| Indistinct | - | - | - | - | - | - | ||
| Angular | 0 | - | 2 | 0 | 0 | 2 | ||
| Microlobulated | 2 | - | 0 | 6 | 1 | 9 | ||
| Spiculated | 0 | - | 0 | 0 | 1 | 1 | ||
| Total | 427 | - | 3 | 6 | 2 | 438 | ||
| Observer 2 | Circumscribed | 420 | - | 1 | 0 | 1 | 426 | 1.06 |
| Indistinct | - | - | - | - | - | - | ||
| Angular | 0 | - | 2 | 0 | 0 | 2 | ||
| Microlobulated | 4 | - | 0 | 6 | 0 | 10 | ||
| Spiculated | - | - | 0 | 0 | 1 | 1 | ||
| Total | 424 | - | 3 | 6 | 2 | 435 | ||
| Observer 3 | Circumscribed | 427 | - | 1 | 0 | 1 | 429 | 0.99 |
| Indistinct | - | - | - | - | - | - | ||
| Angular | 0 | - | 2 | 0 | 0 | 2 | ||
| Microlobulated | 2 | - | 0 | 6 | 0 | 8 | ||
| Spiculated | 0 | - | 0 | 0 | 1 | 1 | ||
| Total | 429 | - | 3 | 6 | 2 | 440 | ||
| HHUS Observers | ABUS (Gold Standard) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anechoic | Isoechoic | Hypoechoic | Hyperechoic | Complex Cystic and Solid | Heterogenous | Total | p Value | ||
| Right Breast | |||||||||
| Observer 1 | Anechoic | 495 | - | 0 | 0 | 0 | 0 | 495 | 1.22 |
| Isoechoic | - | - | - | - | - | - | - | ||
| Hypoechoic | 6 | - | 19 | 0 | 0 | 0 | 25 | ||
| Hyperechoic | 0 | - | 0 | 10 | 0 | 0 | 10 | ||
| Complex | 0 | - | 0 | 0 | 1 | 0 | 1 | ||
| Cystic and Solid | |||||||||
| Heterogenous | 0 | - | 0 | 0 | 0 | 5 | 5 | ||
| Total | 501 | - | 19 | 10 | 1 | 5 | 536 | ||
| Observer 2 | Anechoic | 499 | - | 0 | 0 | 0 | 0 | 499 | 1.03 |
| Isoechoic | - | - | - | - | - | - | - | ||
| Hypoechoic | 5 | - | 19 | 0 | 0 | 0 | 24 | ||
| Hyperechoic | 0 | - | 0 | 10 | 0 | 0 | 11 | ||
| Complex | 0 | - | 0 | 0 | 1 | 0 | 1 | ||
| Cystic and Solid | |||||||||
| Heterogenous | 0 | - | 0 | 0 | 0 | 5 | 5 | ||
| Total | 504 | - | 19 | 10 | 1 | 5 | 539 | ||
| Observer 3 | Anechoic | 502 | - | 1 | 0 | 0 | 0 | 502 | 1.14 |
| Isoechoic | - | - | - | - | - | - | - | ||
| Hypoechoic | 4 | - | 19 | 0 | 0 | 0 | 23 | ||
| Hyperechoic | 0 | - | 0 | 11 | 0 | 0 | 11 | ||
| Complex | 0 | - | 0 | 0 | 1 | 0 | 1 | ||
| Cystic and Solid | |||||||||
| Heterogenous | 0 | - | 0 | 0 | 0 | 5 | 5 | ||
| Total | 502 | - | 19 | 11 | 1 | 5 | 541 | ||
| Left Breast | |||||||||
| Observer 1 | Anechoic | 408 | - | 0 | 0 | - | 0 | 408 | 0.06 |
| Isoechoic | - | - | - | - | - | - | - | ||
| Hypoechoic | 5 | - | 17 | 0 | - | 0 | 22 | ||
| Hyperechoic | 0 | - | 0 | 6 | - | 0 | 6 | ||
| Complex | 0 | - | 0 | 0 | - | - | 0 | ||
| Cystic and Solid | |||||||||
| Heterogenous | - | - | - | - | - | 2 | 2 | ||
| Total | 413 | - | 17 | 6 | - | 2 | 438 | ||
| Observer 2 | Anechoic | 407 | - | 0 | 0 | - | 0 | 407 | 0.34 |
| Isoechoic | - | - | - | - | - | - | - | ||
| Hypoechoic | 3 | - | 17 | 0 | - | 0 | 20 | ||
| Hyperechoic | 0 | - | 0 | 6 | - | 0 | 6 | ||
| Complex | - | - | - | - | - | - | - | ||
| Cystic and Solid | |||||||||
| Heterogenous | 0 | - | 0 | 0 | - | 2 | 2 | ||
| Total | 410 | - | 17 | 6 | - | 2 | 435 | ||
| Observer 3 | Anechoic | 412 | - | 0 | 0 | - | 0 | 412 | 0.33 |
| Isoechoic | - | - | - | - | - | - | - | ||
| Hypoechoic | 3 | - | 17 | 0 | - | 0 | 20 | ||
| Hyperechoic | 0 | - | 0 | 6 | - | 0 | 6 | ||
| Complex | - | - | - | - | - | - | - | ||
| Cystic and Solid | |||||||||
| Heterogenous | 0 | - | 0 | 0 | - | 2 | 2 | ||
| Total | 415 | - | 16 | 6 | - | 2 | 440 | ||
| HHUS Observers | ABUS (Gold Standard) | ||||||
|---|---|---|---|---|---|---|---|
| Enhancement | Shadowing | Combined | Absent | Total | p Value | ||
| Right Breast | |||||||
| Observer 1 | Enhancement | 493 | 0 | - | - | 493 | 0.21 |
| Shadowing | 0 | 33 | - | - | 33 | ||
| Combined | - | - | - | - | - | ||
| Absent | 10 | 0 | - | - | 10 | ||
| Total | 503 | 33 | - | - | 536 | ||
| Observer 2 | Enhancement | 495 | 0 | - | - | 495 | 0.93 |
| Shadowing | 0 | 33 | - | - | 33 | ||
| Combined | - | - | - | - | - | ||
| Absent | 11 | 0 | - | - | 11 | ||
| Total | 506 | 33 | - | - | 539 | ||
| Observer 3 | Enhancement | 497 | 0 | - | - | 497 | 0.97 |
| Shadowing | 0 | 33 | - | - | 33 | ||
| Combined | - | - | - | - | - | ||
| Absent | 11 | 0 | - | - | 11 | ||
| Total | 508 | 33 | - | - | 541 | ||
| Left Breast | |||||||
| Observer 1 | Enhancement | 407 | 0 | 0 | - | 407 | 0.06 |
| Shadowing | 0 | 24 | 0 | - | 24 | ||
| Combined | 0 | 0 | 1 | - | 1 | ||
| Absent | 6 | 0 | 0 | - | 6 | ||
| Total | 413 | 24 | 1 | - | 438 | ||
| Observer 2 | Enhancement | 407 | 0 | 0 | - | 407 | 0.51 |
| Shadowing | 0 | 24 | 0 | - | 24 | ||
| Combined | 0 | 0 | 1 | - | 1 | ||
| Absent | 3 | 0 | 0 | - | - | ||
| Total | 410 | 24 | 1 | - | 435 | ||
| Observer 3 | Enhancement | 411 | 0 | 0 | - | 411 | 0.06 |
| Shadowing | 0 | 24 | 0 | - | 24 | ||
| Combined | 0 | 0 | 1 | - | 1 | ||
| Absent | 4 | 0 | 0 | - | 4 | ||
| Total | 415 | 24 | 1 | - | 440 | ||
| HHUS Observers | ABUS (Gold Standard) | ||||||
|---|---|---|---|---|---|---|---|
| No Calcifications | Calcifications in Mass | Calcifications Out of Mass | Intraductal Calcifications | Total | p Value | ||
| Right Breast | |||||||
| Observer 1 | No Calcifications | 514 | 2 | 2 | - | 518 | 0.87 |
| Calcifications in Mass | 0 | 8 | 0 | - | 8 | ||
| Calcifications Out of Mass | 5 | 0 | 5 | - | 10 | ||
| Intraductal Calcifications | - | - | - | - | - | ||
| Total | 519 | 10 | 7 | - | 536 | ||
| Observer 2 | No Calcifications | 522 | 2 | 3 | - | 527 | 0.82 |
| Calcifications in Mass | 0 | 7 | 1 | - | 8 | ||
| Calcifications Out of Mass | 1 | 0 | 3 | - | 4 | ||
| Intraductal Calcifications | - | - | - | - | - | ||
| Total | 523 | 9 | 7 | - | 539 | ||
| Observer 3 | No Calcifications | 523 | 2 | 3 | - | 528 | 0.91 |
| Calcifications in Mass | 0 | 8 | 0 | - | 8 | ||
| Calcifications Out of Mass | 1 | 0 | 4 | - | 5 | ||
| Intraductal Calcifications | - | - | - | - | - | ||
| Total | 493 | 10 | 7 | - | 541 | ||
| Left Breast | |||||||
| Observer 1 | No Calcifications | 423 | 1 | 1 | - | 425 | 0.43 |
| Calcifications in Mass | 2 | 6 | 0 | - | 8 | ||
| Calcifications Out of Mass | 1 | 0 | 4 | - | 5 | ||
| Intraductal Calcifications | - | - | - | - | - | ||
| Total | 426 | 7 | 5 | - | 438 | ||
| Observer 2 | No Calcifications | 419 | 1 | 1 | - | 421 | 0.43 |
| Calcifications in Mass | 4 | 6 | 0 | - | 10 | ||
| Calcifications Out of Mass | 0 | 0 | 4 | - | 4 | ||
| Intraductal Calcifications | - | - | - | - | - | ||
| Total | 423 | 7 | 5 | - | 435 | ||
| Observer 3 | No Calcifications | 425 | 1 | 1 | - | 427 | 0.37 |
| Calcifications in Mass | 3 | 6 | 0 | - | 9 | ||
| Calcifications Out of Mass | 0 | 0 | 4 | - | 4 | ||
| Intraductal Calcifications | - | - | - | - | - | ||
| Total | 428 | 7 | 5 | - | 440 | ||
| HHUS Observers | ABUS (Gold Standard) | |||||||
|---|---|---|---|---|---|---|---|---|
| BIRADS1 | BIRADS2 | BIRADS3 | BIRADS4 | BIRADS5 | Total | p Value | ||
| Right Breast | ||||||||
| Observer 1 | BIRADS1 | - | - | - | - | - | - | 0.23 |
| BIRADS2 | - | 490 | 2 | 0 | - | 492 | ||
| BIRADS3 | - | 9 | 29 | 0 | - | 38 | ||
| BIRADS4 | - | 0 | 0 | 6 | - | 6 | ||
| BIRADS 5 | - | - | - | - | - | - | ||
| Total | - | 499 | 31 | 6 | - | 536 | ||
| Observer 2 | BIRADS1 | - | - | - | - | - | - | 0.33 |
| BIRADS2 | - | 492 | 2 | 0 | - | 494 | ||
| BIRADS3 | - | 10 | 29 | 0 | - | 39 | ||
| BIRADS4 | - | 0 | 0 | 6 | - | 6 | ||
| BIRADS 5 | - | - | - | - | - | - | ||
| Total | - | 502 | 31 | 6 | - | 539 | ||
| Observer 3 | BIRADS1 | - | - | - | - | - | - | 0.23 |
| BIRADS2 | - | 500 | 2 | 0 | - | 502 | ||
| BIRADS3 | - | 4 | 28 | 0 | - | 32 | ||
| BIRADS4 | - | 0 | 1 | 6 | - | 7 | ||
| BIRADS 5 | - | - | - | - | - | - | ||
| Total | - | 504 | 31 | 6 | - | 541 | ||
| Left Breast | ||||||||
| Observer 1 | BIRADS1 | - | - | - | - | - | - | 0.3 |
| BIRADS2 | - | 408 | 1 | 0 | - | 409 | ||
| BIRADS3 | - | 4 | 21 | 0 | - | 25 | ||
| BIRADS4 | - | 4 | 0 | 3 | - | 4 | ||
| BIRADS 5 | - | - | - | - | - | - | ||
| Total | - | 413 | 22 | 4 | - | 438 | ||
| Observer 2 | BIRADS1 | - | - | - | - | - | - | 0.21 |
| BIRADS2 | - | 406 | 1 | 0 | - | 407 | ||
| BIRADS3 | - | 3 | 21 | 0 | - | 24 | ||
| BIRADS4 | - | 1 | 0 | 3 | - | 4 | ||
| BIRADS 5 | - | - | - | - | - | - | ||
| Total | - | 410 | 22 | 3 | - | 435 | ||
| Observer 3 | BIRADS1 | - | - | - | - | - | - | 0.24 |
| BIRADS2 | - | 411 | 1 | 0 | - | 412 | ||
| BIRADS3 | - | 3 | 21 | 0 | - | 24 | ||
| BIRADS4 | - | 1 | 0 | 3 | - | 4 | ||
| BIRADS 5 | - | - | - | - | - | - | ||
| Total | - | 415 | 22 | 3 | - | 440 | ||
| BIRADS Lexicon | κ Value(R/L) | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ABUS | HHUS Observers | ||||||||
| Observer 1 | Observer 2 | Observer 3 | |||||||
| Shape | 0.97 | 0.95 | 0.90 | 0.92 | 0.92 | 0.92 | 0.95 | 0.90 | >0.05 |
| Orientation | 0.90 | 0.92 | 0.70 | 0.70 | 0.70 | 0.87 | 0.70 | 0.66 | <0.05 |
| Margin | 0.95 | 0.84 | 0.84 | 0.75 | 0.93 | 0.80 | 0.93 | 0.82 | >0.05 |
| Echo Pattern | 0.96 | 0.93 | 0.90 | 0.92 | 0.94 | 0.90 | 0.94 | 0.92 | >0.05 |
| Posterior Acoustic | 0.88 | 0.95 | 0.86 | 0.89 | 0.85 | 0.94 | 0.85 | 0.92 | >0.05 |
| Calcification | 0.92 | 0.83 | 0.84 | 0.81 | 0.90 | 0.76 | 0.81 | 0.80 | >0.05 |
| BIRADS Assessment | 0.92 | 0.86 | 0.90 | 0.84 | 0.90 | 0.85 | 0.92 | 0.84 | >0.05 |
| HHUS Observers | ABUS (Gold Standard) | ||||||
|---|---|---|---|---|---|---|---|
| RUIQ | RLIQ | RLOQ | RUOQ | Total | p Value | ||
| Right Breast | |||||||
| Observer 1 | RUIQ | 135 | 0 | 0 | 21 | 156 | 0.12 |
| RLIQ | 1 | 76 | 0 | 0 | 77 | ||
| RLOQ | 0 | 0 | 136 | 1 | 137 | ||
| RUOQ | 6 | 0 | 1 | 159 | 166 | ||
| Total | 142 | 76 | 137 | 181 | 536 | ||
| Observer 2 | RUIQ | 135 | 3 | 1 | 21 | 160 | 0.11 |
| RLIQ | 0 | 75 | 1 | 0 | 76 | ||
| RLOQ | 0 | 0 | 133 | 0 | 133 | ||
| RUOQ | 5 | 0 | 1 | 164 | 170 | ||
| Total | 140 | 78 | 136 | 185 | 539 | ||
| Observer 3 | RUIQ | 140 | 0 | 0 | 19 | 159 | 0.22 |
| RLIQ | 1 | 75 | 0 | 0 | 76 | ||
| RLOQ | 0 | 1 | 137 | 1 | 139 | ||
| RUOQ | 1 | 0 | 1 | 165 | 167 | ||
| Total | 142 | 76 | 138 | 185 | 541 | ||
| Left Breast | LUOQ | LLOQ | LIQ | LUIQ | Total | p Value | |
| Observer 1 | LUOQ | 125 | 0 | 0 | 10 | 135 | 0.06 |
| LLOQ | 0 | 114 | 3 | 0 | 117 | ||
| LLIQ | 0 | 0 | 97 | 0 | 97 | ||
| LUIQ | 5 | 0 | 0 | 84 | 89 | ||
| Total | 130 | 114 | 100 | 94 | 438 | ||
| Observer 2 | LUOQ | 119 | 0 | 0 | 8 | 127 | 0.1 |
| LLOQ | 0 | 113 | 0 | 1 | 114 | ||
| LLIQ | 0 | 0 | 101 | 1 | 102 | ||
| LUIQ | 6 | 0 | 0 | 86 | 92 | ||
| Total | 125 | 113 | 101 | 96 | 435 | ||
| Observer 3 | LUOQ | 126 | 0 | 0 | 9 | 135 | 1.02 |
| LLOQ | 0 | 115 | 0 | 0 | 115 | ||
| LLIQ | 0 | 0 | 99 | 1 | 100 | ||
| LUIQ | 3 | 0 | 0 | 87 | 90 | ||
| Total | 129 | 115 | 99 | 97 | 440 | ||
| Parameters | HHUS | p Value | |||
|---|---|---|---|---|---|
| ABUS | Observer 1 | Observer 2 | Observer 3 | ||
| Sensitivity % | 99 | 97 | 97 | 98 | 0.60 |
| Specificity% | 97 | 90 | 93 | 96 | |
| PPV % | 96 | 80 | 90 | 94 | 0.67 |
| NPV % | 94 | 73 | 73 | 76 | |
| Accuracy % | 97 | 95 | 95 | 97 | 0.67 |
| AUC | 0.99 | 0.98 | 0.97 | 0.97 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibraheem, S.A.; Mahmud, R.; Mohamad Saini, S.; Abu Hassan, H.; Keiteb, A.S. Diagnostic Performance of Prototype Handheld Ultrasound According to the Fifth Edition of BI-RADS for Breast Ultrasound Compared with Automated Breast Ultrasound among Females with Positive Lumps. Diagnostics 2023, 13, 1065. https://doi.org/10.3390/diagnostics13061065
Ibraheem SA, Mahmud R, Mohamad Saini S, Abu Hassan H, Keiteb AS. Diagnostic Performance of Prototype Handheld Ultrasound According to the Fifth Edition of BI-RADS for Breast Ultrasound Compared with Automated Breast Ultrasound among Females with Positive Lumps. Diagnostics. 2023; 13(6):1065. https://doi.org/10.3390/diagnostics13061065
Chicago/Turabian StyleIbraheem, Shahad A., Rozi Mahmud, Suraini Mohamad Saini, Hasyma Abu Hassan, and Aysar Sabah Keiteb. 2023. "Diagnostic Performance of Prototype Handheld Ultrasound According to the Fifth Edition of BI-RADS for Breast Ultrasound Compared with Automated Breast Ultrasound among Females with Positive Lumps" Diagnostics 13, no. 6: 1065. https://doi.org/10.3390/diagnostics13061065
APA StyleIbraheem, S. A., Mahmud, R., Mohamad Saini, S., Abu Hassan, H., & Keiteb, A. S. (2023). Diagnostic Performance of Prototype Handheld Ultrasound According to the Fifth Edition of BI-RADS for Breast Ultrasound Compared with Automated Breast Ultrasound among Females with Positive Lumps. Diagnostics, 13(6), 1065. https://doi.org/10.3390/diagnostics13061065






