Organ-Specific Positron Emission Tomography Scanners for Breast Imaging: Comparison between the Performances of Prior and Novel Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Organ-Specific, Ring-Shaped PET Scanners
2.1.1. PMT-Based PET Scanner: Elmammo™
2.1.2. SiPM-Based TOF-PET Scanner: BresTome™
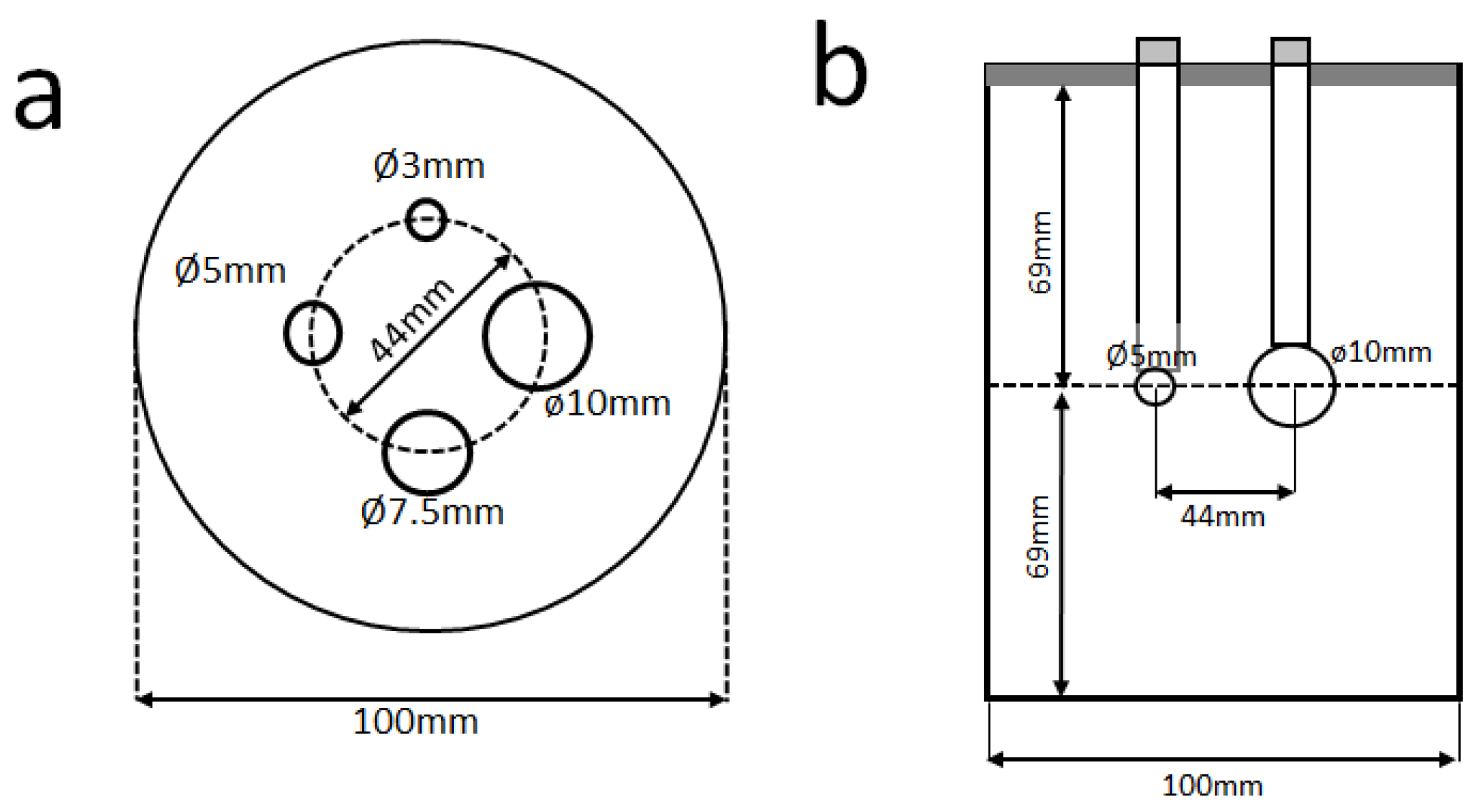
2.2. Data Acquisition
2.3. Image Reconstruction
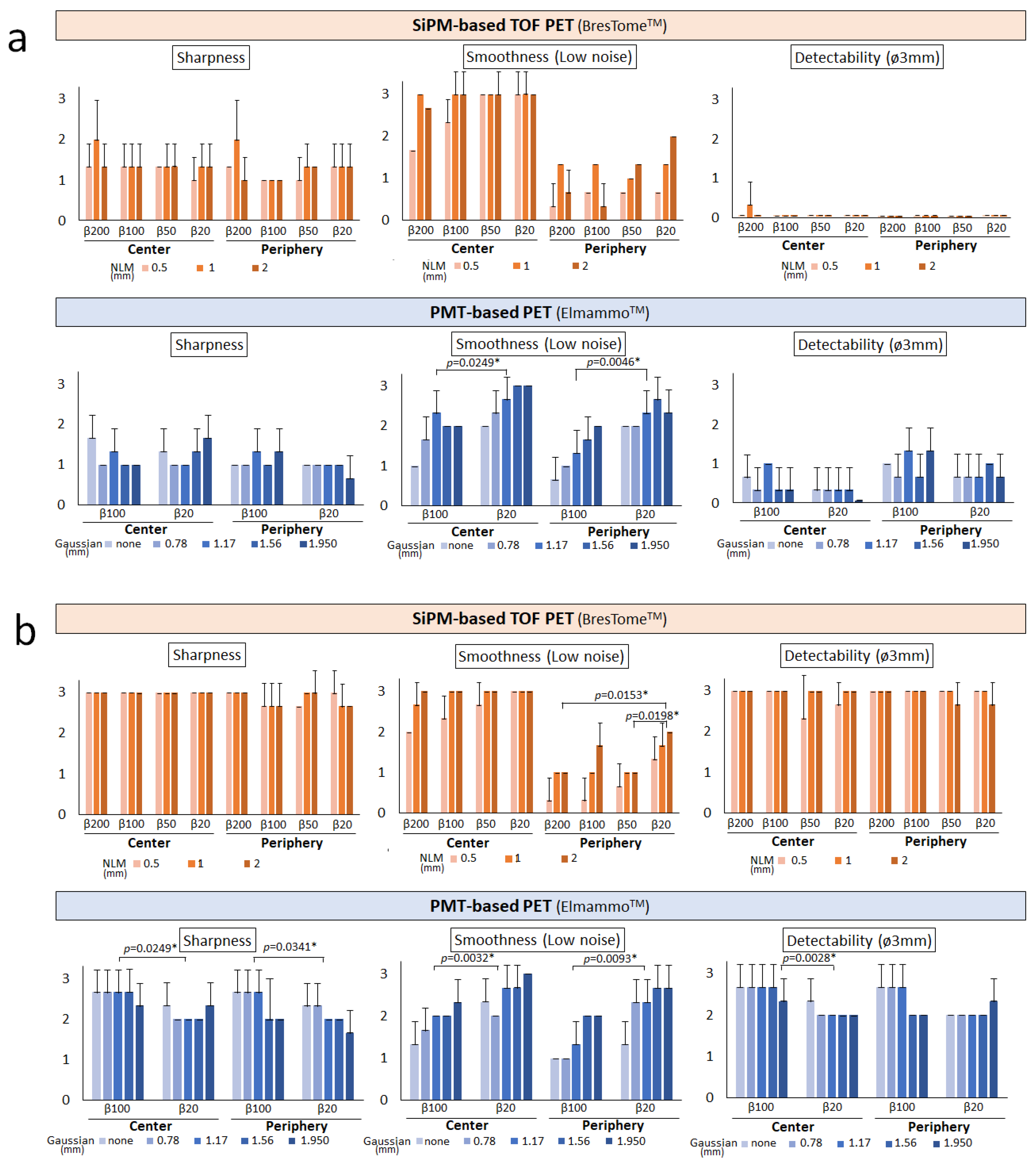
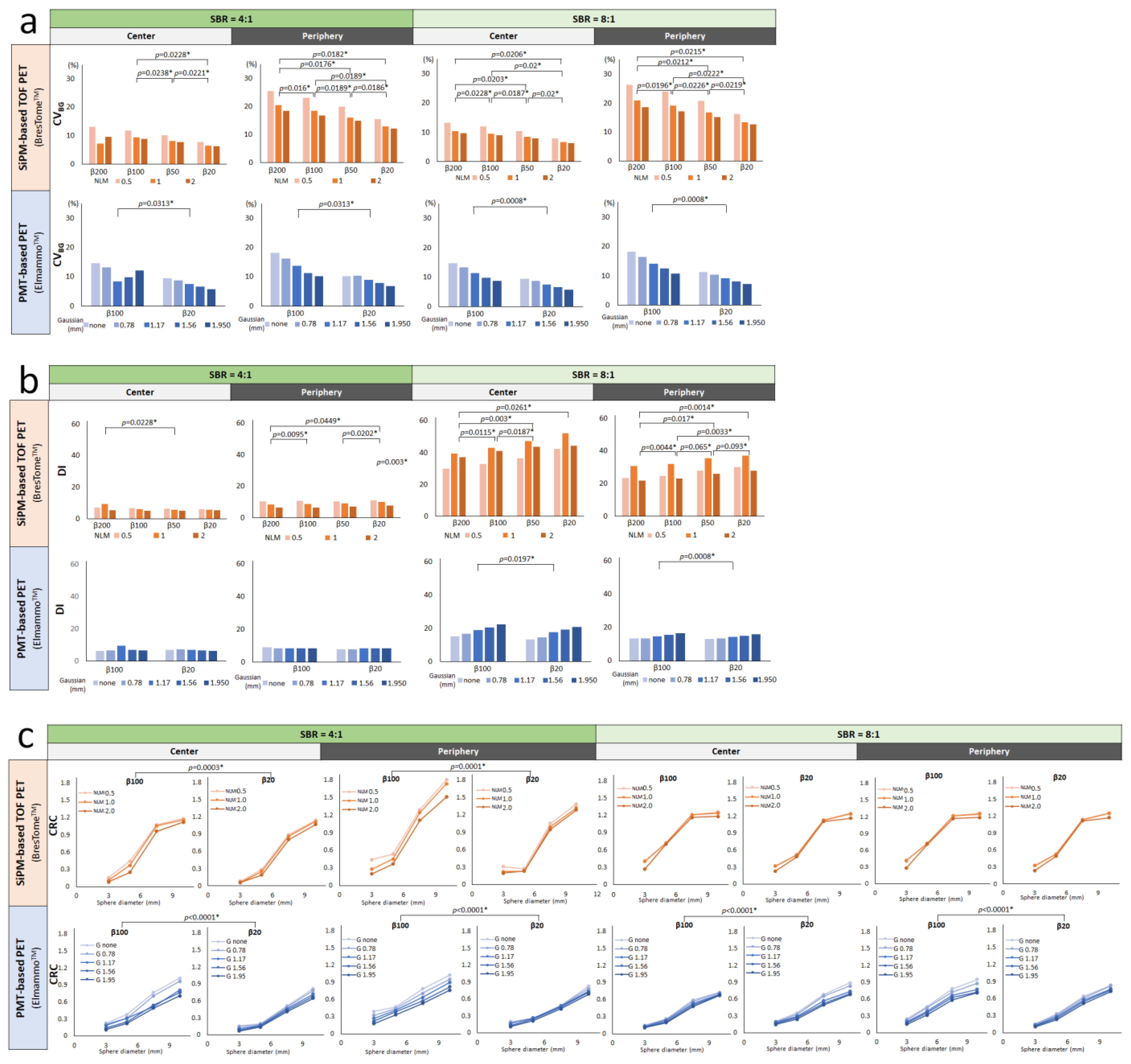
2.4. Analysis of Image Quality
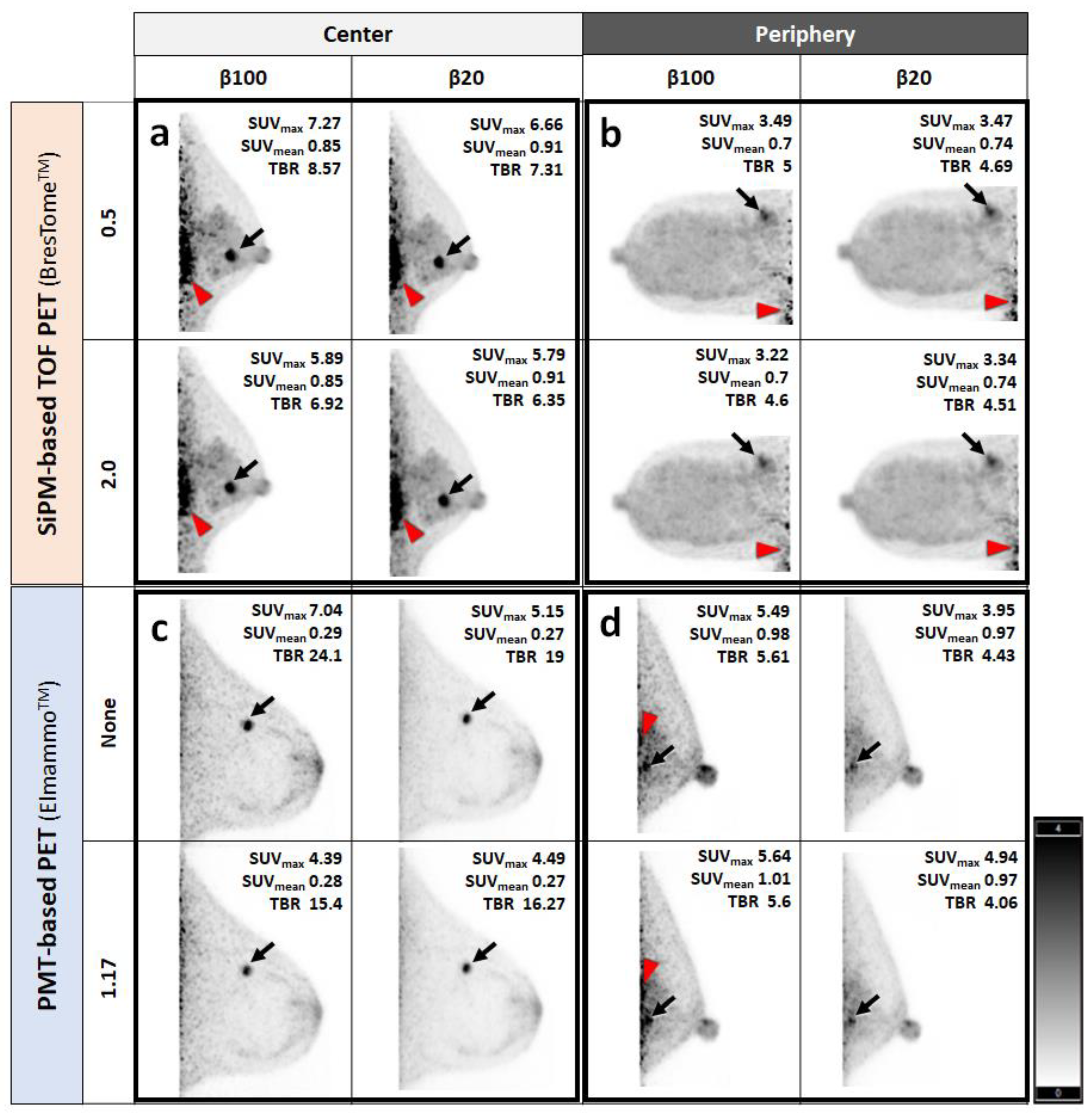
2.5. Human Imaging
3. Results
Visual Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Satoh, Y.; Motosugi, U.; Imai, M.; Onishi, H. Comparison of dedicated breast positron emission tomography and whole-body positron emission tomography/computed tomography images: A common phantom study. Ann. Nucl. Med. 2020, 34, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Satoh, Y.; Motosugi, U.; Omiya, Y.; Onishi, H. Unexpected abnormal uptake in the breasts at dedicated breast PET: Incidentally detected small cancers or nonmalignant features? AJR Am. J. Roentgenol. 2019, 212, 443–449. [Google Scholar] [CrossRef]
- Sueoka, S.; Sasada, S.; Masumoto, N.; Emi, A.; Kadoya, T.; Okada, M. Performance of dedicated breast positron emission tomography in the detection of small and low-grade breast cancer. Breast Cancer Res. Treat. 2021, 187, 125–133. [Google Scholar] [CrossRef]
- Koyasu, H.; Goshima, S.; Noda, Y.; Nishibori, H.; Takeuchi, M.; Matsunaga, K.; Yamada, T.; Matsuo, M. The feasibility of dedicated breast PET for the assessment of residual tumor after neoadjuvant chemotherapy. Jpn. J. Radiol. 2019, 37, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, Y.; Yanagawa, M.; Fujita, Y.; Honma, K.; Tanei, T.; Shimoda, M.; Miyake, T.; Naoi, Y.; Kim, S.J.; Shimazu, K.; et al. Prediction of pathological complete response after neoadjuvant chemotherapy in breast cancer: Comparison of diagnostic performances of dedicated breast PET, whole-body PET, and dynamic contrast-enhanced MRI. Breast Cancer Res. Treat. 2021, 188, 107–115. [Google Scholar] [CrossRef]
- Watanabe, M.; Saito, A.; Isobe, T.; Ote, K.; Yamada, R.; Moriya, T.; Omura, T. Performance evaluation of a high-resolution brain PET scanner using four-layer MPPC DOI detectors. Phys. Med. Biol. 2017, 62, 7148–7166. [Google Scholar] [CrossRef]
- Tashima, H.; Yoshida, E.; Iwao, Y.; Wakizaka, H.; Maeda, T.; Seki, C.; Kimura, Y.; Takado, Y.; Higuchi, M.; Suhara, T.; et al. First prototyping of a dedicated PET system with the hemisphere detector arrangement. Phys. Med. Biol. 2019, 64, 065004. [Google Scholar] [CrossRef]
- Ishii, K.; Hanaoka, K.; Watanabe, S.; Morimoto-Ishikawa, D.; Yamada, T.; Kaida, H.; Yamakawa, Y.; Minagawa, S.; Takenouchi, S.; Ohtani, A.; et al. High-resolution silicon photomultiplier time-of-flight dedicated head PET system for clinical brain studies. J. Nucl. Med. 2023, 64, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, T.; Kobayashi, T.; Yamakawa, Y.; Hanaoka, K.; Watanabe, S.; Morimoto-Ishikawa, D.; Yamada, T.; Kaida, H.; Ishii, K. Initial evaluation of a new maximum-likelihood attenuation correction factor-based attenuation correction for time-of-flight brain PET. Ann. Nucl. Med. 2022, 36, 420–426. [Google Scholar] [CrossRef]
- Morimoto-Ishikawa, D.; Hanaoka, K.; Watanabe, S.; Yamada, T.; Yamakawa, Y.; Minagawa, S.; Takenouchi, S.; Ohtani, A.; Mizuta, T.; Kaida, H.; et al. Evaluation of the performance of a high-resolution time-of-flight PET system dedicated to the head and breast according to NEMA NU 2-2012 standard. EJNMMI Phys. 2022, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Murayama, H.; Kitamura, K.; Yamaya, T.; Yoshida, E.; Omura, T.; Kawai, H.; Inadama, N.; Orita, N. A four-Layer depth of interaction detector block for small animal PET. IEEE Trans. Nucl. Sci. 2004, 51, 2537–2542. [Google Scholar] [CrossRef]
- Yamakawa, Y.K.K. Attenuation correction using level set method for application specific PET scanners. IEEE Nucl. Sci. Symp. Med. Imaging Conf. Rec. 2011, 3130–3132. [Google Scholar] [CrossRef]
- Bailey, D.L.; Meikle, S.R. A convolution-subtraction scatter correction method for 3D PET. Phys. Med. Biol. 1994, 39, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Miyake, K.K.; Matsumoto, K.; Inoue, M.; Nakamoto, Y.; Kanao, S.; Oishi, T.; Kawase, S.; Kitamura, K.; Yamakawa, Y.; Akazawa, A.; et al. Performance evaluation of a new dedicated breast PET scanner using NEMA NU4-2008 standards. J. Nucl. Med. 2014, 55, 1198–1203. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, Y.; Satake, H.; Ishigaki, S.; Shimamoto, K.; Uota, F.; Tadokoro, M.; Sato, T.; Kato, K.; Ishiguchi, T.; Naganawa, S. Physiological background parenchymal uptake of 18F-FDG in normal breast tissues using dedicated breast PET: Correlation with mammographic breast composition, menopausal status, and menstrual cycle. Ann. Nucl. Med. 2022, 36, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Satoh, Y.; Motosugi, U.; Imai, M.; Omiya, Y.; Onishi, H. Evaluation of image quality at the detector’s edge of dedicated breast positron emission tomography. EJNMMI Phys. 2021, 8, 5. [Google Scholar] [CrossRef]
- Tanaka, E.; Kudo, H. Subset-dependent relaxation in block-iterative algorithms for image reconstruction in emission tomography. Phys. Med. Biol. 2003, 48, 1405–1422. [Google Scholar] [CrossRef]
- Satoh, Y.; Imai, M.; Hirata, K.; Asakawa, Y.; Ikegawa, C.; Onishi, H. Optimal relaxation parameters of dynamic row-action maximum likelihood algorithm and post-smoothing filter for image reconstruction of dedicated breast PET. Ann. Nucl. Med. 2021, 35, 608–616. [Google Scholar] [CrossRef]
- Hirata, K.; Kobayashi, K.; Wong, K.P.; Manabe, O.; Surmak, A.; Tamaki, N.; Huang, S.C. A semi-automated technique determining the liver standardized uptake value reference for tumor delineation in FDG PET-CT. PLoS ONE 2014, 9, e105682. [Google Scholar] [CrossRef] [Green Version]
- Satoh, Y.; Imai, M.; Ikegawa, C.; Hirata, K.; Abo, N.; Kusuzaki, M.; Oyama-Manabe, N.; Onishi, H. Effect of radioactivity outside the field of view on image quality of dedicated breast positron emission tomography: Preliminary phantom and clinical studies. Ann. Nucl. Med. 2022, 36, 1010–1018. [Google Scholar] [CrossRef]
- Kamitani, T.; Yabuuchi, H.; Kanemaki, Y.; Tozaki, M.; Sonomura, T.; Mizukoshi, W.; Nakata, W.; Shimono, T.; Urano, M.; Yamano, T.; et al. Effects of menstrual cycle on background parenchymal enhancement and detectability of breast cancer on dynamic contrast-enhanced breast MRI: A multicenter study of an Asian population. Eur. J. Radiol. 2019, 110, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Ashrafinia, S.; Mohy-Ud-Din, H.; Karakatsanis, N.A.; Jha, A.K.; Casey, M.E.; Kadrmas, D.J.; Rahmim, A. Generalized PSF modeling for optimized quantitation in PET imaging. Phys. Med. Biol. 2017, 62, 5149–5179. [Google Scholar] [CrossRef]
- Park, H.; Yi, M.; Lee, J.S. Silicon photomultiplier signal readout and multiplexing techniques for positron emission tomography: A review. Biomed. Eng. Lett. 2022, 12, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zheng, C.; Cui, B.; Qi, Z.; Zhao, Z.; An, Y.; Qiao, L.; Han, Y.; Zhou, Y.; Lu, J. Multiparametric imaging hippocampal neurodegeneration and functional connectivity with simultaneous PET/MRI in Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2440–2452. [Google Scholar] [CrossRef] [Green Version]
- Frost, G.R.; Longo, V.; Li, T.; Jonas, L.A.; Judenhofer, M.; Cherry, S.; Koutcher, J.; Lekaye, C.; Zanzonico, P.; Li, Y.-M. Author correction: Hybrid PET/MRI enables high-spatial resolution, quantitative imaging of amyloid plaques in an Alzheimer’s disease mouse model. Sci. Rep. 2020, 10, 13826. [Google Scholar] [CrossRef] [PubMed]



| PET System | SiPM-Based TOF Scanner | PMT-Based Scanner |
|---|---|---|
| Product name | BresTome™ | Elmammo™ |
| FOV, mm | 162 | 156.5 |
| Transaxial FOV, mm | 264 | 185 |
| Gantry diameter | 300 | 195 |
| Crystal size, mm3 | 2.1 × 2.1 × 15 | 1.44 × 1.44 × 18 |
| Material of crystal | LGSO | LGSO |
| Photo device | SiPM | PMT |
| No. of DOI layers | 1 | 4 |
| TOF technology | Available | n.a. |
| Matrix size | 240 (x) × 240 (y) × 148 (z) | 236 (x) × 236 (y) × 200 (z) |
| Voxel size, mm | 1.1 | 0.78 |
| Spatial resolution at a 5 mm offset in the center of the FOV * | 1.0–2.5 mm | ≤1.5 mm |
| Sensitivity at 0 cm in the center of the FOV, cps/kBq * | 0.06–0.11 cps/Bq (5–10%) | 0.09–0.13 cps/Bq |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satoh, Y.; Hanaoka, K.; Ikegawa, C.; Imai, M.; Watanabe, S.; Morimoto-Ishikawa, D.; Onishi, H.; Ito, T.; Komoike, Y.; Ishii, K. Organ-Specific Positron Emission Tomography Scanners for Breast Imaging: Comparison between the Performances of Prior and Novel Models. Diagnostics 2023, 13, 1079. https://doi.org/10.3390/diagnostics13061079
Satoh Y, Hanaoka K, Ikegawa C, Imai M, Watanabe S, Morimoto-Ishikawa D, Onishi H, Ito T, Komoike Y, Ishii K. Organ-Specific Positron Emission Tomography Scanners for Breast Imaging: Comparison between the Performances of Prior and Novel Models. Diagnostics. 2023; 13(6):1079. https://doi.org/10.3390/diagnostics13061079
Chicago/Turabian StyleSatoh, Yoko, Kohei Hanaoka, Chihiro Ikegawa, Masamichi Imai, Shota Watanabe, Daisuke Morimoto-Ishikawa, Hiroshi Onishi, Toshikazu Ito, Yoshifumi Komoike, and Kazunari Ishii. 2023. "Organ-Specific Positron Emission Tomography Scanners for Breast Imaging: Comparison between the Performances of Prior and Novel Models" Diagnostics 13, no. 6: 1079. https://doi.org/10.3390/diagnostics13061079





