Multifunctional Evaluation Technology for Diagnosing Malfunctions of Regional Pelvic Floor Muscles Based on Stretchable Electrode Array Probe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
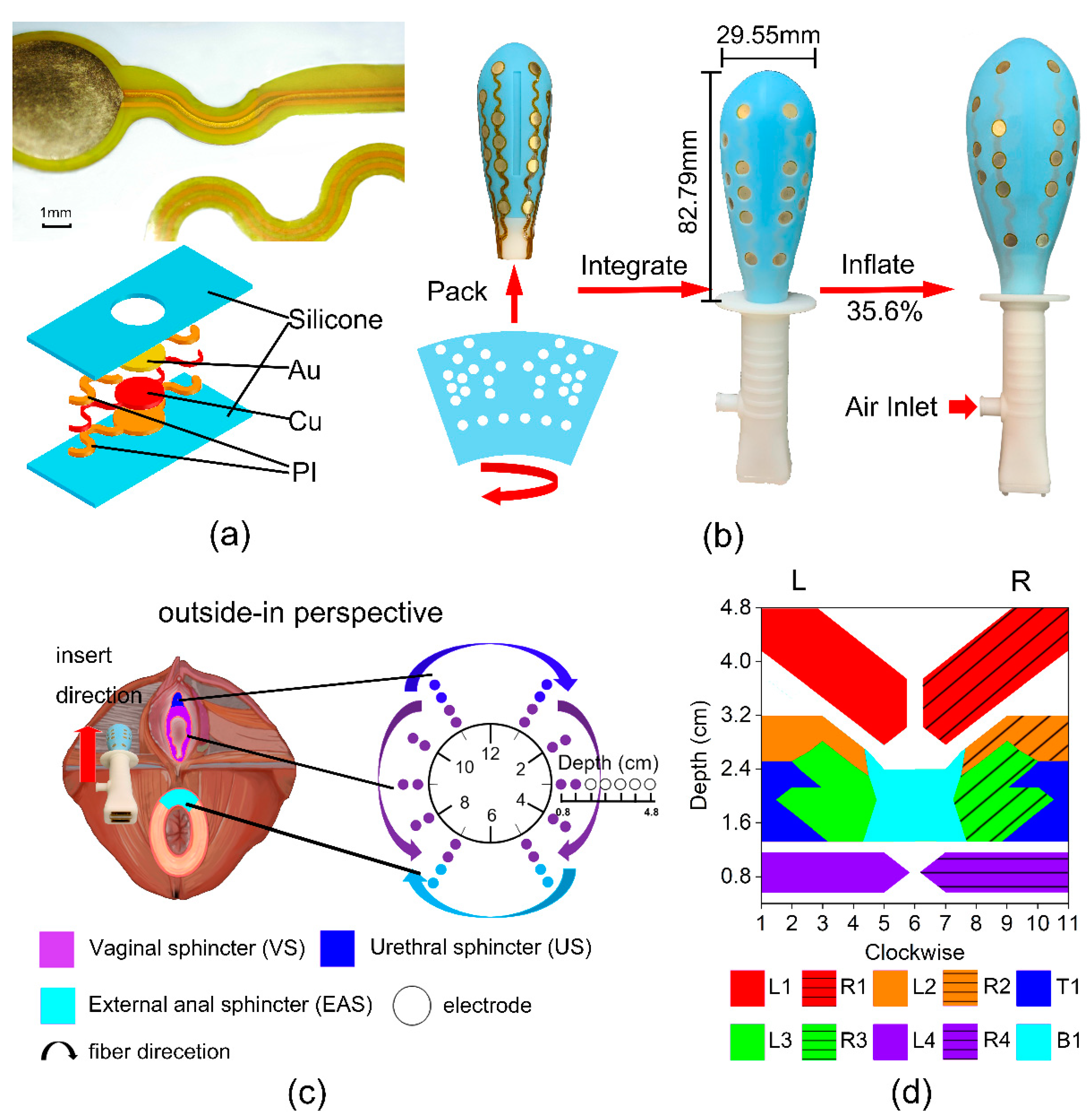
2.2. Airbag-Type Stretchable Electrode Array Probe
2.3. Evaluation System in MET
2.4. Experimental Protocol
2.5. Statistical Analysis
3. Results
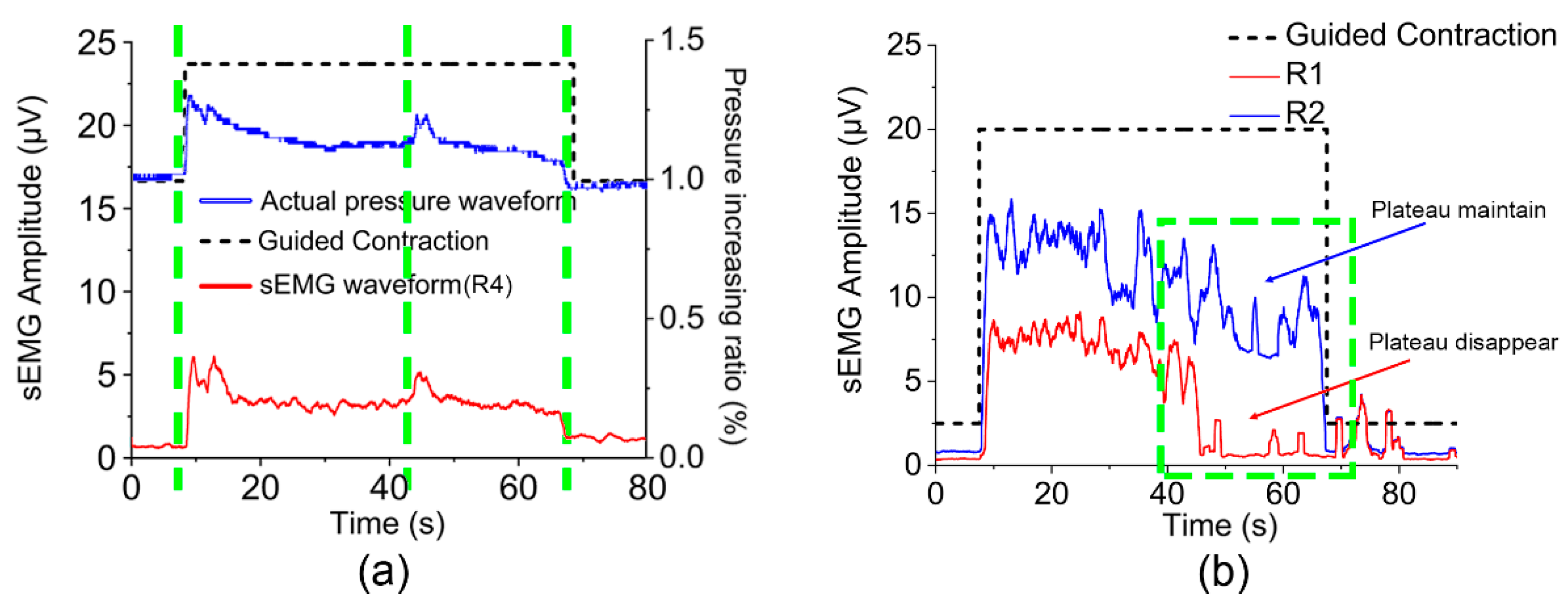
3.1. Accurate Signal Acquisition of ASEA
3.2. Pressure Feedback Analysis
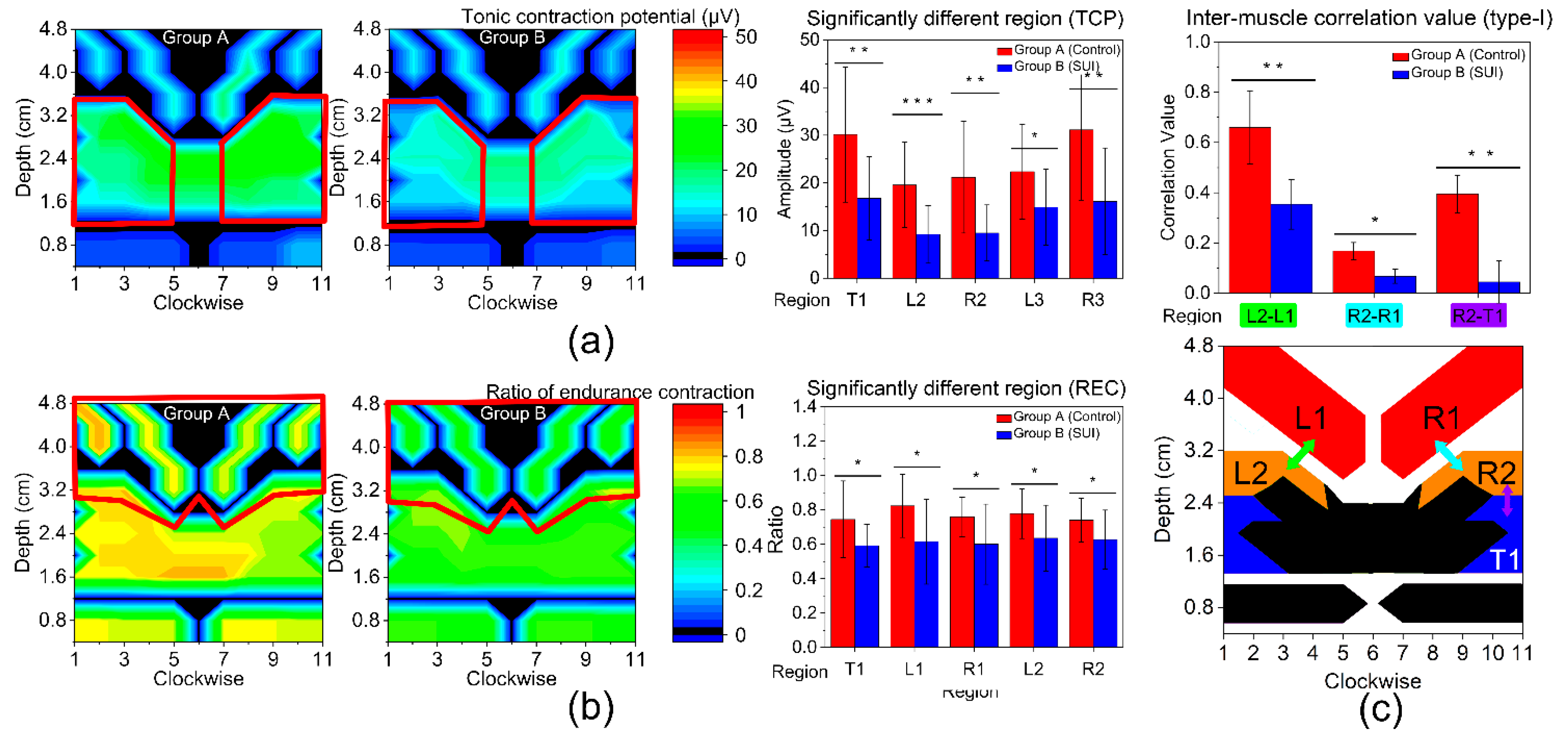
3.3. Type-I Muscle Function
3.4. Type-II Muscle Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lukacz, E.S.; Santiago-Lastra, Y.; Albo, M.E.; Brubaker, L. Urinary Incontinence in Women: A Review. JAMA 2017, 318, 1592–1604. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, A.H.; Taylor, A.W.; Wilson, D.H.; Wilson, D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. BJOG 2000, 107, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M. Stress Incontinence in Women. N. Engl. J. Med. 2021, 384, 2428–2436. [Google Scholar] [CrossRef]
- Hagen, S.; Glazener, C.; McClurg, D.; Macarthur, C.; Elders, A.; Herbison, P.; Wilson, D.; Toozs-Hobson, P.; Hemming, C.; Hay-Smith, J.; et al. Pelvic floor muscle training for secondary prevention of pelvic organ prolapse (PREVPROL): A multicentre randomised controlled trial. Lancet 2017, 389, 393–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, Y.; Brown, H.W.; Brubaker, L.; Cornu, J.N.; Daly, J.O.; Cartwright, R. Urinary incontinence in women. Nat. Rev. Dis. Prim. 2017, 3, 17042. [Google Scholar] [CrossRef] [Green Version]
- Saldana Ruiz, N.; Kaiser, A.M. Fecal incontinence—Challenges and solutions. World J. Gastroenterol. 2017, 23, 11–24. [Google Scholar] [CrossRef]
- Dietz, H.P. Ultrasound in the assessment of pelvic organ prolapse. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 54, 12–30. [Google Scholar] [CrossRef]
- Ptaszkowski, K.; Małkiewicz, B.; Zdrojowy, R.; Paprocka-Borowicz, M.; Ptaszkowska, L. Assessment of the Elastographic and Electromyographic of Pelvic Floor Muscles in Postmenopausal Women with Stress Urinary Incontinence Symptoms. Diagnostics 2021, 11, 2051. [Google Scholar] [CrossRef]
- Castellanos-Lopez, E.; Castillo-Merino, C.; Abuin-Porras, V.; Lopez-Lopez, D.; Romero-Morales, C. Ultrasonography Comparison of Pelvic Floor and Abdominal Wall Muscles in Women with and without Dyspareunia: A Cross-Sectional Study. Diagnostics 2022, 12, 1827. [Google Scholar] [CrossRef]
- Sicilia-Gomez, C.; Fernandez-Carnero, S.; Martin-Perez, A.; Cuenca-Zaldivar, N.; Naranjo-Cinto, F.; Pecos-Martin, D.; Cervera-Cano, M.; Nunez-Nagy, S. Abdominal and Pelvic Floor Activity Related to Respiratory Diaphragmatic Activity in Subjects with and without Non-Specific Low Back Pain. Diagnostics 2022, 12, 2530. [Google Scholar] [CrossRef]
- Del Vescovo, R.; Piccolo, C.L.; Vecchia, N.D.; Giurazza, F.; Cazzato, R.L.; Grasso, R.F.; Zobel, B.B. MRI role in morphological and functional assessment of the levator ani muscle: Use in patients affected by stress urinary incontinence (SUI) before and after pelvic floor rehabilitation. Eur. J. Radiol. 2014, 83, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Peschers, U.M. Evaluation of Pelvic Floor Muscle Strength Using Four Different Techniques. Int. Urogynecol. J. 2001, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, R.R.; Hodges, P.W. Contraction of the pelvic floor muscles during abdominal maneuvers. Arch. Phys. Med. Rehabil. 2001, 82, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-de-Souza, D.P.; Alcaraz-Clariana, S.; Garcia-Luque, L.; Carmona-Perez, C.; Garrido-Castro, J.L.; Cruz-Medel, I.; Camargo, P.R.; Alburquerque-Sendin, F. Absolute and Relative Reliability of the Assessment of the Muscle Mechanical Properties of Pelvic Floor Muscles in Women with and without Urinary Incontinence. Diagnostics 2021, 11, 2315. [Google Scholar] [CrossRef]
- Zhang, Y.; Timm, G.W. 3D Internal Muscle Activities Imaging Based on Multi-Channel Surface EMG Recordings: Surface EMG-Based Muscles Activities Imaging Technique. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010. [Google Scholar]
- Koenig, I.; Eichelberger, P.; Leitner, M.; Moser, H.; Kuhn, A.; Taeymans, J.; Radlinger, L. Pelvic floor muscle activity patterns in women with and without stress urinary incontinence while running. Ann. Phys. Rehabil. Med. 2020, 63, 495–499. [Google Scholar] [CrossRef]
- de Paiva, F.A.; Ferreira, K.R.; Barbosa, M.A.; Barbosa, A.C. Masticatory Myoelectric Side Modular Ratio Asymmetry during Maximal Biting in Women with and without Temporomandibular Disorders. Biosensors 2022, 12, 654. [Google Scholar] [CrossRef]
- Braekken, I.H.; Stuge, B.; Tveter, A.T.; Bo, K. Reliability, validity and responsiveness of pelvic floor muscle surface electromyography and manometry. Int. Urogynecol. J. 2021, 32, 3267–3274. [Google Scholar] [CrossRef]
- Keshwani, N.; McLean, L. State of the art review: Intravaginal probes for recording electromyography from the pelvic floor muscles. Neurourol. Urodyn. 2015, 34, 104–112. [Google Scholar] [CrossRef]
- Dias, N.; Zhang, C.; Spitznagle, T.; Lai, H.H.; Zhang, Y. High-Density Surface Electromyography Assessment of Pelvic Floor Dysfunction in Women with Interstitial Cystitis/Bladder Pain Syndrome. J. Urol. 2020, 204, 1275–1283. [Google Scholar] [CrossRef]
- Cescon, C.; Riva, D.; Zacesta, V.; Drusany-Staric, K.; Martsidis, K.; Protsepko, O.; Baessler, K.; Merletti, R. Effect of vaginal delivery on the external anal sphincter muscle innervation pattern evaluated by multichannel surface EMG: Results of the multicentre study TASI-2. Int. Urogynecol. J. 2014, 25, 1491–1499. [Google Scholar] [CrossRef]
- Wang, S.M.; Dong, S.R.; Li, W.J.; Cen, J.; Zhu, H.M.; Fu, C.Y.; Jin, H.; Li, Y.C.; Feng, X.; Luo, J.K.; et al. Physiology-Based Stretchable Electronics Design Method for Accurate Surface Electromyography Evaluation. Adv. Sci. 2021, 8, 2004987. [Google Scholar] [CrossRef]
- Chimera, N.J.; Holmes, M.W.R.; Gabriel, D.A. Anthropometrics and electromyography as predictors for maximal voluntary isometric wrist torque: Considerations for ergonomists. Appl. Ergon. 2021, 97, 103496. [Google Scholar] [CrossRef] [PubMed]
- Camardella, C.; Junata, M.; Tse, K.C.; Frisoli, A.; Tong, R.K. How Many Muscles? Optimal Muscles Set Search for Optimizing Myocontrol Performance. Front. Comput. Neurosci. 2021, 15, 668579. [Google Scholar] [CrossRef] [PubMed]
- Halski, T.; Ptaszkowski, K.; Slupska, L.; Dymarek, R.; Paprocka-Borowicz, M. Relationship between lower limb position and pelvic floor muscle surface electromyography activity in menopausal women: A prospective observational study. Clin. Interv. Aging 2017, 12, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Ptaszkowski, K.; Malkiewicz, B.; Zdrojowy, R.; Paprocka-Borowicz, M.; Ptaszkowska, L. The Prognostic Value of the Surface Electromyographic Assessment of Pelvic Floor Muscles in Women with Stress Urinary Incontinence. J. Clin. Med. 2020, 9, 1967. [Google Scholar] [CrossRef]
- Ballmer, C.; Eichelberger, P.; Leitner, M.; Moser, H.; Luginbuehl, H.; Kuhn, A.; Radlinger, L. Electromyography of pelvic floor muscles with true differential versus faux differential electrode configuration. Int. Urogynecol. J. 2020, 31, 2051–2059. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Glazer, H.I.; Hacad, C.R. The Glazer Protocol: Evidence-Based Medicine Pelvic Floor Muscle (PFM) Surface Electromyography (SEMG). Biofeedback 2012, 40, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Madill, S.J.; Harvey, M.A.; McLean, L. Women with SUI demonstrate motor control differences during voluntary pelvic floor muscle contractions. Int. Urogynecol. J. Pelvic. Floor Dysfunct. 2009, 20, 447–459. [Google Scholar] [CrossRef]
- Luginbuehl, H.; Baeyens, J.P.; Kuhn, A.; Christen, R.; Oberli, B.; Eichelberger, P.; Radlinger, L. Pelvic floor muscle reflex activity during coughing—An exploratory and reliability study. Ann. Phys. Rehabil. Med. 2016, 59, 302–307. [Google Scholar] [CrossRef]
- Hacad, C.R.; Glazer, H.I. The Glazer Intrapelvic Surface Electromyography (SEMG) Protocol in a Case of Male Urinary Incontinence and a Case of Female Hypoactive Sexual Desire Disorder. Biofeedback 2012, 40, 80–95. [Google Scholar] [CrossRef] [Green Version]
- Blomquist, J.L.; Carroll, M.; Munoz, A.; Handa, V.L. Pelvic floor muscle strength and the incidence of pelvic floor disorders after vaginal and cesarean delivery. Am. J. Obstet. Gynecol. 2020, 222, 62.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baracho, S.M.; Barbosa da Silva, L.; Baracho, E.; Lopes da Silva Filho, A.; Sampaio, R.F.; Mello de Figueiredo, E. Pelvic floor muscle strength predicts stress urinary incontinence in primiparous women after vaginal delivery. Int. Urogynecol. J. 2012, 23, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Rocca Rossetti, S. Functional anatomy of pelvic floor. Arch. Ital. Urol. Androl. 2016, 88, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.T.; Kim, K.H. Pelvic floor muscle exercise and training for coping with urinary incontinence. J. Exerc. Rehabil. 2021, 17, 379–387. [Google Scholar] [CrossRef]
- Delancey, J. Urethral and Bladder Neck Closure Mechanisms—Reply. Am. J. Obstet. Gynecol. 1995, 173, 347–348. [Google Scholar] [CrossRef]
- Delancey, J.O.L. Structural Support of the Urethra as It Relates To Stress Urinary-Incontinence—The Hammock Hypothesis. Am. J. Obstet. Gynecol. 1994, 170, 1713–1723. [Google Scholar] [CrossRef]
- Hegde, A.; Aguilar, V.C.; Davila, G.W. Levator ani defects in patients with stress urinary incontinence: Three-dimensional endovaginal ultrasound assessment. Int. Urogynecol. J. 2017, 28, 85–93. [Google Scholar] [CrossRef]
- Falah-Hassani, K.; Reeves, J.; Shiri, R.; Hickling, D.; McLean, L. The pathophysiology of stress urinary incontinence: A systematic review and meta-analysis. Int. Urogynecol. J. 2021, 32, 501–552. [Google Scholar] [CrossRef]
- Wesnes, S.L.; Lose, G. Preventing urinary incontinence during pregnancy and postpartum: A review. Int. Urogynecol. J. 2013, 24, 889–899. [Google Scholar] [CrossRef]
- Gomelsky, A.; Coco, C.T.; Dmochowski, R.R. Urinary incontinence in women: Non-pharmacologic approaches and newer pharmacotherapies. Minerva Med. 2014, 105, 263–274. [Google Scholar] [CrossRef] [PubMed]


| Variables | Group A N = 15 | Group B N = 15 | p-Value |
|---|---|---|---|
| Age (years) | 29.80 ± 4.63 | 29.33 ± 4.94 | 0.819 |
| BMI (kg/m2) | 21.82 ± 2.18 | 22.81 ± 2.94 | 0.174 |
| Waist-hip ratio | 0.90 ± 0.05 | 0.88 ± 0.05 | 0.503 |
| No. of births (n) | 1.40 ± 0.51 | 1.33 ± 0.49 | 0.478 |
| Variables | Group A N = 15 | Group B N = 15 | p-Value |
|---|---|---|---|
| Rapid Contra. PIR (%) | 2.66 ± 1.85 | 1.34 ± 0.91 | 0.023 |
| Tonic Contra. PIR (%) | 1.14 ± 0.58 | 0.41 ± 0.22 | <0.001 |
| Endurance Contra. PIR (%) | 1.13 ± 0.68 | 0.57 ± 0.40 | 0.012 |
| Rapid Contra. AT (s) | 0.80 ± 0.28 | 1.16 ± 0.36 | 0.006 |
| Tonic Contra. AT (s) | 0.70 ± 0.28 | 1.12 ± 0.38 | 0.002 |
| Endurance Contra. AT (s) | 0.90 ± 0.36 | 1.20 ± 0.38 | 0.036 |
| Tonic Contraction Potential (TCP), μV | Ratio of Endurance Contraction (REC) | |||||
|---|---|---|---|---|---|---|
| Region | Group A N = 15 | Group B N = 15 | p-Value | Group A N = 15 | Group B N = 15 | p-Value |
| T1 (US) | 30.15 ± 14.19 | 16.78 ± 8.73 | 0.004 | 0.74 ± 0.22 | 0.59 ± 0.12 | 0.028 |
| B1 (EAS) | 17.33 ± 9.26 | 14.47 ± 8.86 | 0.396 | 0.71 ± 0.12 | 0.61 ± 0.16 | 0.063 |
| L1 (IC) | 12.95 ± 7.10 | 12.12 ± 10.30 | 0.800 | 0.82 ± 0.19 | 0.62 ± 0.25 | 0.014 |
| R1 (IC) | 12.18 ± 4.24 | 10.27 ± 8.42 | 0.438 | 0.76 ± 0.12 | 0.60 ± 0.23 | 0.025 |
| L2 (PC) | 19.57 ± 8.94 | 9.20 ± 6.06 | <0.001 | 0.78 ± 0.15 | 0.64 ± 0.19 | 0.029 |
| R2 (PC) | 21.17 ± 11.72 | 9.48 ± 5.89 | 0.002 | 0.74 ± 0.13 | 0.63 ± 0.17 | 0.049 |
| L3 (PR) | 22.36 ± 9.96 | 14.90 ± 7.98 | 0.031 | 0.78 ± 0.14 | 0.70 ± 0.33 | 0.363 |
| R3 (PR) | 31.14 ± 14.75 | 16.12 ± 11.22 | 0.004 | 0.67 ± 0.12 | 0.64 ± 0.15 | 0.583 |
| L4 (VS) | 4.12 ± 1.79 | 3.67 ± 1.44 | 0.452 | 0.75 ± 0.28 | 0.65 ± 0.21 | 0.274 |
| R4 (VS) | 4.03 ± 1.85 | 3.73 ± 1.36 | 0.619 | 0.74 ± 0.34 | 0.64 ± 0.29 | 0.434 |
| Rapid Contraction Potential (RCP), μV | Variability of Rapid Contractions (VRC) | |||||
|---|---|---|---|---|---|---|
| Region | Group A N = 15 | Group B N = 15 | p-Value | Group A N = 15 | Group B N = 15 | p-Value |
| T1 (US) | 38.66 ± 12.75 | 26.75 ± 9.66 | 0.008 | 2.01 ± 1.08 | 3.08 ± 2.55 | 0.145 |
| B1 (EAS) | 27.02 ± 10.87 | 22.97 ± 9.28 | 0.282 | 3.38 ± 2.64 | 4.04 ± 2.92 | 0.526 |
| L1 (IC) | 13.76 ± 11.20 | 11.96 ± 6.06 | 0.588 | 1.45 ± 1.35 | 1.79 ± 1.31 | 0.485 |
| R1 (IC) | 10.57 ± 3.40 | 9.79 ± 5.08 | 0.628 | 1.26 ± 0.96 | 1.53 ± 1.11 | 0.480 |
| L2 (PC) | 21.15 ± 10.06 | 20.72 ± 12.59 | 0.918 | 1.91 ± 1.63 | 1.81 ± 1.79 | 0.881 |
| R2 (PC) | 41.96 ± 13.08 | 27.50 ± 13.38 | 0.006 | 1.70 ± 1.61 | 1.66 ± 1.46 | 0.066 |
| L3 (PR) | 33.77 ± 16.50 | 25.52 ± 10.04 | 0.109 | 2.34 ± 1.54 | 2.96 ± 2.73 | 0.446 |
| R3 (PR) | 20.23 ± 9.13 | 12.82 ± 5.95 | 0.014 | 1.19 ± 0.83 | 3.46 ± 2.57 | 0.003 |
| L4 (VS) | 9.24 ± 4.05 | 9.20 ± 3.58 | 0.975 | 1.07 ± 0.64 | 1.27 ± 0.59 | 0.375 |
| R4 (VS) | 8.87 ± 3.54 | 8.36 ± 3.16 | 0.682 | 1.48 ± 1.29 | 1.35 ± 0.82 | 0.732 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Yang, L.; Jiang, H.; Xia, J.; Li, W.; Zhang, Z.; Zhang, S.; Jin, H.; Luo, J.; Dong, S.; et al. Multifunctional Evaluation Technology for Diagnosing Malfunctions of Regional Pelvic Floor Muscles Based on Stretchable Electrode Array Probe. Diagnostics 2023, 13, 1158. https://doi.org/10.3390/diagnostics13061158
Wang S, Yang L, Jiang H, Xia J, Li W, Zhang Z, Zhang S, Jin H, Luo J, Dong S, et al. Multifunctional Evaluation Technology for Diagnosing Malfunctions of Regional Pelvic Floor Muscles Based on Stretchable Electrode Array Probe. Diagnostics. 2023; 13(6):1158. https://doi.org/10.3390/diagnostics13061158
Chicago/Turabian StyleWang, Shengming, Luoqi Yang, Haofei Jiang, Jie Xia, Wenjuan Li, Zujuan Zhang, Shaomin Zhang, Hao Jin, Jikui Luo, Shurong Dong, and et al. 2023. "Multifunctional Evaluation Technology for Diagnosing Malfunctions of Regional Pelvic Floor Muscles Based on Stretchable Electrode Array Probe" Diagnostics 13, no. 6: 1158. https://doi.org/10.3390/diagnostics13061158





