Non-Aneurysmal Perimesencephalic Subarachnoid Hemorrhage: A Literature Review
Abstract
:1. Introduction
2. Epidemiology
3. Risk and Causality Factors
4. Pathogenic Sequence and Causes
5. Clinical Factors, Long-Term Functional and Cognitive Outcomes
6. Diagnosis and Treatment Approach
7. A Detailed Overview of Complications
7.1. Vasoactive Complications
7.2. Hydrocephalus and Fluid Build-Up
8. Our Clinical Experience and General Outcomes
9. Discussion and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PMSAH | Perimesencephalic subarachnoid hemorrhage. |
| PMH | Perimesencephalic hemorrhage. |
| aSAH | aneurysmal subarachnoid hemorrhage. |
| NAPMSAH | Non-aneurysmal perimesencephalic suabrrachnoid hemorrhage. |
| CT | Computer tomography. |
| DSA | digital subtraction angiography (addressing point 25). |
| MR | Magnetic resonance. |
| CSF | Cerebrospinal fluid |
References
- Purves. Neuroscience; Sinauer Associates: Sunderland, MA, USA, 2004. [Google Scholar]
- Flaherty, M.L.; Haverbusch, M.; Kissela, B.; Kleindorfer, D.; Schneider, A.; Sekar, P.; Moomaw, C.J.; Sauerbeck, L.; Broderick, J.P.; Woo, D. Perimesencephalic Subarachnoid Hemorrhage: Incidence, Risk Factors, and Outcome. J. Stroke Cerebrovasc. Dis. 2005, 14, 267–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, B.; Wu, X.; Brackett, A.; Malhotra, A. Meta-analysis of recent literature on utility of follow-up imaging in isolated perimesencephalic hemorrhage. Clin. Neurol. Neurosurg. 2019, 180, 111–116. [Google Scholar] [CrossRef]
- Mohan, M.; Islim, A.; Dulhanty, L.; Parry-Jones, A.; Patel, H. CT angiogram negative perimesencephalic subarachnoid hemorrhage: Is a subsequent DSA necessary? A systematic review. J. Neurointerventional Surg. 2019, 11, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.L.; Schweizer, T.A. Spontaneous subarachnoid haemorrhage. Lancet 2017, 389, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Alén, J.F.; Lagares, A.; Lobato, R.D.; Gómez, P.A.; Rivas, J.J.; Ramos, A. Comparison between perimesencephalic nonaneurysmal subarachnoid hemorrhage and subarachnoid hemorrhage caused by posterior circulation aneurysms. J. Neurosurg. 2003, 98, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Amer, R.R.; Bakhsh, E.A. Nonaneurysmal Perimesencephalic Subarachnoid Hemorrhage as an Atypical Initial Presentation of Cerebral Venous Sinus Thrombosis: A Case Report. Am. J. Case Rep. 2018, 19, 472–477. [Google Scholar] [CrossRef]
- Galli, F.; Pandolfi, M.; Liguori, A.; Gurgitano, M.; Sberna, M. Bleeding of Perivascular Spaces in Midbrain of a Young Patient With Head Trauma. Cureus 2021, 13, e12884. [Google Scholar] [CrossRef]
- Buell, T.J.; Ramesh, A.; Ding, D.; Raper, D.M.S.; Chen, C.J.; Starke, R.M.; Mukherjee, S.; Crowley, R.W. Dilated Virchow-Robin Spaces Mimicking a Brainstem Arteriovenous Malformation. J. Neurosci. Rural. Pract. 2017, 8, 291–293. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Tzourio, C.; Soumaré, A.; Mazoyer, B.; Dufouil, C.; Chabriat, H. Severity of Dilated Virchow-Robin Spaces Is Associated With Age, Blood Pressure, and MRI Markers of Small Vessel Disease. Stroke 2010, 41, 2483–2490. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, T.H.; Solomon, R.A. Perimesencephalic nonaneurysmal subarachnoid hemorrhage: Review of the literature. Neurosurgery 1996, 39, 433–440, discussion 440. [Google Scholar]
- Hirashima, Y.; Takashima, S.; Matsumura, N.; Kurimoto, M.; Origasa, H.; Endo, S. Right sylvian fissure subarachnoid hemorrhage has electrocardiographic consequences. Stroke 2001, 32, 2278–2281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Gijn, J.; Rinkel, G.J.E. Subarachnoid haemorrhage: Diagnosis, causes and management. Brain A J. Neurol. 2001, 124 Pt 2, 249–278. [Google Scholar] [CrossRef] [PubMed]
- Kleinpeter, G.; Lehr, S. Characterization of risk factor differences in perimesencephalic subarachnoid hemorrhage. Min-Minim. Invasive Neurosurg. 2003, 46, 142–148. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Z.; Zhang, W.B.; Wang, X.; Lenahan, C.; Fang, Y.; Luo, Y.; Liu, Y.; Mei, S.; Chen, S.; et al. Development of a nomogram for predicting clinical outcome in patients with angiogram-negative subarachnoid hemorrhage. CNS Neurosci. Ther. 2021, 27, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, B.J.; Son, W.; Kwak, Y.; Park, J.; Park, S.H.; Kim, Y.S.; Kang, D.H. Retrospective 6 month-outcomes and prognostic factors following spontaneous angiogram-negative non-perimesencephalic subarachnoid hemorrhage. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2022, 96, 12–20. [Google Scholar] [CrossRef]
- Beseoglu, K.; Pannes, S.; Steiger, H.J.; Hanggi, D. Long-term outcome and quality of life after nonaneurysmal subarachnoid hemorrhage. Acta Neurochir. 2010, 152, 409–416. [Google Scholar] [CrossRef]
- Sarabia, E.; Lagares, A.; Fernandez-Alen, J.A.; Arikan, F.; Vilalta, J.; Ibanez, J.; Maillo, A.; Gabarros, A.; Dominiguez, J.; Horcajadas, A.; et al. Idiopathic subarachnoid hemorrhage: A multicentre series of 220 patients. Neurocirurgia 2010, 21, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.S.; Feigin, V.; Bennett, D.; Lin, R.B.; Hankey, G.; Jamrozik, K. Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS) Group: Active and passive smoking and the risk of subarachnoid hemorrhage: An international population-based case-control study. Stroke 2004, 35, 633–637. [Google Scholar] [CrossRef] [Green Version]
- Naidech, A.M.; Rosenberg, N.F.; Maas, M.B.; Bendok, B.R.; Batjer, H.H.; Nemeth, A.J. Predictors of hemorrhage volume and disability after perimesencephalic subarachnoid hemorrhage. Neurology 2012, 78, 811–815. [Google Scholar] [CrossRef]
- Gupta, S.K.; Gupta, R.; Khosla, V.K.; Mohindra, S.; Chhabra, R.; Khandelwal, N.; Gupta, V.; Mukherjee, K.K.; Tewari, M.K.; Mathuriya, S.N.; et al. Nonaneurysmal nonperimesencephalic subarachnoid hemorrhage: Is it a benign entity? Surg. Neurol. 2009, 71, 566–571, discussion 2009, 571, 572. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, J.H.; Qin, X. Perimesencephalic subarachnoid hemorrhage: Risk factors, clinical presentations, and outcome. Acta Neurochir. Suppl. 2011, 110, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Caeiro, L.; Santos, C.O.; Ferro, J.M.; Figueira, M.L. Neuropsychiatric disturbances in acute subarachnoid haemorrhage. Eur. J. Neurol. 2011, 18, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Hirano, K.; Kamada, M.; Imamura, K.; Ishii, N.; Sekihara, Y.; Suzuki, Y.; Ishii, R. Perimesencephalic nonaneurysmal subarachnoid haemorrhage and variations in the veins. Neuroradiology 2002, 44, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, A.; Schweizer, T.A.; Spears, J.; Cusimano, M.; Macdonald, R.L. Nonaneurysmal perimesencephalic subarachnoid hemorrhage: Diagnosis, pathophysiology, clinical characteristics, and long-term outcome. World Neurosurg. 2014, 82, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Shad, A.; Rourke, T.J.; Jahromi, A.H.; Green, A.L. Straight sinus stenosis as a proposed cause of perimesencephalic non-aneurysmal haemorrhage. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2008, 15, 839–841. [Google Scholar] [CrossRef]
- Mathews, M.S.; Brown, D.; Brant-Zawadzki, M. Perimesencephalic nonaneurysmal hemorrhage associated with vein of Galen stenosis. Neurology 2008, 70 Pt 2, 2410–2411. [Google Scholar] [CrossRef]
- Hegazi, M.O.; Ahmed, S.; Sakr, M.G.; Hassanien, O.A. Anticoagulation for cerebral venous thrombosis with subarachnoid hemorrhage: A case report. Med. Princ. Pract. 2010, 19, 73–75. [Google Scholar] [CrossRef]
- Roman-Filip, C.C.; Stîngaciu, A.R.; Catană, M.G.; Dan, A.A.; Bălaşa, A.F.; Juravle, C.; Grosu, F. Atypical posterior circulation strokes: A case-based review of rare anatomical variations involved. Rom. J. Morphol. Embryol. 2021, 62, 289–293. [Google Scholar] [CrossRef]
- Maier, S.; Motataianu, A.; Bajko, Z.; Romaniuc, A.; Balasa, A. Pontine cavernoma haemorrhage at 24 weeks of pregnancy that resulted in eight-and-a-half syndrome. Acta Neurol. Belg. 2019, 119, 471–474. [Google Scholar] [CrossRef]
- Van der Schaaf, I.C.; Velthuis, B.K.; Gouw, A.; Rinkel, G.J. Venous drainage in perimesencephalic hemorrhage. Stroke 2004, 35, 1614–1618. [Google Scholar] [CrossRef] [Green Version]
- Griessenauer, C.J.; Loukas, M.; Scott, J.A.; Tubbs, R.S.; Cohen-Gadol, A.A. The artery of Davidoff and Schechter: An anatomical study with neurosurgical case correlates. Br. J. Neurosurg. 2013, 27, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.S. Dural arteriovenous fistula supplied by the artery of Davidoff and Schechter. Radiol. Case Rep. 2015, 5, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gioppo, A.; Faragò, G.; Caldiera, V.; Caputi, L.; Cusin, A.; Ciceri, E. Medial tentorial dural arteriovenous fistula embolization: Single experience with embolic liquid polymer SQUID and review of the literature. World Neurosurg. 2017, 107, 1050.e1–1050.e7. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Delen, E.; Korfali, E. Perimesencephalic subarachnoid hemorrhage: Etiologies, risk factors, and necessity of the second angiogram. Asian J. Neurosurg. 2016, 11, 50–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sattur, M.G.; Gunasekaran, A.; Spiotta, A.M.; Lena, J.R. Basilar Artery Perforator Aneurysms and their Contemporary Management. Neurol. India 2020, 68, 1301–1306. [Google Scholar]
- Schwartz, T.H.; Yoon, S.S.; Cutruzzola, F.W.; Goodman, R.R. Third ventriculostomy: Post-operative ventricular size and outcome. Min-Minim. Invasive Neurosurg. 1996, 39, 122–129. [Google Scholar] [CrossRef]
- Madureira, S.; Canhão, P.; Guerreiro, M.; Ferro, J.M. Cognitive and emotional consequences of perimesencephalic subarachnoid hemorrhage. J. Neurol. 2000, 247, 862–867. [Google Scholar] [CrossRef]
- Fu, F.W.; Rao, J.; Zheng, Y.Y.; Song, L.; Chen, W.; Zhou, Q.H.; Yang, J.G.; Ke, J.Q.; Zheng, G.Q. Perimesencephalic nonaneurysmal subarachnoid hemorrhage caused by transverse sinus thrombosis: A case report and review of literature. Medicine 2017, 96, e7374. [Google Scholar] [CrossRef]
- Marquardt, G.; Niebauer, T.; Schick, U.; Lorenz, R. Long term follow up after perimesencephalic subarachnoid haemorrhage. J. Neurol. Neurosurg. Psychiatry 2000, 69, 127–130. [Google Scholar] [CrossRef] [Green Version]
- Greebe, P.; Rinkel, G.J.; Algra, A. Anosmia after perimesencephalic nonaneurysmal hemorrhage. Stroke 2009, 40, 2885–2886. [Google Scholar] [CrossRef] [Green Version]
- Lago, A.; López-Cuevas, R.; Tembl, J.I.; Fortea, G.; Górriz, D.; Aparici, F.; Parkhutik, V. Short- and long-term outcomes in non-aneurysmal non-perimesencephalic subarachnoid hemorrhage. Neurol. Res. 2016, 38, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Greebe, P.; Rinkel, G.J. Life expectancy after perimesencephalic subarachnoid hemorrhage. Stroke 2007, 38, 1222–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godefroy, O.; Fickl, A.; Roussel, M.; Auribault, C.; Bugnicourt, J.M.; Lamy, C.; Canaple, S.; Petitnicolas, G. Is the Montreal Cognitive Assessment superior to the Mini-Mental State Examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke 2011, 42, 1712–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweizer, T.A.; Al-Khindi, T.; Loch Macdonald, R. Diffusion tensor imaging as a surrogate marker for outcome after perimesencephalic subarachnoid hemorrhage. Clin. Neurol. Neurosurg. 2012, 114, 798–800. [Google Scholar] [CrossRef]
- Cho, M.K.; Jang, S.H. Diffusion Tensor Imaging Studies on Spontaneous Subarachnoid Hemorrhage-Related Brain Injury: A Mini-Review. Front. Neurol. 2020, 11, 283. [Google Scholar] [CrossRef]
- Sabri, M.; Ai, J.; Knight, B.; Tariq, A.; Jeon, H.; Shang, X.; Marsden, P.A.; Loch Macdonald, R. Uncoupling of endothelial nitric oxide synthase after experimental subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2011, 31, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Backes, D.; Rinkel, G.J.; Kemperman, H.; Linn, F.H.; Vergouwen, M.D. Time-dependent test characteristics of head computed tomography in patients suspected of nontraumatic subarachnoid hemorrhage. Stroke 2012, 43, 2115–2119. [Google Scholar] [CrossRef] [Green Version]
- Long, B.; Koyfman, A.; Runyon, M.S. Subarachnoid Hemorrhage: Updates in Diagnosis and Management. Emerg. Med. Clin. North Am. 2017, 35, 803–824. [Google Scholar] [CrossRef]
- Carpenter, C.R.; Hussain, A.M.; Ward, M.J.; Zipfel, G.J.; Fowler, S.; Pines, J.M.; Sivilotti, M.L. Spontaneous Subarachnoid Hemorrhage: A Systematic Review and Meta-analysis Describing the Diagnostic Accuracy of History, Physical Examination, Imaging, and Lumbar Puncture With an Exploration of Test Thresholds. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2016, 23, 963–1003. [Google Scholar] [CrossRef] [Green Version]
- Sailer, A.M.; Wagemans, B.A.; Nelemans, P.J.; de Graaf, R.; van Zwam, W.H. Diagnosing intracranial aneurysms with MR angiography: Systematic review and meta-analysis. Stroke 2014, 45, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Potter, C.A.; Fink, K.R.; Ginn, A.L.; Haynor, D.R. Perimesencephalic Hemorrhage: Yield of Single versus Multiple DSA Examinations—A Single-Center Study and Meta-Analysis. Radiology 2016, 281, 858–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutinho, J.M.; Sacho, R.H.; Schaafsma, J.D.; Agid, R.; Krings, T.; Radovanovic, I.; Matouk, C.C.; Mikulis, D.J.; Mandell, D.M. High-Resolution Vessel Wall Magnetic Resonance Imaging in Angiogram-Negative Non-Perimesencephalic Subarachnoid Hemorrhage. Clin. Neuroradiol. 2017, 27, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Mattay, R.R.; Saucedo, J.F.; Lehman, V.T.; Xiao, J.; Obusez, E.C.; Raymond, S.B.; Fan, Z.; Song, J.W. Current Clinical Applications of Intracranial Vessel Wall MR Imaging. Semin. Ultrasound CT MR 2021, 42, 463–473. [Google Scholar] [CrossRef]
- Reis, G.Z.; de Moraes Machado, F.S.; de Paula, W.K.; Ribas, F.D.; Dos Reis, F.I. Hydrocephalous as a Complication of Perimesencephalic Nonaneurysmal Subarachnoid Hemorrhage. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2020, 29, 105381. [Google Scholar] [CrossRef]
- Marupudi, N.I.; Mittal, S. Diagnosis and Management of Hyponatremia in Patients with Aneurysmal Subarachnoid Hemorrhage. J. Clin. Med. 2015, 4, 756–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, J.P.; Kim, S.E.; Chai, C.L.; Hong, E.P.; Yang, J.S.; Kang, S.H.; Choi, H.J.; Cho, Y.J. Seizure incidence of angiogram-negative subarachnoid hemorrhage: An updated meta-analysis. J. Chin. Med. Assoc. 2020, 83, 466–470. [Google Scholar] [CrossRef]
- Cánovas, D.; Gil, A.; Jato, M.; de Miquel, M.; Rubio, F. Clinical outcome of spontaneous non-aneurysmal subarachnoid hemorrhage in 108 patients. Eur. J. Neurol. 2012, 19, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Samaniego, E.A.; Dabus, G.; Fuentes, K.; Linfante, I. Endovascular treatment of severe vasospasm in nonaneurysmal perimesencephalic subarachnoid hemorrhage. Neurocritical Care 2011, 15, 537–541. [Google Scholar] [CrossRef]
- Hsu, W.; Pradilla, G.; Garonzik, I.M.; Conway, J.E. Pretruncal non-aneurysmal subarachnoid hemorrhage causing basilar artery vasospasm. Neurocritical Care 2010, 13, 256–260. [Google Scholar] [CrossRef]
- Daou, B.J.; Khalsa, S.S.S.; Anand, S.K.; Williamson, C.A.; Cutler, N.S.; Aaron, B.L.; Srinivasan, S.; Rajajee, V.; Sheehan, K.; Pandey, A.S. Volumetric quantification of aneurysmal subarachnoid hemorrhage independently predicts hydrocephalus and seizures. J. Neurosurg. 2021, 135, 1155–1163. [Google Scholar] [CrossRef]
- Chou, S.H. Subarachnoid Hemorrhage. Continuum 2021, 27, 1201–1245. [Google Scholar] [CrossRef] [PubMed]
- Merkel, H.; Lindner, D.; Gaber, K.; Ziganshyna, S.; Jentzsch, J.; Mucha, S.; Gerhards, T.; Sari, S.; Stock, A.; Vothel, F.; et al. Standardized Classification of Cerebral Vasospasm after Subarachnoid Hemorrhage by Digital Subtraction Angiography. J. Clin. Med. 2022, 11, 2011. [Google Scholar] [CrossRef] [PubMed]
- Nesvick, C.L.; Oushy, S.; Rinaldo, L.; Wijdicks, E.F.; Lanzino, G.; Rabinstein, A.A. Clinical complications and outcomes of angiographically negative subarachnoid hemorrhage. Neurology 2019, 92, e2385–e2394. [Google Scholar] [CrossRef] [PubMed]
- Prat, D.; Goren, O.; Bruk, B.; Bakon, M.; Hadani, M.; Harnof, S. Description of the vasospasm phenomena following perimesencephalic nonaneurysmal subarachnoid hemorrhage. BioMed Res. Int. 2013, 2013, 371063. [Google Scholar] [CrossRef]
- Mohan, M.; Islim, A.I.; Rasul, F.T.; Rominiyi, O.; deSouza, R.M.; Poon, M.T.C.; Jamjoom, A.A.B.; Kolias, A.G.; Woodfield, J.; Patel, K.; et al. British Neurosurgical Trainee Research Collaborative. Subarachnoid haemorrhage with negative initial neurovascular imaging: A systematic review and meta-analysis. Acta Neurochir. 2019, 161, 2013–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, C.M.; Kistler, J.P.; Davis, J.M. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980, 6, 1–9. [Google Scholar] [CrossRef]
- Wilson, D.A.; Nakaji, P.; Abla, A.A.; Uschold, T.D.; Fusco, D.J.; Oppenlander, M.E.; Albuquerque, F.C.; McDougall, C.G.; Zabramski, J.M.; Spetzler, R.F. A simple and quantitative method to predict symptomatic vasospasm after subarachnoid hemorrhage based on computed tomography: Beyond the Fisher scale. Neurosurgery 2012, 71, 869–875. [Google Scholar] [CrossRef]
- Konczalla, J.; Kashefiolasl, S.; Brawanski, N.; Lescher, S.; Senft, C.; Platz, J.; Seifert, V. Cerebral vasospasm and delayed cerebral infarctions in 225 patients with non-aneurysmal subarachnoid hemorrhage: The underestimated risk of Fisher 3 blood distribution. J. Neurointerventional Surg. 2016, 8, 1247–1252. [Google Scholar] [CrossRef]
- Schuss, P.; Hadjiathanasiou, A.; Brandecker, S.; Wispel, C.; Borger, V.; Guresir, A.; Vatter, H.; Güresir, E. Risk factors for shunt dependency in patients suffering from spontaneous, non-aneurysmal subarachnoid hemorrhage. Neurosurg. Rev. 2019, 42, 139–145. [Google Scholar] [CrossRef]
- Samuelsson, J.; Sunila, M.; Rentzos, A.; Nilsson, D. Intra-arterial nimodipine for severe cerebral vasospasm after aneurysmal subarachnoid haemorrhage—neurological and radiological outcome. Neuroradiol. J. 2022, 35, 213–219. [Google Scholar] [CrossRef]
- Chen, S.; Yang, Q.; Chen, G.; Zhang, J.H. An update on inflammation in the acute phase of intracerebral hemorrhage. Transl. Stroke Res. 2014, 6, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Luo, J.; Reis, C.; Manaenko, A.; Zhang, J. Hydrocephalus after Subarachnoid Hemorrhage: Pathophysiology, Diagnosis, and Treatment. BioMed Res. Int. 2017, 2017, 8584753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, P.; Raya, A.; Zipfel, G.J.; Dhar, R. Factors Associated with Acute and Chronic Hydrocephalus in Nonaneurysmal Subarachnoid Hemorrhage. Neurocritical Care 2016, 24, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Mensing, L.A.; Vergouwen, M.D.I.; Laban, K.G.; Ruigrok, Y.M.; Velthuis, B.K.; Algra, A.; Gabriel, J.E. Perimesencephalic hemorrhage: A review of epidemiology, risk factors, presumed cause, clinical course, and outcome. Stroke 2018, 49, 1363–1370. [Google Scholar] [CrossRef]
- Hou, K.; Yu, J. Current status of perimesencephalic non-aneurysmal subarachnoid hemorrhage. Front. Neurol. 2022, 13, 960702. [Google Scholar] [CrossRef]
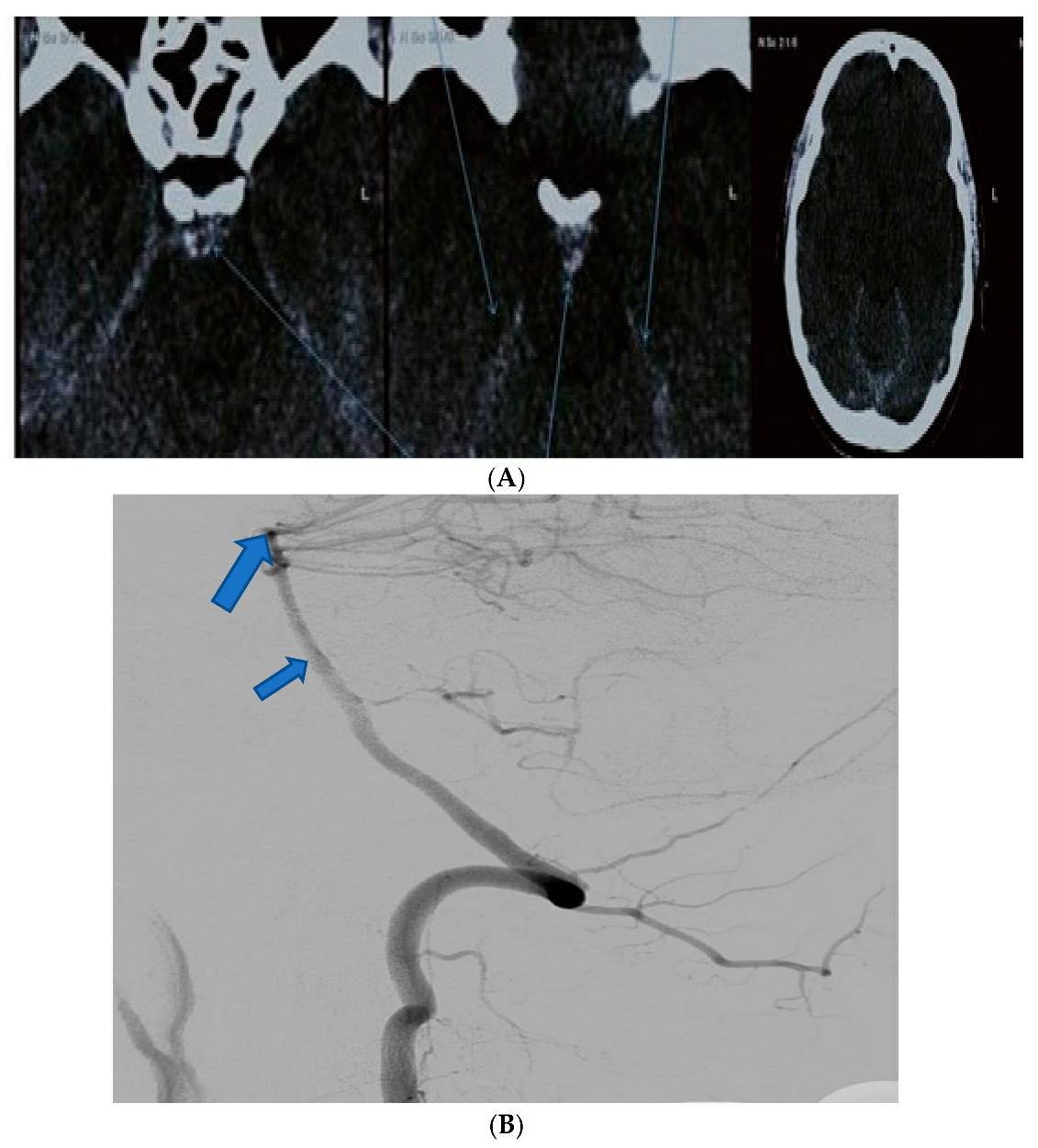
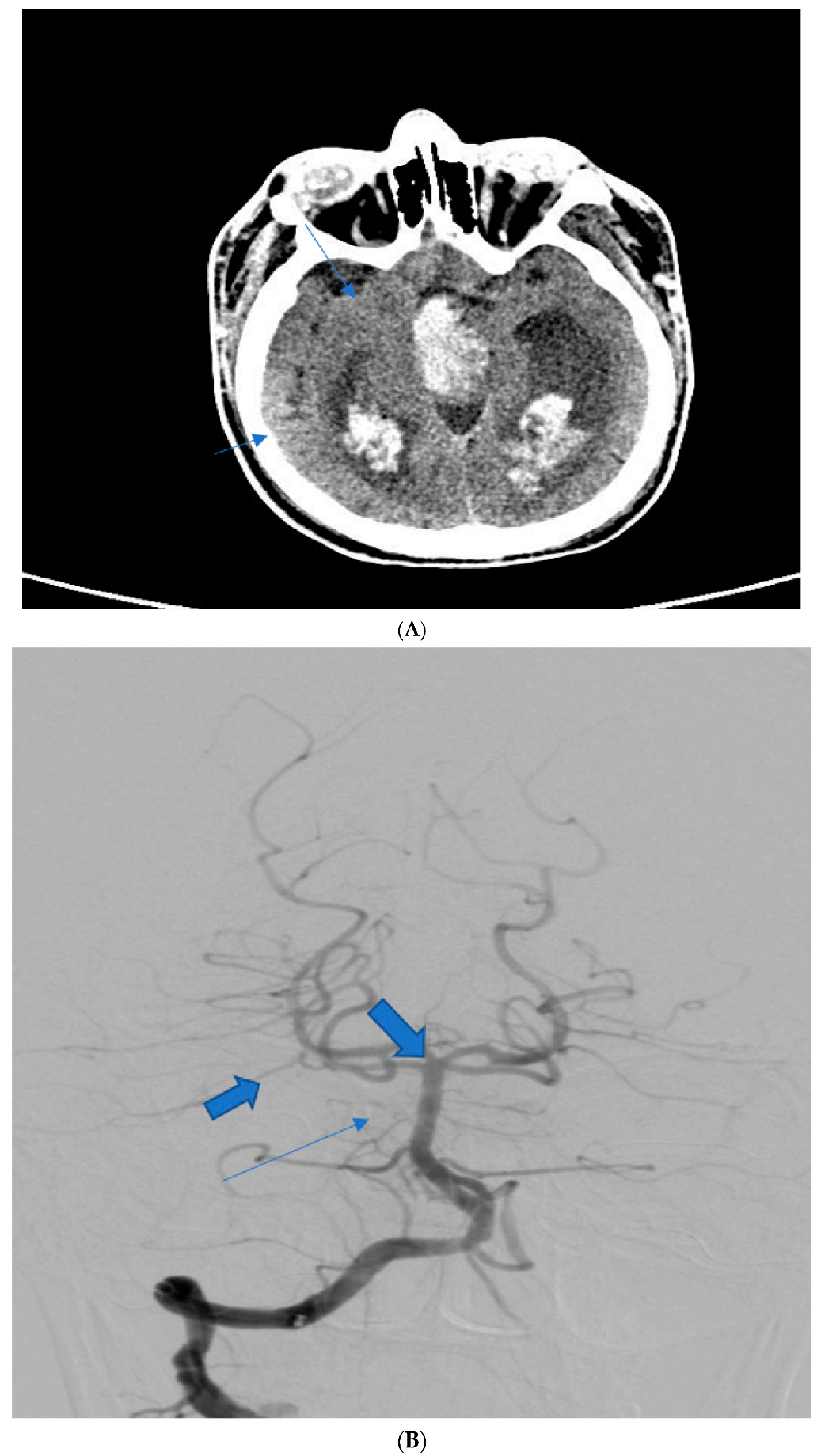
| Patient Age | Patient Sex | Presenting Symptoms | Diagnosis Method | Hunt Hess Scale at Diagnosis | Modified Fischer Scale at Diagnosis | Complications | Rankin Scale at Discharge | Given Treatment |
|---|---|---|---|---|---|---|---|---|
| 54 | F | Diplopia, headache | Native CT scan, CTA | I | I | Mild vasospasm, mild hydrocephaly | 2 | Nimodipine |
| 61 1 | M | Flaccid tetraplegia, altered Mental status | Native CT scan, DSA | IV | IV | Severe vasospasm, hydrocephalus | 6 (deceased) | Nimodipine, mannitol |
| 37 | M | Headache, diplopia | Native CT scan, DSA angiography | II | II | None | 3 | Nimodipine |
| 48 | F | 3rd nerve palsy, pupillary anomalies | Native CT scan, CTA | II | II | Mild vasospasm | 2 | Nimodipine |
| 41 2 | M | Headache, diplopia | Native CT scan, DSA | III | III | Mild vasospasm | 3 | Nimodipine, mannitol |
| 59 | M | 3rd nerve paresis, pupillary anomalies, hemiplegia | Native CT scan, DSA angiography | V | IV | Hydrocephalus, moderate vasospasm | 4 | Nimodipine, mannitol |
| 44 | F | Right internuclear ophthalmoplegia | Native CT scan, CTA | III | I | None | 1 | Nimodipine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roman-Filip, I.; Morosanu, V.; Bajko, Z.; Roman-Filip, C.; Balasa, R.I. Non-Aneurysmal Perimesencephalic Subarachnoid Hemorrhage: A Literature Review. Diagnostics 2023, 13, 1195. https://doi.org/10.3390/diagnostics13061195
Roman-Filip I, Morosanu V, Bajko Z, Roman-Filip C, Balasa RI. Non-Aneurysmal Perimesencephalic Subarachnoid Hemorrhage: A Literature Review. Diagnostics. 2023; 13(6):1195. https://doi.org/10.3390/diagnostics13061195
Chicago/Turabian StyleRoman-Filip, Iulian, Valentin Morosanu, Zoltan Bajko, Corina Roman-Filip, and Rodica Ioana Balasa. 2023. "Non-Aneurysmal Perimesencephalic Subarachnoid Hemorrhage: A Literature Review" Diagnostics 13, no. 6: 1195. https://doi.org/10.3390/diagnostics13061195
APA StyleRoman-Filip, I., Morosanu, V., Bajko, Z., Roman-Filip, C., & Balasa, R. I. (2023). Non-Aneurysmal Perimesencephalic Subarachnoid Hemorrhage: A Literature Review. Diagnostics, 13(6), 1195. https://doi.org/10.3390/diagnostics13061195







